The Anti-Oxidant Defense System of the Marine Polar Ciliate Euplotes nobilii: Characterization of the MsrB Gene Family
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cells
2.2. DNA Isolation and Sequencing
2.3. RNA Extraction and Gene Expression Analysis
2.4. Sequence Analysis and Phylogenetic Relationships
3. Results
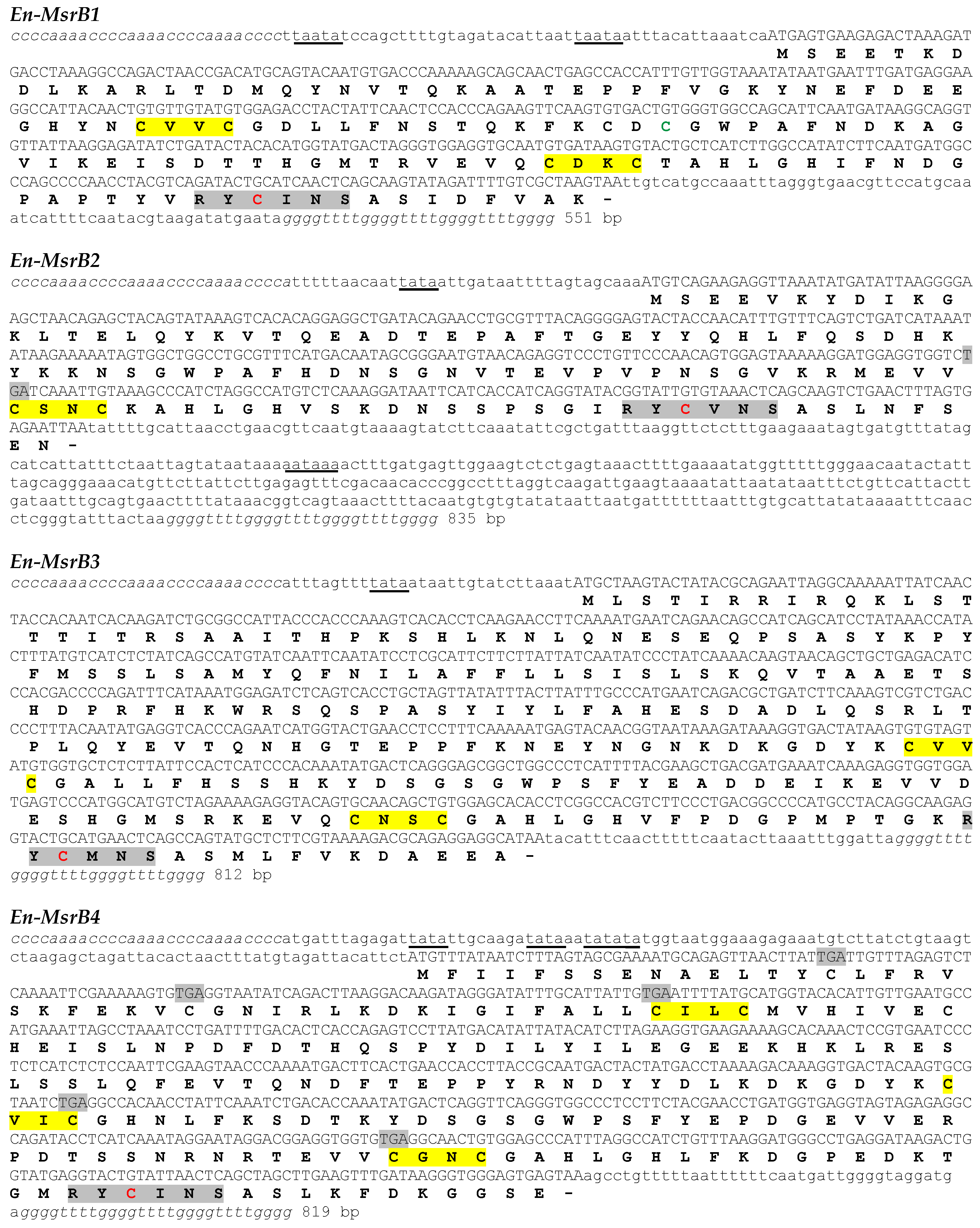
3.1. Gene Identification and Nucleotide Sequences
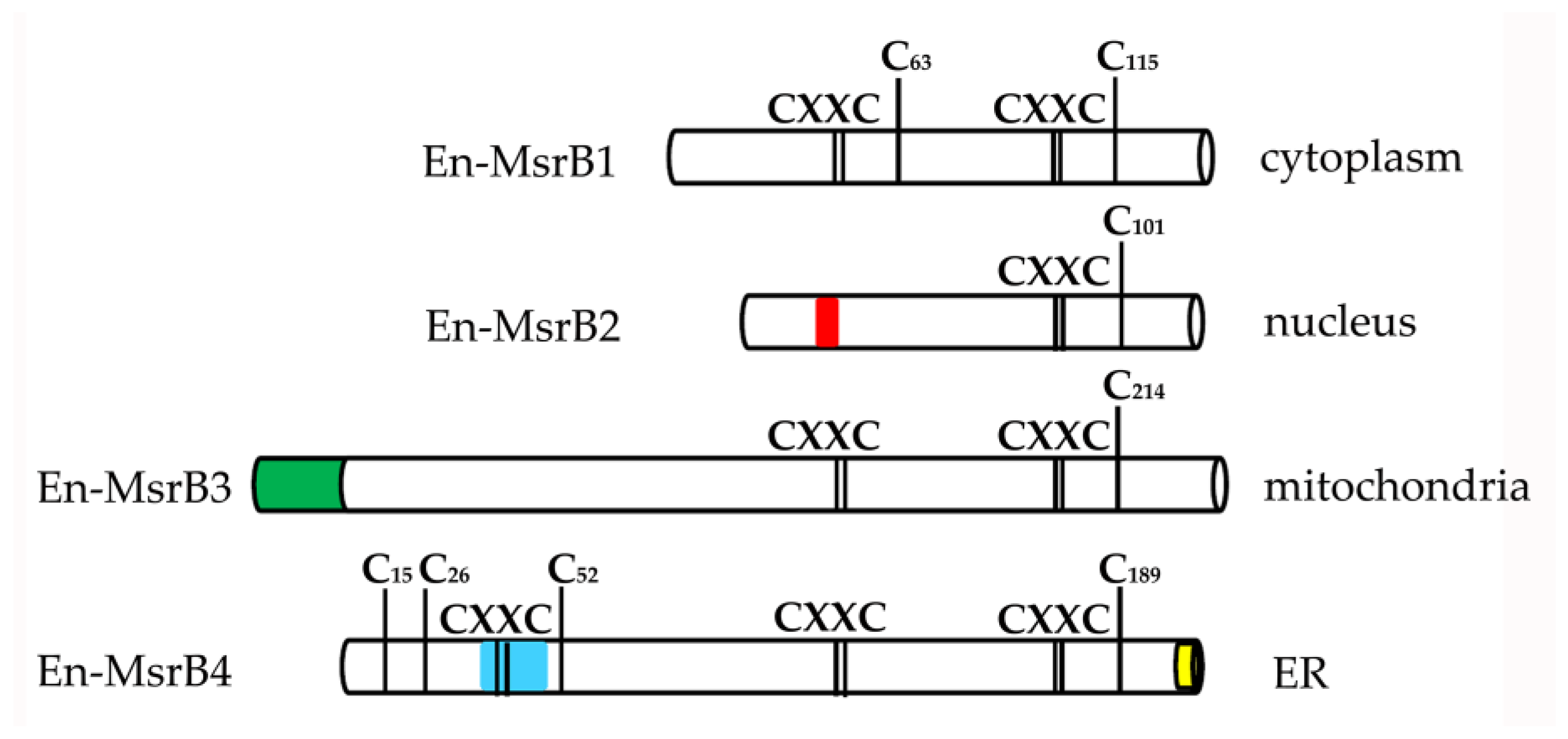
3.2. Amino Acid Sequences
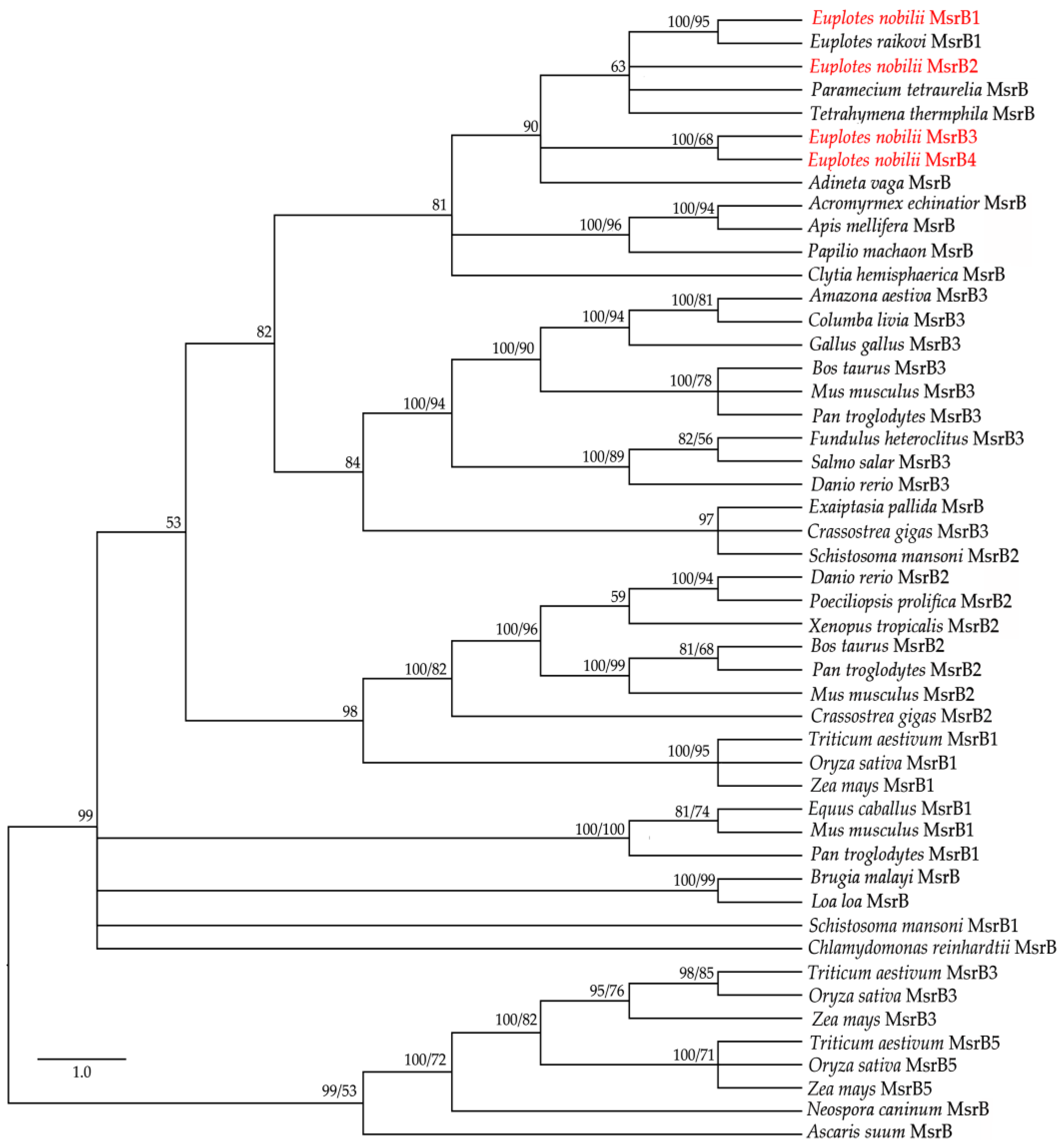
3.3. Phylogenetic Relationships
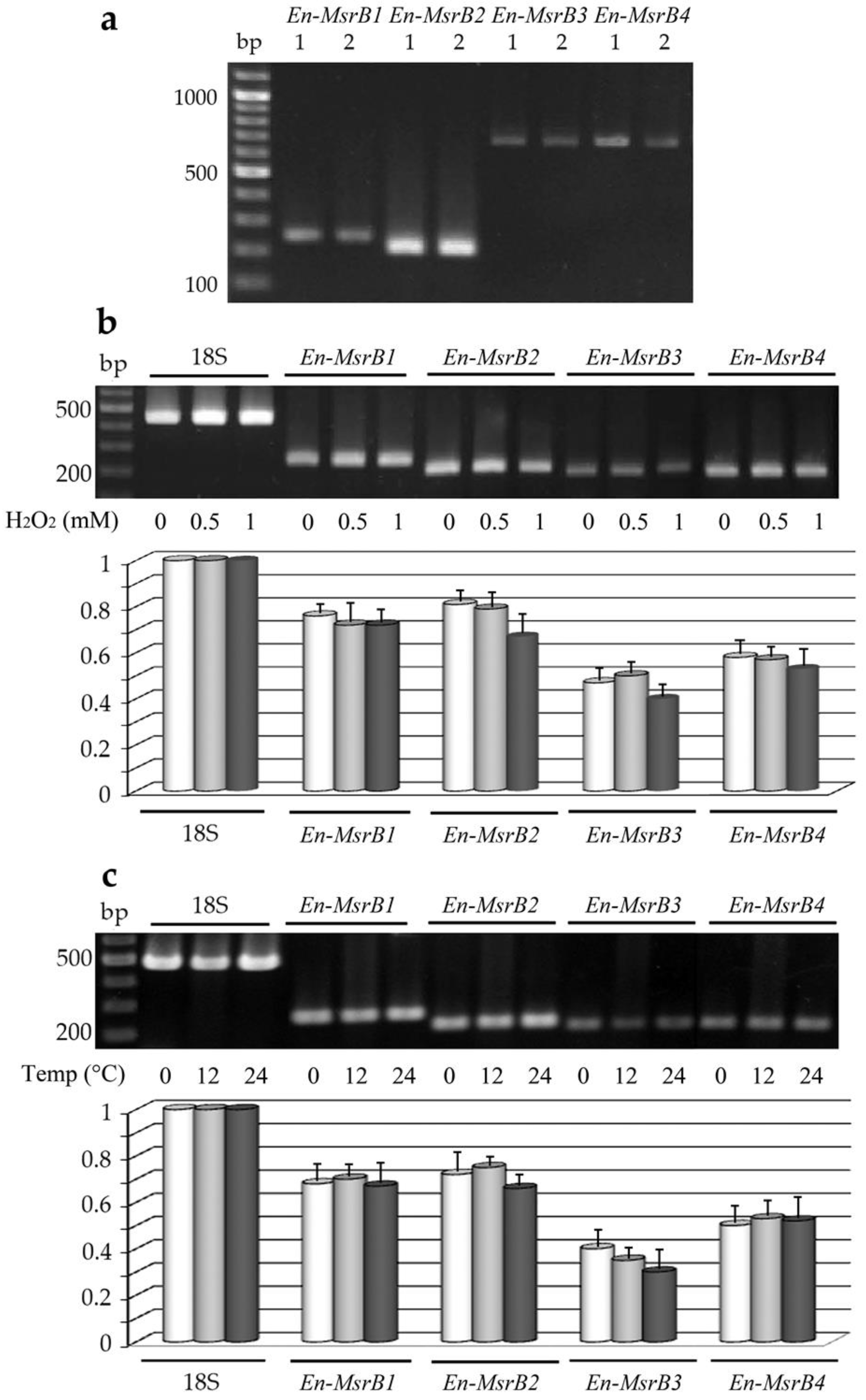
3.4. Gene Expression
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix
| Species | Form | Accession Number |
|---|---|---|
| Euplotes nobilii | B1 | AFZ61876.1 |
| Euplotes nobilii | B2 | KY311562 1 |
| Euplotes nobilii | B3 | KY311563 1 |
| Euplotes nobilii | B4 | KY311564 1 |
| Acromyrmex echinatior | B | GL888413.1 |
| Adineta vaga | B | EU637017.1 |
| Amazona aestiva | B3 | LMAW01002576.1 |
| Apis mellifera | B | XM_003251284.3 |
| Ascaris suum | B | JI212431.1 |
| Bos taurus | B2 | GJ062270.1 |
| Bos taurus | B3 | GJ060799.1 |
| Brugia malayi | B | XM_001900358.1 |
| Chlamydomonas reinhardtii | B | EDP03924.1 |
| Clytia hemisphaerica | B | GBGP01000067.1 |
| Columba livia | B3 | KB375322.1 |
| Crassostrea gigas | B2 | JH821652.1 |
| Crassostrea gigas | B3 | JH816866.1 |
| Danio rerio | B2 | NM_212921.2 |
| Danio rerio | B3 | BC071530.1 |
| Equus caballus | B1 | NM_001170424.1 |
| Euplotes raikovi | B1 | JX978448.1 |
| Exaiptasia pallida | B | LJWW01000100.1 |
| Fundulus heteroclitus | B3 | GCES01009679.1 |
| Gallus gallus | B3 | NM_001199578.1 |
| Loa loa | B | JH712120.1 |
| Mus musculus | B1 | NM_013759.2 |
| Mus musculus | B2 | BC021619.1 |
| Mus musculus | B3 | NM_177092.4 |
| Neospora caninum | B | LN714486.1 |
| Oryza sativa | B1 | LOC_Os06g2776 |
