Elucidating the Potential Biological Impact of Cellulose Nanocrystals
Abstract
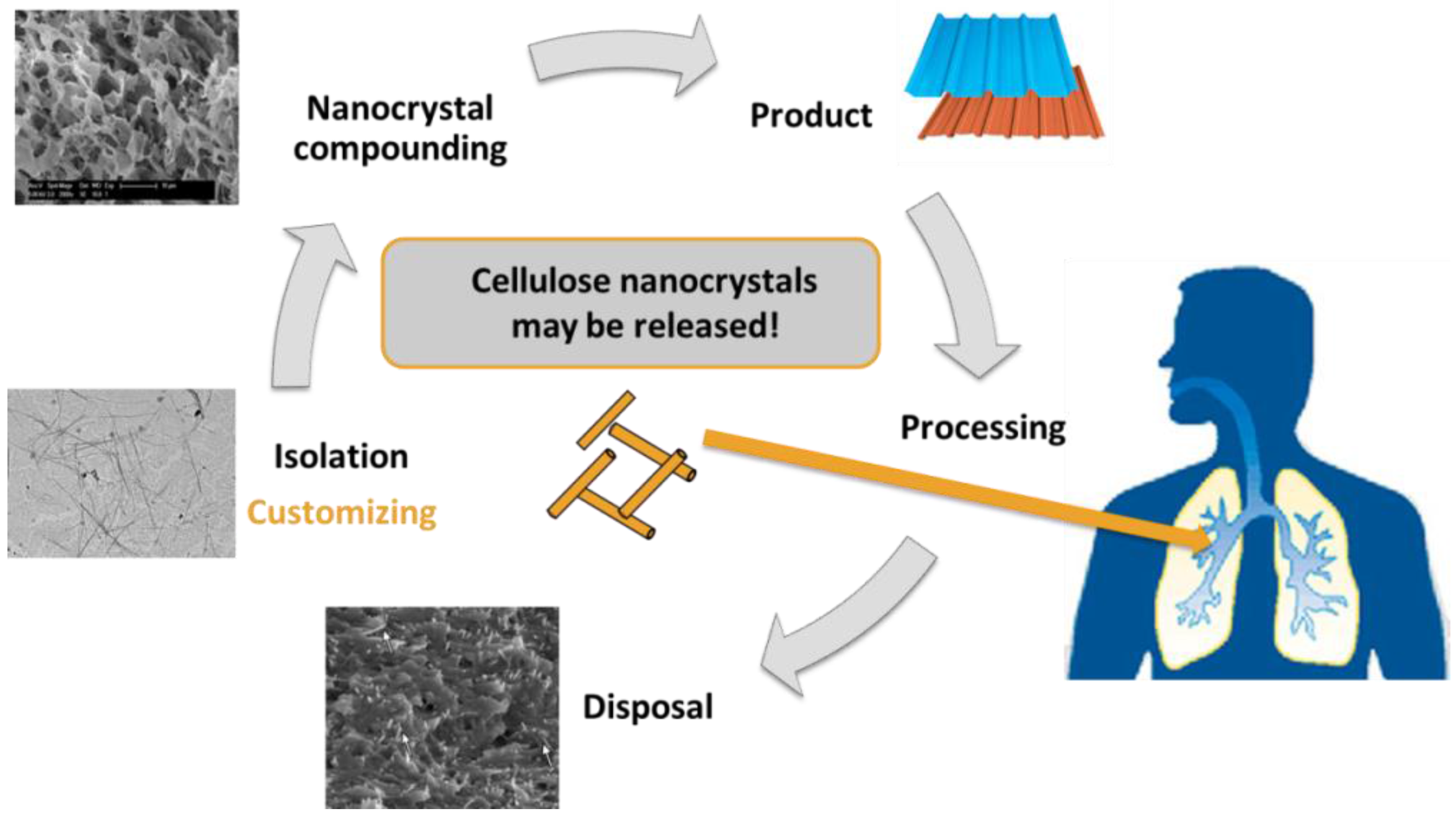
:1. Introduction
2. Life-Cycle and Human Exposure of CNCs
3. Characterising CNC Exposure
4. How to Determine the Potential Biological Impact of Nanocellulose
5. Summary and Outlook
- Assess and quantify what and if the released dose at each stage of the material’s life-cycle is a potential mode for environmental as well as human exposure (e.g., inhalation and skin contact).
- At each stage of the life-cycle of nanocellulose undertaken, thorough characterisation of the released nanomaterial (if any) and decipher between single nanocellulose nanofibers, polymer composite released nanocellulose nanofibers and micron-sized particles. Several parameters need to be analyzed, the most relevant factors being: the dimensions (width, length, aspect ratio), colloidal stability on the studied medium, surface chemistry, specific surface area and degree of crystallinity (directly related to the stiffness of the material).
- In order to achieve the characterisation of the materials at every life-cycle stage, reliable and representative methods must be used (as suggested in Table 1). The need to develop alternative or adapted methods for every nanomaterial, especially nanocellulose remains and is the responsibility of the field to progress. New protocols need to be established for the facile characterization and determination of nanoparticle size and determination of surface chemistry on the nanoscale, which allow for a simple and realistic comparison between studies.
- Understanding of the acute and chronic effects of nanocellulose exposure, particularly during occupational exposure (i.e., isolation stage) in order to comprehend the ability for nanocellulose to either contribute to, or exacerbate pre-existing disease states.
- Determine the biomolecular and biochemical mechanisms that drive, if any, the (adverse) biological effects following nanocellulose exposure.
