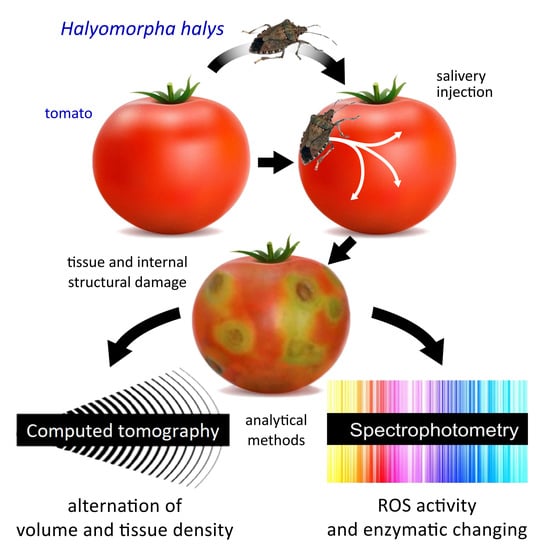
Analysis of the Destructive Effect of the Halyomorpha halys Saliva on Tomato by Computer Tomographical Imaging and Antioxidant Capacity Measurement
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling and Experimental Setting
2.2. CT-Imaging and Post-Processing
2.3. Spectrophotometric Stress Assessment Assay
2.4. Statistical Analysis
3. Results
3.1. Estimation of Volume Change by CT
3.2. Determination of Inner Structure Alteration Using CT
3.3. Results of Stress Indicating Analytical Methods
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAOSTAT. Value of Agricultural Production, Tomato. Available online: http://www.fao.org/faostat/en/#search/tomato (accessed on 11 September 2021).
- Josifov, M.; Kerzhner, I.M. Heteroptera aus Korea. II. Teil (Aradidae, Berytidae, Lygaeidae, Pyrrhocoridae, Rhopalidae, Alydidae, Coreidae, Urostylidae, Acanthosomatidae, Scutelleridae, Pentatomidae, Cydnidae, Plataspidae). Fragm. Faun. 1978, 23, 137–196. [Google Scholar] [CrossRef]
- Hoebeke, E.R.; Carter, M.E. Halyomorpha halys (Stål) (Heteroptera: Pentatomidae): A polyphagous plant pest from Asia newly detected in North America. Proc. Entomol. Soc. Wash. 2003, 105, 225–237. [Google Scholar]
- Haye, T.; Wyniger, D.; Gariepy, T. Recent range expansion of brown marmorated stink bug in Europe. In Proceedings of the 8th International Conference on Urban Pests, Zurich, Switzerland, 20–23 July 2014; pp. 309–314. [Google Scholar]
- Watanabe, M.; Arakawa, R.; Shinagawa, Y.; Okazawa, T. Overwintering flight of brown-marmorated stink bug, Halyomorpha mista to the buildings. Med. Entomol. Zool. 1994, 45, 25–31. [Google Scholar] [CrossRef] [Green Version]
- Inkley, D.B. Characteristics of home invasion by the brown marmorated stink bug (Hemiptera: Pentatomidae). J. Entomol. Sci. 2012, 47, 125–130. [Google Scholar] [CrossRef]
- Wallner, A.M.; Hamilton, G.C.; Nielsen, A.L.; Hahn, N.; Green, E.J.; Rodriguez-Saona, C.R. Landscape factors facilitating the invasive dynamics and distribution of the brown marmorated stink bug, Halyomorpha halys (Hemiptera: Pentatomidae), after arrival in the United States. PLoS ONE 2014, 9, e95691. [Google Scholar] [CrossRef]
- Leskey, T.C.; Nielsen, A.L. Impact of the invasive brown marmorated stink bug in North America and Europe: History, biology, ecology, and management. Ann. Rev. Entomol. 2018, 63, 599–618. [Google Scholar] [CrossRef] [Green Version]
- Wermelinger, B.; Wyniger, D.; Forster, B. First records of an invasive bug in Europe: Halyomorpha halys Stål (Heteroptera: Pentatomidae), a new pest on woody ornamentals and fruit trees? Mitt. Schweiz. Entomol. Ges. 2008, 81, 1–8. [Google Scholar]
- Nielsen, A.L.; Hamilton, G.C.; Matadha, D. Developmental rate estimation and life table analysis for Halyomorpha halys (Hemiptera: Pentatomidae). Environ. Entomol. 2008, 37, 348–355. [Google Scholar] [CrossRef]
- Rice, K.B.; Bergh, C.J.; Bergmann, E.J.; Biddinger, D.J.; Dieckhoff, C.; Dively, G.; Fraser, H.; Gariepy, T.; Hamilton, G.; Haye, T.; et al. Biology, ecology, and management of brown marmorated stink bug (Hemiptera: Pentatomidae). J. Integr. Pest Manag. 2014, 5, A1–A13. [Google Scholar] [CrossRef]
- Kuhar, T.P.; Kamminga, K.L.; Whalen, J.; Dively, G.P.; Brust, G.; Hooks, C.R.R.; Hamilton, G.; Herbert, D.A. The pest potential of brown marmorated stink bug on vegetable crops. Plant Health Progr. 2012, 13, 41. [Google Scholar] [CrossRef] [Green Version]