| Oryza sativa | B3 | LOC_Os05g33510 |
| Oryza sativa | B5 | LOC_Os03g24600 |
| Pan troglodytes | B1 | NM_001114751.1 |
| Pan troglodytes | B2 | GABE01001715.1 |
| Pan troglodytes | B3 | GABE01000482.1 |
| Papilio machaon | B | XM_014504817.1 |
| Paramecium tetraurelia | B | XP_001426263 |
| Poeciliopsis prolifica | B2 | GBYX01192342.1 |
| Salmo salar | B3 | XM_014153858.1 |
| Schistosoma mansoni | B1 | AY669150.1 |
| Schistosoma mansoni | B2 | AY669149.1 |
| Tetrahymena thermophila | B | XP_001019714.4 |
| Triticum aestivum | B1 | KP030665 |
| Triticum aestivum | B3 | KP030667 |
| Triticum aestivum | B5 | KP030670 |
| Zea mays | B1 | GRMZM2G025322 |
| Zea mays | B3 | GRMZM2G089308 |
| Zea mays | B5 | GRMZM2G577677 |
| Xenopus tropicalis | B2 | BC171252.1 |
| Paramecium tetraurelia | B | XP_001426263 |
References
- Vogt, W. Oxidation of methionyl residues in proteins: Tools, targets, and reversal. Free Radic. Biol. Med. 1995, 18, 93–105. [Google Scholar] [CrossRef]
- Friguet, B. Oxidized protein degradation and repair in ageing and oxidative stress. Febs Lett. 2006, 580, 2910–2916. [Google Scholar] [CrossRef]
- Stadtman, E.R.; Levine, R.L. Protein oxidation. Ann. N. Y. Acad. Sci. 2000, 899, 191–208. [Google Scholar] [CrossRef] [PubMed]
- Moskovitz, J. Methionine sulfoxide reductases: Ubiquitous enzymes involved in antioxidant defense, protein regulation, and prevention of aging-associated diseases. Biochim. Biophys. Acta 2005, 1703, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Valbonesi, A.; Luporini, P. Description of two new species of Euplotes and Euplotes rariseta from Antarctica. Polar Biol. 1990, 11, 47–53. [Google Scholar] [CrossRef]
- Di Giuseppe, G.; Erra, F.; Dini, F.; Alimenti, C.; Vallesi, A.; Pedrini, B.; Wüthrich, K.; Luporini, P. Antarctic and Arctic populations of the ciliate Euplotes nobilii show common pheromone-mediated cell-cell signaling and cross-mating. Proc. Natl. Acad. Sci. USA 2011, 108, 3181–3186. [Google Scholar] [CrossRef] [PubMed]