- The application of realistic doses in contrast to overload situations on target organ (in vitro) or related systems has to be the aim in any hazard assessment study.
- Relate the exposure dose effect and associated biochemical effects to the specific characteristics of the nanocellulose investigated in order to determine the specific physical and/or chemical characteristics that might be driving the possible hazardous response measured.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Voet, D.; Voet, J.G. Biochemistry, 4th ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2010. [Google Scholar]
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994. [Google Scholar] [CrossRef] [PubMed]
- Eichhorn, S.J.; Dufresne, A.; Aranguren, M.; Marcovich, N.E.; Capadona, J.R.; Rowan, S.J.; Weder, C.; Thielemans, W.; Roman, M.; Renneckar, S.; et al. Review: Current international research into cellulose nanofibres and nanocomposites. J. Mater. Sci. 2010, 45, 1–33. [Google Scholar] [CrossRef]
- Araki, J.; Wada, M.; Kuga, S.; Okano, T. Flow properties of microcrystalline cellulose suspension prepared by acid treatment of native cellulose. Colloids Surf. A Physicochem. Eng. Asp. 1998, 142, 75–82. [Google Scholar] [CrossRef]
- Dong, X.; Revol, J.-F.; Gray, D. Effect of microcrystallite preparation conditions on the formation of colloid crystals of cellulose. Cellulose 1998, 5, 19–32. [Google Scholar] [CrossRef]
- Camarero-Espinosa, S.; Kuhnt, T.; Foster, E.J.; Weder, C. Isolation of thermally stable cellulose nanocrystals by phosphoric acid hydrolysis. Biomacromolecules 2013, 14, 1223–1230. [Google Scholar] [CrossRef] [PubMed]
- Jorfi, M.; Roberts, M.N.; Foster, E.J.; Weder, C. Physiologically responsive, mechanically adaptive bio-nanocomposites for biomedical applications. ACS Appl. Mater. Interfaces 2013, 5, 1517–1526. [Google Scholar] [CrossRef] [PubMed]
- Mueller, S.; Weder, C.; Foster, E.J. Isolation of cellulose nanocrystals from pseudostems of banana plants. RSC Adv. 2014, 4, 907–915. [Google Scholar] [CrossRef]
- De Souza Lima, M.M.; Wong, J.T.; Paillet, M.; Borsali, R.; Pecora, R. Translational and rotational dynamics of rodlike cellulose whiskers. Langmuir 2002, 19, 24–29. [Google Scholar] [CrossRef]
- Jorfi, M.; Foster, E.J. Recent advances in nanocellulose for biomedical applications. J. Appl. Polym. Sci. 2015, 132, 41719. [Google Scholar] [CrossRef]
- Lin, N.; Dufresne, A. Nanocellulose in biomedicine: Current status and future prospect. Eur. Polym. J. 2014, 59, 302–325. [Google Scholar] [CrossRef]
- Camarero-Espinosa, S.; Rothen-Rutishauser, B.; Weder, C.; Foster, E.J. Directed cell growth in multi-zonal scaffolds for cartilage tissue engineering. Biomaterials 2016, 74, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Biyani, M.V.; Foster, E.J.; Weder, C. Light-healable supramolecular nanocomposites based on modified cellulose nanocrystals. ACS Macro Lett. 2013, 2, 236–240. [Google Scholar] [CrossRef]
- Padalkar, S.; Capadona, J.R.; Rowan, S.J.; Weder, C.; Won, Y.-H.; Stanciu, L.A.; Moon, R.J. Natural biopolymers: Novel templates for the synthesis of nanostructures. Langmuir 2010, 26, 8497–8502. [Google Scholar] [CrossRef] [PubMed]
- Van den Berg, O.; Schroeter, M.; Capadona, J.R.; Weder, C. Nanocomposites based on cellulose whiskers and (semi)conducting conjugated polymers. J. Mater. Chem. 2007, 17, 2746–2753. [Google Scholar] [CrossRef]
- Gawryla, M.D.; van den Berg, O.; Weder, C.; Schiraldi, D.A. Clay aerogel/cellulose whisker nanocomposites: A nanoscale wattle and daub. J. Mater. Chem. 2009, 19, 2118–2124. [Google Scholar] [CrossRef]
- Li, Y.; Ren, H.; Ragauskas, A.J. Rigid polyurethane foam/cellulose whisker nancomposites: Preparation, characterization and properties. J. Nanosci. Nanotechnol. 2011, 11, 6904–6911. [Google Scholar] [CrossRef] [PubMed]
- Heath, L.; Thielemans, W. Cellulose nanowhisker aerogels. Green Chem. 2010, 12, 1448–1453. [Google Scholar] [CrossRef]
- Capadona, J.R.; Shanmuganathan, K.; Tyler, D.J.; Rowan, S.J.; Weder, C. Stimuli-responsive polymer nanocomposites inspired by the sea cucumber dermis. Science 2008, 319, 1370–1374. [Google Scholar] [CrossRef] [PubMed]
- Shanmuganathan, K.; Capadona, J.R.; Rowan, S.J.; Weder, C. Bio-inspired mechanically-adaptive nanocomposites derived from cotton cellulose whiskers. J. Mater. Chem. 2010, 20, 180–186. [Google Scholar] [CrossRef]
- Shanmuganathan, K.; Capadona, J.R.; Rowan, S.J.; Weder, C. Biomimetic mechanically adaptive nanocomposites. Prog. Polym. Sci. 2010, 35, 212–222. [Google Scholar] [CrossRef]
- Rusli, R.; Shanmuganathan, K.; Rowan, S.J.; Weder, C.; Eichhorn, S.J. Stress-transfer in anisotropic and environmentally adaptive cellulose whisker nanocomposites. Biomacromolecules 2010, 11, 762–768. [Google Scholar] [CrossRef] [PubMed]
- Mendez, J.; Annamalai, P.K.; Eichhorn, S.J.; Rusli, R.; Rowan, S.J.; Foster, E.J.; Weder, C. Bioinspired mechanically adaptive polymer nanocomposites with water-activated shape-memory effect. Macromolecules 2011, 44, 6827–6835. [Google Scholar] [CrossRef]