- Leskey, T.C.; Short, B.D.; Butler, B.R.; Wright, S.E. Impact of the invasive brown marmorated stink bug, Halyomorpha halys (Stål), in mid-Atlantic tree fruit orchards in the United States: Case studies of commercial management. Psyche J. Entomol. 2012, 535062. [Google Scholar] [CrossRef] [Green Version]
- Bariselli, M.; Bugiani, R.; Maistrello, L. Distribution and damage caused by Halyomorpha halys in Italy. EPPO Bull. 2016, 46, 332–334. [Google Scholar] [CrossRef]
- Sharma, A.; Khan, A.N.; Subrahmanyam, S.; Raman, A.; Taylor, G.S.; Fletcher, M.J. Salivary proteins of plant-feeding hemipteroids-implication in phytophagy. Bull. Entomol. Res. 2013, 104, 117–136. [Google Scholar] [CrossRef] [PubMed]
- Peiffer, M.; Felton, G.W. Insights into the saliva of the brown marmorated stink bug Halyomorpha halys (Hemiptera: Pentatomidae). PLoS ONE 2014, 9, e88483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Labandeira, C.C.; Phillips, T.L. Insect fluid-feeding on upper pennsylvanian tree ferns (Palaeodictyoptera, Marattiales) and the early history of the piercing-and-sucking functional feeding group. Ann. Entomol. Soc. Am. 1996, 89, 157–183. [Google Scholar] [CrossRef] [Green Version]
- Wheeler, A.G. Biology of the Plant Bugs; Cornell University Press: New York, NY, USA, 2001. [Google Scholar]
- Wicklund, T.; Rosenfeld, H.J.; Martinsen, B.K.; Sundfør, M.W.; Lea, P.; Bruun, T.; Blomhoff, R.; Haffner, K. Antioxidant capacity and colour of strawberry jam as influenced by cultivar and storage conditions. LWT Food Sci. Technol. 2005, 38, 387–391. [Google Scholar] [CrossRef]
- Loayza, F.E.; Brecht, J.K.; Simonne, A.H.; Plotto, A.; Baldwin, E.A.; Bai, J.; Lon-Kan, E. Enhancement of the antioxidant capacity of ripe tomatoes by the application of a hot water treatment at the mature-green stage. Postharvest Biol. Technol. 2020, 161, 111054. [Google Scholar] [CrossRef]
- Jones, J.B., Jr.; Case, V.W. Sampling, handling, and analyzing plant tissue samples. Soil Test. Plant Anal. 1990, 3, 389–427. [Google Scholar]
- Keszthelyi, S.; Pónya, Z.; Csóka, Á.; Bázár, G.; Morschhauser, T.; Donkó, T. Non-destructive imaging and spectroscopic techniques to investigate the hidden-lifestyle arthropod pests: A review. J. Plant Dis. Prot. 2020, 127, 283–295. [Google Scholar] [CrossRef] [Green Version]
- Alba-Alejandre, I.; Alba-Tercedor, J.; Vega, F.E. Observing the devastating coffee berry borer (Hypothenemus hampei) inside the coffee berry using micro-computed tomography. Sci. Rep. 2018, 8, 17033. [Google Scholar] [CrossRef]
- Booth, S.; Kurtz, B.; Heer, M.I.; Mooney, S.J.; Sturrock, C.J. Tracking wireworm burrowing behaviour in soil over time using 3D X-ray computed tomography. Pest Manag. Sci. 2020, 76, 2653–2662. [Google Scholar] [CrossRef] [PubMed]
- Taylor, F.W.; Wagner, F.G.; McMillin, C.W.; Morgan, I.L.; Hopkins, F.F. Locating knots by industrial tomography. A feasibility study. For. Prod. J. 1984, 34, 42–46. [Google Scholar]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghasemi, A.; Zahediasl, S. Normality tests for statistical analysis: A guide for non-statisticians. Int. J. Endocrinol. Metabol. 2012, 10, 486–489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- SPSS Inc. SPSS for Windows, version 7.0; SPSS Inc.: Chicago, IL, USA, 2007. [Google Scholar]
- Farinha, A.O.; Branco, M.; Pereira, M.F.; Auger-Rozenberg, M.A.; Maurício, A.; Yart, A.; Guerreiro, V.; Sousa, E.M.R.; Roques, A. Micro X-ray computed tomography suggests cooperative feeding among adult invasive bugs Leptoglossus occidentalis on mature seeds of stone pine Pinus pinea. Agric. For. Entomol. 2018, 20, 18–27. [Google Scholar] [CrossRef] [Green Version]