- Di Giuseppe, G.; Barbieri, M.; Vallesi, A.; Luporini, P.; Dini, F. Phylogeographical pattern of Euplotes nobilii, a protist ciliate with a bipolar biogeographical distribution. Mol. Ecol. 2013, 22, 4029–4037. [Google Scholar] [CrossRef] [PubMed]
- Di Giuseppe, G.; Dini, F.; Vallesi, A.; Luporini, P. Genetic relationships in bipolar species of the protist ciliate, Euplotes. Hydrobiologia 2015, 761, 71–83. [Google Scholar] [CrossRef]
- Jiang, J.M.; Zhang, Q.Q.; Warren, A.; Al-Rasheid, K.A.S.; Song, W.B. Morphology and SSU rRNA gene-based phylogeny of two marine Euplotes species, E. orientalis spec. nov. and E. raikovi Agamaliev, 1966 (Ciliophora, Euplotida). Eur. J. Protistol. 2010, 46, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Dobri, N.; Ngueng Oumarou, E.E.; Alimenti, C.; Vallesi, A. Polar and non-polar species of the protozoan ciliate Euplotes behave differently in response to environmental oxidative stress. Ital. J. Zool. 2014, 81, 409–414. [Google Scholar] [CrossRef]
- Dobri, N.; Ngueng Oumarou, E.E.; Alimenti, C.; Ortenzi, C.; Luporini, P.; Vallesi, A. Methionine sulfoxide reduction in ciliates: Characterization of the ready-to-use methionine sulfoxide-R-reductase genes in Euplotes. Gene 2013, 515, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Jahn, C.L.; Klobutcher, L.A. Genome remodeling in ciliated protozoa. Ann. Rev. Microbiol. 2002, 56, 489–520. [Google Scholar] [CrossRef] [PubMed]
- Vallesi, A.; Di Pretoro, B.; Ballarini, P.; Apone, F.; Luporini, P. A novel protein kinase from the ciliate Euplotes raikovi with close structural identity to the mammalian intestinal and male-germ cell kinases: Characterization and functional implications in the autocrine pheromone signaling loop. Protist 2010, 161, 250–263. [Google Scholar] [CrossRef] [PubMed]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.D.; et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 24, 3389–3402. [Google Scholar] [CrossRef]