- Dagnon, K.L.; Shanmuganathan, K.; Weder, C.; Rowan, S.J. Water-triggered modulus changes of cellulose nanofiber nanocomposites with hydrophobic polymer matrices. Macromolecules 2012, 45, 4707–4715. [Google Scholar] [CrossRef]
- Way, A.E.; Hsu, L.; Shanmuganathan, K.; Weder, C.; Rowan, S.J. Ph-responsive cellulose nanocrystal gels and nanocomposites. ACS Macro Lett. 2012, 1, 1001–1006. [Google Scholar] [CrossRef]
- Shatkin, J.A.; Wegner, T.H.; Bilek, E.T.; Cowie, J. Market projections of cellulose nanomaterial-enabled products-part 1: Applications. Tappi J. 2014, 13, 9–16. [Google Scholar]
- Davis, C.S.; Grolman, D.L.; Karim, A.; Gilman, J.W. What do we still need to understand to commercialize cellulose nanomaterials? Green Mater. 2015, 3, 53–58. [Google Scholar] [CrossRef]
- Stone, V.; Pozzi-Mucelli, S.; Tran, L.; Aschberger, K.; Sabella, S.; Vogel, U.; Poland, C.; Balharry, D.; Fernandes, T.; Gottardo, S.; et al. Its-nano—Prioritising nanosafety research to develop a stakeholder driven intelligent testing strategy. Part. Fibre Toxicol. 2014, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Krug, H.F. Nanosafety research—Are we on the right track? Angew. Chem. Int. Ed. 2014, 53, 12304–12319. [Google Scholar] [CrossRef] [PubMed]
- Arts, J.H.E.; Hadi, M.; Irfan, M.-A.; Keene, A.M.; Kreiling, R.; Lyon, D.; Maier, M.; Michel, K.; Petry, T.; Sauer, U.G.; et al. A decision-making framework for the grouping and testing of nanomaterials (DF4nanoGrouping). Regul. Toxicol. Pharmacol. 2015, 71, S1–S27. [Google Scholar] [CrossRef] [PubMed]
- Shatkin, J.A.; Kim, B. Cellulose nanomaterials: Life cycle risk assessment, and environmental health and safety roadmap. Environ. Sci. Nano 2015, 2, 477–499. [Google Scholar] [CrossRef]
- Nowack, B.; Brouwer, C.; Geertsma, R.E.; Heugens, E.H.W.; Ross, B.L.; Toufektsian, M.-C.; Wijnhoven, S.W.P.; Aitken, R.J. Analysis of the occupational, consumer and environmental exposure to engineered nanomaterials used in 10 technology sectors. Nanotoxicology 2012, 7, 1152–1156. [Google Scholar] [CrossRef] [PubMed]
- Rusli, R.; Eichhorn, S.J. Determination of the stiffness of cellulose nanowhiskers and the fiber-matrix interface in a nanocomposite using raman spectroscopy. Appl. Phys. Lett. 2008, 93, 033111. [Google Scholar] [CrossRef]
- Sturcova, A.; Davies, G.R.; Eichhorn, S.J. Elastic modulus and stress-transfer properties of tunicate cellulose whiskers. Biomacromolecules 2005, 6, 1055–1061. [Google Scholar] [CrossRef] [PubMed]
- Favier, V.; Chanzy, H.; Cavaille, J.Y. Polymer nanocomposites reinforced by cellulose whiskers. Macromolecules 1995, 28, 6365–6367. [Google Scholar] [CrossRef]
- Losert, S.; von Goetz, N.; Bekker, C.; Fransman, W.; Wijnhoven, S.W.P.; Delmaar, C.; Hungerbuhler, K.; Ulrich, A. Human exposure to conventional and nanoparticle-containing sprays—A critical review. Environ. Sci. Technol. 2014, 48, 5366–5378. [Google Scholar] [CrossRef] [PubMed]
- Maynard, A.D.; Baron, P.A.; Foley, M.; Shvedova, A.A.; Kisin, E.R.; Castranova, V. Exposure to carbon nanotube material: Aerosol release during the handling of unrefined single-walled carbon nanotube material. J. Toxicol. Environ. Health A 2004, 67, 87–107. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Choi, Y.C.; Shin, J.H.; Lee, J.H.; Lee, Y.; Park, S.Y.; Baek, J.E.; Park, J.D.; Ahn, K.; Yu, I.J. Health surveillance study of workers who manufacture multi-walled carbon nanotubes. Nanotoxicology 2015, 9, 802–811. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Huang, J.-Q.; Zhao, M.-Q.; Qian, W.-Z.; Wei, F. Carbon nanotube mass production: Principles and processes. ChemSusChem 2011, 4, 864–889. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Kanoh, S.; Motoyoshi, K.; Aida, S. Diffuse lung disease caused by cotton fibre inhalation but distinct from byssinosis. Thorax 2004, 59, 1095–1097. [Google Scholar] [CrossRef] [PubMed]
- Steffi, F.; Jurgen, S. Environmental, health and safety aspects of nanotechnology—Implications for the R&D in (small) companies. Sci. Technol. Adv. Mater. 2007, 8, 12–18. [Google Scholar]
- Kuhlbusch, T.A.J.; Asbach, C.; Fissan, H.; Göhler, D.; Stintz, M. Nanoparticle exposure at nanotechnology workplaces: A review. Part. Fibre Toxicol. 2011, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- Pietroiusti, A.; Magrini, A. Engineered nanoparticles at the workplace: Current knowledge about workers’ risk. Occup. Med. 2014, 64, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Methner, M.; Hodson, L.; Geraci, C. Nanoparticle emission assessment technique (NEAT) for the identification and measurement of potential inhalation exposure to engineered nanomaterials—Part A. J. Occup. Environ. Hyg. 2009, 7, 127–132. [Google Scholar] [CrossRef] [PubMed]
- NIOSH. Current Intelligence Bulletin 65: Occupational Exposure to Carbon Nanotubes and Nanofibers. Available online: http://www.cdc.gov/niosh/docs/2013-145/ (accessed on 12 September 2015).