- Stahl, J.M.; Scaccini, D.; Pozzebon, A.; Daane, K.M. Comparing the feeding damage of the invasive brown marmorated stink bug to a native stink bug and leaf footed bug on California pistachios. Insects 2020, 11, 688. [Google Scholar] [CrossRef]
- Agustí, N.; Cohen, A.C. Lygus hesperus and L. lineolaris (Hemiptera: Miridae), phytophages, zoophages, or omnivores: Evidence of feeding adaptations suggested by the salivary and midgut digestive enzymes. J. Entomol. Sci. 2000, 35, 176–186. [Google Scholar] [CrossRef]
- Tan, X.; Xu, X.; Gao, Y.; Yang, Q.; Zhu, Y.; Wang, J.; Wan, F.; Zhou, H. Levels of salivary enzymes of Apolygus lucorum (Hemiptera: Miridae), from 1st instar nymph to adult, and their potential relation to bug feeding. PLoS ONE 2016, 11, e0168848. [Google Scholar] [CrossRef]
- Zhu, Y.C.; Yao, J.; Luttrell, R. Identification of genes potentially responsible for extra-oral digestion and overcoming plant defense from salivary glands of the tarnished plant bug (Hemiptera: Miridae) using cDNA sequencing. J. Insect Sci. 2016, 16, 60. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.M.; Lim, U.T. Evaluation of mature soybean pods as a food source for two pod-sucking bugs, Riptortus pedestris (Hemiptera: Alydidae) and Halyomorpha halys (Hemiptera: Pentatomidae). PLoS ONE 2017, 12, e0176187. [Google Scholar] [CrossRef] [Green Version]
- Chrysargyris, A.; Xylia, P.; Antoniou, O.; Tzortzakis, M. Climate change due to heat and drought stress can alter the physiology of Maratheftiko local Cyprian grapevine variety. J. Water Clim. Change 2018, 9, 715–727. [Google Scholar] [CrossRef]
- Guinan, K.J.; Sujeeth, N.; Copeland, R.B.; Jones, P.W.; O’Brien, N.M.; Sharma, H.S.S.; Prouteau, P.F.J.; O’Sullivan, J.T. Discrete roles for extracts of Ascophyllum nodosum in enhancing plant growth and tolerance to abiotic and biotic stresses. Acta Hortic. 2013, 109, 127–135. [Google Scholar] [CrossRef]
- Sparks, M.E.; Bansal, R.; Benoit, J.B.; Blackburn, M.B.; Chao, H.; Chen, M.; Cheng, S.; Childers, C.; Dinh, H.; Doddapaneni, H.V.; et al. Brown marmorated stink bug, Halyomorpha halys (Stål), genome: Putative underpinnings of polyphagy, insecticide resistance potential and biology of a top worldwide pest. BMC Genom. 2020, 21, 227. [Google Scholar] [CrossRef]
- Salvador, M.J.; Lopes, G.N.; Nascimento Filho, V.F.; Zucchi, O.L. Quality control of commercial tea by x-ray fluorescence. X-ray Spectrom. 2002, 31, 141–144. [Google Scholar] [CrossRef]
- Musaev, F.B.; Soldatenko, A.V.; Krivenkov, L.V.; Bukharov, A.F.; Beletskiy, S.L.; Kezimana, P. Assessment of vegetable seeds quality by micro-focus X-ray analysis. Res. Crop. 2020, 21, 604–610. [Google Scholar]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keszthelyi, S.; Gibicsár, S.; Jócsák, I.; Fajtai, D.; Donkó, T. Analysis of the Destructive Effect of the Halyomorpha halys Saliva on Tomato by Computer Tomographical Imaging and Antioxidant Capacity Measurement. Biology 2022, 11, 1070. https://doi.org/10.3390/biology11071070
Keszthelyi S, Gibicsár S, Jócsák I, Fajtai D, Donkó T. Analysis of the Destructive Effect of the Halyomorpha halys Saliva on Tomato by Computer Tomographical Imaging and Antioxidant Capacity Measurement. Biology. 2022; 11(7):1070. https://doi.org/10.3390/biology11071070
Chicago/Turabian StyleKeszthelyi, Sándor, Szilvia Gibicsár, Ildikó Jócsák, Dániel Fajtai, and Tamás Donkó. 2022. "Analysis of the Destructive Effect of the Halyomorpha halys Saliva on Tomato by Computer Tomographical Imaging and Antioxidant Capacity Measurement" Biology 11, no. 7: 1070. https://doi.org/10.3390/biology11071070