- Notredame, C.; Higgins, D.G.; Heringa, J. T-coffee: A novel method for fast and accurate multiple sequence alignment. J. Mol. Biol. 2000, 302, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. ProtTest 3: Fast selection of best-fit models of protein evolution. Bioinformatics 2011, 27, 1164–1165. [Google Scholar] [CrossRef] [PubMed]
- Ferro, D.; Bakiu, R.; De Pittà, C.; Boldrin, F.; Cattalini, F.; Pucciarelli, S.; Miceli, C.; Santovito, G. Cu, Zn superoxide dismutases from Tetrahymena thermophila: Molecular evolution and gene expression of the first line of antioxidant defenses. Protist 2015, 166, 131–145. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Lewis, P.O.; Fan, Y.; Kuo, L.; Chen, M.H. Improving marginal likelihood estimation for Bayesian phylogenetic model selection. Syst. Biol. 2011, 60, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; Mark, P.V.D.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Guindon, S.; Dufayard, J.F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Meyer, F.; Schmidt, H.J.; Plümper, E.; Hasilik, A.; Mersmann, G.; Meyer, H.E.; Engström, A.; Heckmann, K. UGA is translated as cysteine in pheromone 3 of Euplotes octocarinatus. Proc. Natl. Acad. Sci. USA 1991, 88, 3758–3761. [Google Scholar] [CrossRef] [PubMed]
- Turanov, A.A.; Lobanov, A.V.; Formenko, D.E.; Morrison, H.G.; Sogin, M.L.; Klobutcher, L.A.; Hatfield, D.L.; Gladyshev, V.N. Genetic code supports targeted insertion of two amino acids by one codon. Science 2009, 323, 259–261. [Google Scholar] [CrossRef] [PubMed]
- Su, D.; Li, Y.; Gladyshev, V.N. Selenocysteine insertion directed by the 3′-UTR SECIS element in Escherichia coli. Nucleic Acids Res. 2005, 33, 2486–2492. [Google Scholar] [CrossRef] [PubMed]