- Morrow, P.E. Possible mechanisms to explain dust overloading of the lungs. Fundam. Appl. Toxicol. 1988, 10, 369–384. [Google Scholar] [CrossRef]
- Endes, C.; Schmid, O.; Kinnear, C.; Mueller, S.; Camarero-Espinosa, S.; Vanhecke, D.; Foster, E.J.; Petri-Fink, A.; Rothen-Rutishauser, B.; Weder, C.; et al. An in vitro testing strategy towards mimicking the inhalation of high aspect ratio nanoparticles. Part. Fibre Toxicol. 2014, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.; Willis, J.; De Martinis, D.; Hansen, B.; Laursen, H.; Sintes, J.R.; Kearns, P.; Gonzalez, M. Science policy considerations for responsible nanotechnology decisions. Nat. Nanotechnol. 2011, 6, 73–77. [Google Scholar] [CrossRef] [PubMed]
- NIOSH. Niosh Potential Occupational Carcinogens. Available online: http://www.cdc.gov/niosh/npg/nengapdxa.html (accessed on 12 September 2015).
- Borm, P.J.; Tran, L.; Donaldson, K. The carcinogenic action of crystalline silica: A review of the evidence supporting secondary inflammation-driven genotoxicity as a principal mechanism. Crit. Rev. Toxicol. 2011, 41, 756–770. [Google Scholar] [CrossRef] [PubMed]
- Thompson, D.; Chen, S.-C.; Wang, J.; Pui, D.Y.H. Aerosol emission monitoring and assessment of potential exposure to multi-walled carbon nanotubes in the manufacture of polymer nanocomposites. Ann. Occup. Hyg. 2015, 59, 1135–1151. [Google Scholar] [CrossRef] [PubMed]
- Schlagenhauf, L.; Chu, B.T.T.; Buha, J.; Nüesch, F.; Wang, J. Release of carbon nanotubes from an epoxy-based nanocomposite during an abrasion process. Environ. Sci. Technol. 2012, 46, 7366–7372. [Google Scholar] [CrossRef] [PubMed]
- Schlagenhauf, L.; Nüesch, F.; Wang, J. Release of carbon nanotubes from polymer nanocomposites. Fibers 2014, 2, 108. [Google Scholar] [CrossRef]
- Bergin, I.L.; Witzmann, F.A. Nanoparticle toxicity by the gastrointestinal route: Evidence and knowledge gaps. Int. J. Biomed. Nanosci. Nanotechnol. 2013, 3, 163–210. [Google Scholar] [CrossRef] [PubMed]
- Muthu, M.S.; Leong, D.T.; Mei, L.; Feng, S.-S. Nanotheranostics—Application and further development of nanomedicine strategies for advanced theranostics. Theranostics 2014, 4, 660–677. [Google Scholar] [CrossRef] [PubMed]
- Walser, T.; Limbach, L.K.; Brogioli, R.; Erismann, E.; Flamigni, L.; Hattendorf, B.; Juchli, M.; Krumeich, F.; Ludwig, C.; Prikopsky, K.; et al. Persistence of engineered nanoparticles in a municipal solid-waste incineration plant. Nat. Nanotechnol. 2012, 7, 520–524. [Google Scholar] [CrossRef] [PubMed]
- Oberdorster, G.; Oberdorster, E.; Oberdorster, J. Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect. 2005, 113, 823–839. [Google Scholar] [CrossRef] [PubMed]
- Bouwmeester, H.; Lynch, I.; Marvin, H.J.; Dawson, K.A.; Berges, M.; Braguer, D.; Byrne, H.J.; Casey, A.; Chambers, G.; Clift, M.J.; et al. Minimal analytical characterization of engineered nanomaterials needed for hazard assessment in biological matrices. Nanotoxicology 2011, 5, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Duffin, R.; Tran, L.; Brown, D.; Stone, V.; Donaldson, K. Proinflammogenic effects of low-toxicity and metal nanoparticles in vivo and in vitro: Highlighting the role of particle surface area and surface reactivity. Inhal. Toxicol. 2007, 19, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.-C.; Lin, S.; Wang, P.C.; Sridhar, R. Techniques for physicochemical characterization of nanomaterials. Biotechnol. Adv. 2014, 32, 711–726. [Google Scholar] [CrossRef] [PubMed]
- Moore, T.L.; Rodriguez-Lorenzo, L.; Hirsch, V.; Balog, S.; Urban, D.; Jud, C.; Rothen-Rutishauser, B.; Lattuada, M.; Petri-Fink, A. Nanoparticle colloidal stability in cell culture media and impact on cellular interactions. Chem. Soc. Rev. 2015, 44, 6287–6305. [Google Scholar] [CrossRef] [PubMed]
- Balog, S.; Rodriguez-Lorenzo, L.; Monnier, C.A.; Obiols-Rabasa, M.; Rothen-Rutishauser, B.; Schurtenberger, P.; Petri-Fink, A. Characterizing nanoparticles in complex biological media and physiological fluids with depolarized dynamic light scattering. Nanoscale 2015, 7, 5991–5997. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, K.; Murphy, F.A.; Duffin, R.; Poland, C.A. Asbestos, carbon nanotubes and the pleural mesothelium: A review of the hypothesis regarding the role of long fibre retention in the parietal pleura, inflammation and mesothelioma. Part. Fibre Toxicol. 2010, 7. [Google Scholar] [CrossRef] [PubMed]
- Ellouk, S.A.; Jaurand, M.C. Review of animal/in vitro data on biological effects of man-made fibers. Environ Health Perspect. 1994, 102, 47–61. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.; Addison, J.; Bolton, R.; Donaldson, K.; Jones, A.; Smith, T. The pathogenicity of long versus short fibre samples of amosite asbestos administered to rats by inhalation and intraperitoneal injection. Br. J. Exp. Pathol. 1986, 67, 415–430. [Google Scholar] [PubMed]
- Stanton, M.F.; Layard, M.; Tegeris, A.; Miller, E.; May, M.; Kent, E. Carcinogenicity of fibrous glass: Pleural response in the rat in relation to fiber dimension. J. Natl. Cancer Inst. 1977, 58, 587–603. [Google Scholar] [PubMed]
- Abbate, S.; Martino, L.B.; Tringali, M.A.; Catania, S.; Albiero, F.; Cavallari, V.; Costa, C.; Giacobbe, G.; Brecciaroli, R.; Giorgianni, C.; et al. Changes induced by exposure of the human lung to glass fibre reinforced plastic. Environ Health Perspect. 2006, 114, 1725–1729. [Google Scholar] [CrossRef] [PubMed]
- Poland, C.A.; Duffin, R.; Kinloch, I.; Maynard, A.; Wallace, W.A.H.; Seaton, A.; Stone, V.; Brown, S.; MacNee, W.; Donaldson, K. Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nat. Nanotechnol. 2008, 3, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Schinwald, A.; Chernova, T.; Donaldson, K. Use of silver nanowires to determine thresholds for fibre length-dependent pulmonary inflammation and inhibition of macrophage migration in vitro. Part. Fibre Toxicol. 2012, 9, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Sacui, I.A.; Nieuwendaal, R.C.; Burnett, D.J.; Stranick, S.J.; Jorfi, M.; Weder, C.; Foster, E.J.; Olsson, R.T.; Gilman, J.W. Comparison of the properties of cellulose nanocrystals and cellulose nanofibrils isolated from bacteria, tunicate, and wood processed using acid, enzymatic, mechanical, and oxidative methods. ACS Appl. Mater. Interfaces 2014, 6, 6127–6138. [Google Scholar] [CrossRef] [PubMed]
- Shanmuganathan, K.; Capadona, J.R.; Rowan, S.J.; Weder, C. Stimuli-responsive mechanically adaptive polymer nanocomposites. ACS Appl. Mater. Interfaces 2010, 2, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Utembe, W.; Potgieter, K.; Stefaniak, A.B.; Gulumian, M. Dissolution and biodurability: Important parameters needed for risk assessment of nanomaterials. Part. Fibre Toxicol. 2015, 12, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Michen, B.; Geers, C.; Vanhecke, D.; Endes, C.; Rothen-Rutishauser, B.; Balog, S.; Petri-Fink, A. Avoiding drying-artifacts in transmission electron microscopy: Characterizing the size and colloidal state of nanoparticles. Sci. Rep. 2015, 5, 9793. [Google Scholar] [CrossRef] [PubMed]
- Dammak, A.; Moreau, C.; Beury, N.; Schwikal, K.; Winter Heiko, T.; Bonnin, E.; Saake, B.; Cathala, B. Elaboration of multilayered thin films based on cellulose nanocrystals and cationic xylans: Application to xylanase activity detection. Holzforschung 2013, 67, 579–586. [Google Scholar] [CrossRef]
- Beck-Candanedo, S.; Roman, M.; Gray, D.G. Effect of reaction conditions on the properties and behavior of wood cellulose nanocrystal suspensions. Biomacromolecules 2005, 6, 1048–1054. [Google Scholar] [CrossRef] [PubMed]
- Lahiji, R.R.; Xu, X.; Reifenberger, R.; Raman, A.; Rudie, A.; Moon, R.J. Atomic force microscopy characterization of cellulose nanocrystals. Langmuir 2010, 26, 4480–4488. [Google Scholar] [CrossRef] [PubMed]
- Podsiadlo, P.; Choi, S.-Y.; Shim, B.; Lee, J.; Cuddihy, M.; Kotov, N.A. Molecularly engineered nanocomposites: Layer-by-layer assembly of cellulose nanocrystals. Biomacromolecules 2005, 6, 2914–2918. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.; Chakrabarty, D. Isolation of nanocellulose from waste sugarcane bagasse (scb) and its characterization. Carbohydr. Polym. 2011, 86, 1291–1299. [Google Scholar] [CrossRef]
- Boluk, Y.; Danumah, C. Analysis of cellulose nanocrystal rod lengths by dynamic light scattering and electron microscopy. J. Nanopart. Res. 2013, 16, 1–7. [Google Scholar] [CrossRef]
- Bondeson, D.; Mathew, A.; Oksman, K. Optimization of the isolation of nanocrystals from microcrystalline cellulose by acid hydrolysis. Cellulose 2006, 13, 171–180. [Google Scholar] [CrossRef]
- Dumanli, A.G.; van der Kooij, H.M.; Kamita, G.; Reisner, E.; Baumberg, J.J.; Steiner, U.; Vignolini, S. Digital color in cellulose nanocrystal films. ACS Appl. Mater. Interfaces 2014, 6, 12302–12306. [Google Scholar] [CrossRef] [PubMed]