- Emanuelsson, O.; Brunak, S.; Heijne, G.V.; Nielsen, H. Locating proteins in the cell using TargetP, SignalP and related tools. Nat. Protoc. 2007, 2, 953–971. [Google Scholar] [CrossRef] [PubMed]
- Nakai, K.; Horton, P. PSORT: A program for detecting sorting signals in proteins and predicting their subcellular localization. Trends Biochem. Sci. 1999, 24, 34–36. [Google Scholar] [CrossRef]
- Lange, A.; Mills, R.E.; Lange, C.J.; Stewart, M.; Devine, S.E.; Corbett, A.H. Classical nuclear localization signals: Definition, function, and interaction with importin α. J. Biol. Chem. 2007, 282, 5101–5105. [Google Scholar] [CrossRef] [PubMed]
- Hespeels, B.; Flot, J.F.; Derzelle, A.; Van Doninck, K. Evidence for Ancient Horizontal Gene Acquisitions in Bdelloid Rotifers of the Genus Adineta. In Evolutionary Biology: Genome Evolution, Speciation, Coevolution and Origin of Life; Pontarotti, P., Ed.; Springer International Publishing: Cham, Switzerland, 2014; pp. 207–225. [Google Scholar]
- Zhu, J.; Ding, P.; Li, Q.; Gao, Y.; Chen, F.; Xia, G. Molecular characterization and expression profile of methionine sulfoxide reductase gene family in maize (Zea mays) under abiotic stresses. Gene 2015, 562, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Gong, Z.H.; Zhang, W.X.; Wang, Y.; Ma, L.T.; Wang, H.F.; Guo, X.Q.; Xu, B.H. Identification and characterization of a novel methionine sulfoxide reductase B gene (AccMsrB) from Apis cerana cerana (Hymenoptera: Apidae). Ann. Entomol. Soc. Am. 2015, 108, 575–584. [Google Scholar] [CrossRef]
- Kobayashi, N.; McEntee, K. Identification of cis and trans components of a novel heat shock stress regulatory pathway in Saccharomyces cerevisiae. Mol. Cell. Biol. 1993, 13, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.P. The antioxidant response element and oxidative stress modifiers in airway diseases. Curr. Mol. Med. 2008, 8, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Ferro, D.; Franchi, N.; Mangano, V.; Bakiu, R.; Cammarata, M.; Parrinello, N.; Santovito, G.; Ballarin, L. Characterization and metal-induced gene transcription of two new copper zinc superoxide dismutases in the solitary ascidian Ciona intestinalis. Aquat. Toxicol. 2013, 140, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Franchi, N.; Ferro, D.; Ballarin, L.; Santovito, G. Expression of genes involved in glutathione biosynthesis in the solitary tunicate Ciona intestinalis exposed to heavy metals. Aquat. Toxicol. 2012, 114, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Levine, R.L. Methionine in proteins defends against oxidative stress. Faseb J. 2009, 23, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Dobri, N.; Candelori, A.; Ricci, F.; Luporini, P.; Vallesi, A. Evidence for methionine-sulfoxide-reductase gene transfer from Alphaproteobacteria to the transcriptionally active (macro) nucleus of the ciliate, Euplotes raikovi. BMC Microbiol. 2014, 14. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.A.; Koc, A.; Cerny, R.L.; Gladyshev, V.N. Reaction mechanism, evolutionary analysis, and role of Zinc in Drosophila methionine-R-sulfoxide reductase. J. Biol. Chem. 2002, 277, 37527–37535. [Google Scholar] [CrossRef] [PubMed]
- Boschi-Muller, S.; Olry, A.; Antoine, M.; Branlant, G. The enzymology and biochemistry of methionine sulfoxide reductases. BBA-Proteins Proteom. 2005, 1703, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Olry, A.; Boschi-Muller, S.; Yu, H.; Burnel, D.; Branlant, G. Insights into the role of the metal binding site in methionine-R-sulfoxide reductases B. Protein Sci. 2005, 14, 2828–2837. [Google Scholar] [CrossRef] [PubMed]
- Neiers, F.; Kriznik, A.; Boschi-Muller, S.; Branlant, G. Evidence for a new sub-class of methionine sulfoxide reductases B with an alternative thioredoxin recognition signature. J. Biol. Chem. 2004, 279, 42462–42468. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Gladyshev, V.N. Selenium and methionine sulfoxide reduction. In Selenium: Its Molecular Biology and Role in Human Health; Hatfield, D.L., Berry, M.J., Gladyshev, V.N., Eds.; Springer: New York, NY, USA, 2011; pp. 481–492. [Google Scholar]
- Tarrago, L.; Laugier, E.; Rey, P. Protein-repairing methionine sulfoxide reductases in photosynthetic organisms: Gene organization, reduction mechanisms, and physiological roles. Mol. Plant 2009, 2, 202–217. [Google Scholar] [CrossRef] [PubMed]
- Tarrago, L.; Laugier, E.; Zaffagnini, M.; Marchand, C.; Le Maréchal, P.; Rouhier, N.; Lemaire, S.D.; Rey, P. Regeneration mechanisms of Arabidopsis thaliana methionine sulfoxide reductases B by glutaredoxins and thioredoxins. J. Biol. Chem. 2009, 284, 18963–18971. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Kim, J.R. Thioredoxin as a reducing agent for mammalian methionine sulfoxide reductases B lacking resolving cysteine. Biochem. Biophys. Res. Commun. 2008, 371, 490–494. [Google Scholar] [CrossRef] [PubMed]
- Tarrago, L.; Laugier, E.; Zaffagnini, M.; Marchand, C.H.; Marechal, P.L.; Lemaire, S.D.; Rey, P. Plant thioredoxin CDSP32 regenerates 1-cys methionine sulfoxide reductase B activity through the direct reduction of sulfenic acid. J. Biol. Chem. 2010, 285, 14964–14972. [Google Scholar] [CrossRef] [PubMed]
- Oumarou, N.; Emilie, E. Mechanisms of Repairing Age-Induced Protein Oxidation in the Protozoan Ciliate Euplotes: Characterization of the Methionine Sulfoxide Reductase Genes. Ph.D. Thesis, University of Camerino, Camerino, Italy, 2011. [Google Scholar]