- Beck, S.; Bouchard, J.; Berry, R. Dispersibility in water of dried nanocrystalline cellulose. Biomacromolecules 2012, 13, 1486–1494. [Google Scholar] [CrossRef] [PubMed]
- Morandi, G.; Heath, L.; Thielemans, W. Cellulose nanocrystals grafted with polystyrene chains through surface-initiated atom transfer radical polymerization (SI-ATRP). Langmuir 2009, 25, 8280–8286. [Google Scholar] [CrossRef] [PubMed]
- Junior de Menezes, A.; Siqueira, G.; Curvelo, A.A.S.; Dufresne, A. Extrusion and characterization of functionalized cellulose whiskers reinforced polyethylene nanocomposites. Polymer 2009, 50, 4552–4563. [Google Scholar] [CrossRef]
- Biyani, M.; Weder, C.; Foster, E.J. Photoswitchable nanocomposites made from coumarin-functionalized cellulose nanocrystals. Polym. Chem. 2014, 5, 5501–5508. [Google Scholar] [CrossRef]
- Habibi, Y.; Goffin, A.L.; Schiltz, N.; Duquesne, E.; Dubois, P.; Dufresne, A. Bionanocomposites based on poly(epsilon-caprolactone)-grafted cellulose nanocrystals by ring-opening polymerization. J. Mater. Chem. 2008, 18, 5002–5010. [Google Scholar] [CrossRef]
- Lu, P.; Hsieh, Y.-L. Preparation and properties of cellulose nanocrystals: Rods, spheres, and network. Carbohydr. Polym. 2010, 82, 329–336. [Google Scholar] [CrossRef]
- Shin, Y.; Exarhos, G.J. Template synthesis of porous titania using cellulose nanocrystals. Mater. Lett. 2007, 61, 2594–2597. [Google Scholar] [CrossRef]
- Beck-Candanedo, S.; Viet, D.; Gray, D. Induced phase separation in cellulose nanocrystal suspensions containing ionic dye species. Cellulose 2006, 13, 629–635. [Google Scholar] [CrossRef]
- Cullen, R.T.; Miller, B.G.; Jones, A.D.; Davis, J.M.G. Toxicity of cellulose fibres. Ann. Occup. Hyg. 2002, 46, 81–84. [Google Scholar] [CrossRef]
- Milton, D.K.; Godleski, J.J.; Feldman, H.A.; Greaves, I.A. Toxicity of intratracheally instilled cotton dust, cellulose, and endotoxin. Am. Rev. Respir. Dis. 1990, 142, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Cullen, R.T.; Searl, A.; Miller, B.G.; Davis, J.M.G.; Jones, A.D. Pulmonary and intraperitoneal inflammation induced by cellulose fibres. J. Appl. Toxicol. 2000, 20, 49–60. [Google Scholar] [CrossRef]
- Clift, M.J.D.; Foster, E.J.; Vanhecke, D.; Studer, D.; Wick, P.; Gehr, P.; Rothen-Rutishauser, B.; Weder, C. Investigating the interaction of cellulose nanofibers derived from cotton with a sophisticated 3d human lung cell coculture. Biomacromolecules 2011, 12, 3666–3673. [Google Scholar] [CrossRef] [PubMed]
- Roman, M. Toxicity of cellulose nanocrystals: A review. Ind. Biotechnol. 2015, 11, 25–33. [Google Scholar] [CrossRef]
- Manke, A.; Wang, L.; Rojanasakul, Y. Mechanisms of nanoparticle-induced oxidative stress and toxicity. BioMed Res. Int. 2013, 2013, 942916. [Google Scholar] [CrossRef] [PubMed]
- Magdolenova, Z.; Collins, A.; Kumar, A.; Dhawan, A.; Stone, V.; Dusinska, M. Mechanisms of genotoxicity. A review of in vitro and in vivo studies with engineered nanoparticles. Nanotoxicology 2014, 8, 233–278. [Google Scholar] [CrossRef] [PubMed]
- Burden, N.; Aschberger, K.; Chaudhry, Q.; Clift, M.J.D.; Doak, S.; Fowler, P.; Johnston, H.; Landsiedel, R.; Rowland, J.; Stone, V. Aligning nanotoxicology with the 3Rs: What is needed to realise the short, medium and long-term opportunities? Nano Today 2016. accepted. [Google Scholar]
- Hartung, T.; Sabbioni, E. Alternative in vitro assays in nanomaterial toxicology. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2011, 3, 545–573. [Google Scholar] [CrossRef] [PubMed]
- Fabbrizi, M.R.; Duff, T.; Oliver, J.; Wilde, C. Advanced in vitro systems for efficacy and toxicity testing in nanomedicine. Eur. J. Nanomed. 2014, 6, 171–183. [Google Scholar] [CrossRef]
- Clift, M.J.; Endes, C.; Vanhecke, D.; Wick, P.; Gehr, P.; Schins, R.P.; Petri-Fink, A.; Rothen-Rutishauser, B. A comparative study of different in vitro lung cell culture systems to assess the most beneficial tool for screening the potential adverse effects of carbon nanotubes. Toxicol. Sci. 2014, 137, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Mihalchik, A.L.; Ding, W.; Porter, D.W.; McLoughlin, C.; Schwegler-Berry, D.; Sisler, J.D.; Stefaniak, A.B.; Snyder-Talkington, B.N.; Cruz-Silva, R.; Terrones, M.; et al. Effects of nitrogen-doped multi-walled carbon nanotubes compared to pristine multi-walled carbon nanotubes on human small airway epithelial cells. Toxicology 2015, 333, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Endes, C.; Mueller, S.; Kinnear, C.; Vanhecke, D.; Foster, E.J.; Petri-Fink, A.; Weder, C.; Clift, M.J.D.; Rothen-Rutishauser, B. Fate of cellulose nanocrystal aerosols deposited on the lung cell surface in vitro. Biomacromolecules 2015, 16, 1267–1275. [Google Scholar] [CrossRef] [PubMed]