- Schrallhammer, M.; Schweikert, M.; Vallesi, A.; Verni, F.; Petroni, P. Detection of a novel subspecies of Francisella noatunensis as endosymbiont of the ciliate Euplotes raikovi. Microb. Ecol. 2011, 61, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Schrallhammer, M. Towards an Understanding of the Evolution and Ecology of Host-Symbiont Interactions of Ciliates and Their Intracellular Bacteria with Perspectives on Cell Biology and Nanobiotechnology. Ph.D. Thesis, Biologisches Institut der Universität Stuttgart, Stuttgart, Germany, 2010. [Google Scholar]

| Primer Combination | Primer Sequences (5’–3’) | Product Size (bp) |
|---|---|---|
| B1FW + B1RV | AAAGGCCAGACTAACCGACA + CATTGCACCTCCACCCTAGT | 249 |
| B2FW + B2RV | AAAGTCACACAGGAGGCTGA + CATGGCCTAGATGGGCTTTA | 208 |
| B2FW + oligo(dT)-AP | AAAGTCACACAGGAGGCTGA + GGCCACGCGTCGACTAGTACT17 | 480 |
| B3FW + B3RV | ATGCTAAGTACTATACGCAGAA + TATGCCTCCTCTGCGTCTTT | 689 |
| B3FW2 + B3RV | CTGGCCCTCATTTTACGAAG + TATGCCTCCTCTGCGTCTTT | 201 |
| B4FW + B4RV | ATGGTAATGGAAAGAGAAATG + ACTCACTCCCACCCTTATCA | 686 |
| B4FW2 + B4RV | GTGGCCCTCCTTCTACGAAC + ACTCACTCCCACCCTTATCA | 200 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ricci, F.; Lauro, F.M.; Grzymski, J.J.; Read, R.; Bakiu, R.; Santovito, G.; Luporini, P.; Vallesi, A. The Anti-Oxidant Defense System of the Marine Polar Ciliate Euplotes nobilii: Characterization of the MsrB Gene Family. Biology 2017, 6, 4. https://doi.org/10.3390/biology6010004
Ricci F, Lauro FM, Grzymski JJ, Read R, Bakiu R, Santovito G, Luporini P, Vallesi A. The Anti-Oxidant Defense System of the Marine Polar Ciliate Euplotes nobilii: Characterization of the MsrB Gene Family. Biology. 2017; 6(1):4. https://doi.org/10.3390/biology6010004
Chicago/Turabian StyleRicci, Francesca, Federico M. Lauro, Joseph J. Grzymski, Robert Read, Rigers Bakiu, Gianfranco Santovito, Pierangelo Luporini, and Adriana Vallesi. 2017. "The Anti-Oxidant Defense System of the Marine Polar Ciliate Euplotes nobilii: Characterization of the MsrB Gene Family" Biology 6, no. 1: 4. https://doi.org/10.3390/biology6010004