- Moreira, S.; Silva, N.B.; Almeida-Lima, J.; Oliveira Rocha, H.A.; Batistuzzo Medeiros, S.R.; Alves, C., Jr.; Gama, F.M. Bc nanofibres: In vitro study of genotoxicity and cell proliferation. Toxicol. Lett. 2009, 189, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.I.; Lee, S.E.; Yang, H.; Jin, Y.H.; Park, C.S.; Park, Y.S. Toxicologic evaluation of bacterial synthesized cellulose in endothelial cells and animals. Mol. Cell. Toxicol. 2010, 6, 373–380. [Google Scholar] [CrossRef]
- Kovacs, T.; Naish, V.; O'Connor, B.; Blaise, C.; Gagne, F.; Hall, L.; Trudeau, V.; Martel, P. An ecotoxicological characterization of nanocrystalline cellulose (ncc). Nanotoxicology 2010, 4, 255–270. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, K.A.; Mena, J.A.; Male, K.B.; Hrapovic, S.; Kamen, A.; Luong, J.H.T. Effect of surface charge on the cellular uptake and cytotoxicity of fluorescent labeled cellulose nanocrystals. ACS Appl. Mater. Interfaces 2010, 2, 2924–2932. [Google Scholar] [CrossRef] [PubMed]
- de Lima, R.; Mattoso, L.H.; Feitosa, L.O.; Maruyama, C.R.; Barga, M.A.; Yamawaki, P.C.; Vieira, I.J.; Teixeira, E.M.; Corrêa, A.C.; Fraceto, L.F. Evaluation of the genotoxicity of cellulose nanofibers. Int. J. Nanomed. 2012, 7, 3555–3565. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Hirani, A.A.; Colacino, K.R.; Lee, Y.W.; Roman, M. Cytotoxicity and cellular uptake of cellulose nanocrystals. Nano LIFE 2012, 2, 1241006. [Google Scholar] [CrossRef]
- Hannukainen, K.S.; Suhonen, S.; Savolainen, K.; Norppa, H. Genotoxicity of nanofibrillated cellulose in vitro as measured by enzyme comet assay. Toxicol. Lett. 2012, 211, S71. [Google Scholar] [CrossRef]
- Male, K.B.; Leung, A.C.W.; Montes, J.; Kamen, A.; Luong, J.H.T. Probing inhibitory effects of nanocrystalline cellulose: Inhibition versus surface charge. Nanoscale 2012, 4, 1373–1379. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.M.; Raposo, N.R.B.; Brayner, R.; Teixeira, E.M.; Oliveira, V.; Quintão, C.C.R.; Camargo, L.S.A.; Mattoso, L.H.C.; Brandão, H.M. Cytotoxicity and expression of genes involved in the cellular stress response and apoptosis in mammalian fibroblast exposed to cotton cellulose nanofibers. Nanotechnology 2013, 24, 075103. [Google Scholar] [CrossRef] [PubMed]
- Catalan, J.; Ilves, M.; Jarventaus, H.; Hannukainen, K.S.; Kontturi, E.; Vanhala, E.; Alenius, H.; Savolainen, K.M.; Norppa, H. Genotoxic and immunotoxic effects of cellulose nanocrystals in vitro. Environ. Mol. Mutagen. 2015, 56, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Hanif, Z.; Ahmed, F.R.; Shin, S.W.; Kim, Y.-K.; Um, S.H. Size- and dose-dependent toxicity of cellulose nanocrystals (CNC) on human fibroblasts and colon adenocarcinoma. Colloids Surf. B 2014, 119, 162–165. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.; Mouton, L.; Yepremian, C.; Coute, A.; Lo, J.; Marconcini, J.; Ladeira, L.; Raposo, N.; Brandao, H.; Brayner, R. Ecotoxicological effects of carbon nanotubes and cellulose nanofibers in chlorella vulgaris. J. Nanobiotechnol. 2014, 12, 15. [Google Scholar] [CrossRef] [PubMed]
- Yanamala, N.; Farcas, M.T.; Hatfield, M.K.; Kisin, E.R.; Kagan, V.E.; Geraci, C.L.; Shvedova, A.A. In vivo evaluation of the pulmonary toxicity of cellulose nanocrystals: A renewable and sustainable nanomaterial of the future. ACS Sustain. Chem. Eng. 2014, 2, 1691–1698. [Google Scholar] [CrossRef] [PubMed]
| Characterization Method | Feature of Nanocellulose Characterised | Limitation regarding Nanocellulose | Limitation Mitigation | References |
|---|---|---|---|---|
| Electron Microscopy (TEM) | Shape & dimension (Best for overall structural analysis, for most samples) | Drying effects when spotting onto EM grids | Alter drying conditions, concentration, BSA-based techniques [73] | [6,13,74] |
| Atomic Force Microscopy (AFM) | Shape & dimension | AFM tip has the potential to overestimate sizes if sharpness is lost | Use height (more accurate), not measured width | [75,76,77] |
| Dynamic Light-Scattering (DLS) | Overall dimensions | Tough to elucidate exact dimensions | Modify with an accurate form factor | [78,79] |
| Optical Photographs | Dispersion/colloidal stability. Observation of aggregates (larger than 300 nm) | Limited by Abbe diffraction limit | Must use electron microscopy for smaller (less than 300 nm) | [80,81] |
| Conductometric Charge Titration | Charge density (Best for surface half ester content determination) | Small (<20 mmol/Kg) is within noise limit | Larger sample size, | [6,82] |
| Elemental Analysis | Elemental content of sample | Common for C, H, N, S, P analysis only | Must be correlated to predicted chemical structure | [6,83,84] |
| Infrared Spectroscopy (IR) | Functional groups (bonds) | Only looks at chemical bonds | Limited to IR active chemical bonds, sensitivity | [78,85] |
| X-ray Photoelectron Spectroscopy | Elements on the surface | Voxel does not allow individual CNC analysis | Does not elucidate groups, only elements | [86] |
| Brunauer, Emmet and Teller method (BET) | Surface area | Cellulose naturally aggregates when dried | Aggregation will lead to lower than individualized CNCs | [87,88] |
| Dye Adhesion | Surface area | Limited by size of dye | Use in conjunction with other techniques (e.g., rough estimation by length × dimension analysis) | [70,89] |
| Inverse Gas Chromatography (IGC) | Surface properties | Cellulose naturally aggregates when dried | Aggregation will lead to lower than individualized CNCs | [71] |
| Nanocellulose Form Studied | Biological Model Used | Endpoint Assessed | Reference |
|---|---|---|---|
| Bacterial cellulose nanofibres (BC-NF) | 3T3 fibroblasts, CHO cells | mutagenicity, proliferation, genotoxicity | [103] |
| Bacterial cellulose nanofibres | HUVEC, C57/Bl6 mice | viability, cytotoxicity, apoptosis/necrosis, cell cycle | [104] |
| Cellulose nanocrystals (CNCs) | Oncorhynchus mykiss hepatocytes, Daphnia magna, Ceriodaphia dubia, Pimephales promelas, Vibrio fischeri, Pseudokirchneriella subcapitata, Hydra attenuata, Danio rerio | genotoxicity, reproduction, survival, growth | [105] |
| CNCs isolated from flay | HEK 293, Sf9 cells | uptake, cytotoxicity | [106] |
| CNCs isolated from cotton and tunicates | 3D model of the pulmonary epithelial airway barrier | cytotoxicity, (pro)inflammatory response | [93] |
| Cellulose nanofibers isolated from caraua/cotton | Allium cepa, primary lymphocytes, 3T3 fibroblasts | Genotoxicity | [107] |
| Plant derived CNCs | HBMEC, bEnd.3, RAW 264.7, MCF-10A, MDA-MB-231, MDA-MB-468, KB, PC-3, C6 cells | uptake, cytotoxicity | [108] |
| Nanofibrillated cellulose (NFC) | BEAS 2B cells | Genotoxicity | [109] |
| CNCs isolated from cotton, flax, hemp | V79 fibroblast, Sf9 cells | Cytotoxicity | [110] |
| Cotton cellulose nanofibres (CNF) | Bovine fibroblasts | cytotoxicity, stress response, apoptosis | [111] |
| CNCs isolated from cotton | BEAS 2B cells, monocyte-derived macrophages | cytotoxicity, genotoxicity, inflammatory response | [112] |
| CNCs isolated from MCC | NIH3T3 fibroblasts, HCT116 cells | cell viability | [113] |
| CNFs isolated from cotton | Chlorella vulgaris | cell viability, growth | [114] |
| CNCs isolated from wood | C57BL/6 mice | pulmonary outcome | [115] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camarero-Espinosa, S.; Endes, C.; Mueller, S.; Petri-Fink, A.; Rothen-Rutishauser, B.; Weder, C.; Clift, M.J.D.; Foster, E.J. Elucidating the Potential Biological Impact of Cellulose Nanocrystals. Fibers 2016, 4, 21. https://doi.org/10.3390/fib4030021
Camarero-Espinosa S, Endes C, Mueller S, Petri-Fink A, Rothen-Rutishauser B, Weder C, Clift MJD, Foster EJ. Elucidating the Potential Biological Impact of Cellulose Nanocrystals. Fibers. 2016; 4(3):21. https://doi.org/10.3390/fib4030021
Chicago/Turabian StyleCamarero-Espinosa, Sandra, Carola Endes, Silvana Mueller, Alke Petri-Fink, Barbara Rothen-Rutishauser, Christoph Weder, Martin James David Clift, and E. Johan Foster. 2016. "Elucidating the Potential Biological Impact of Cellulose Nanocrystals" Fibers 4, no. 3: 21. https://doi.org/10.3390/fib4030021
APA StyleCamarero-Espinosa, S., Endes, C., Mueller, S., Petri-Fink, A., Rothen-Rutishauser, B., Weder, C., Clift, M. J. D., & Foster, E. J. (2016). Elucidating the Potential Biological Impact of Cellulose Nanocrystals. Fibers, 4(3), 21. https://doi.org/10.3390/fib4030021








