The Significance and Insignificance of Carbon Nanotube-Induced Inflammation
Abstract
:1. Introduction
2. Immune Responses upon Pulmonary Exposure
2.1. Considerations for CNT Inhalation
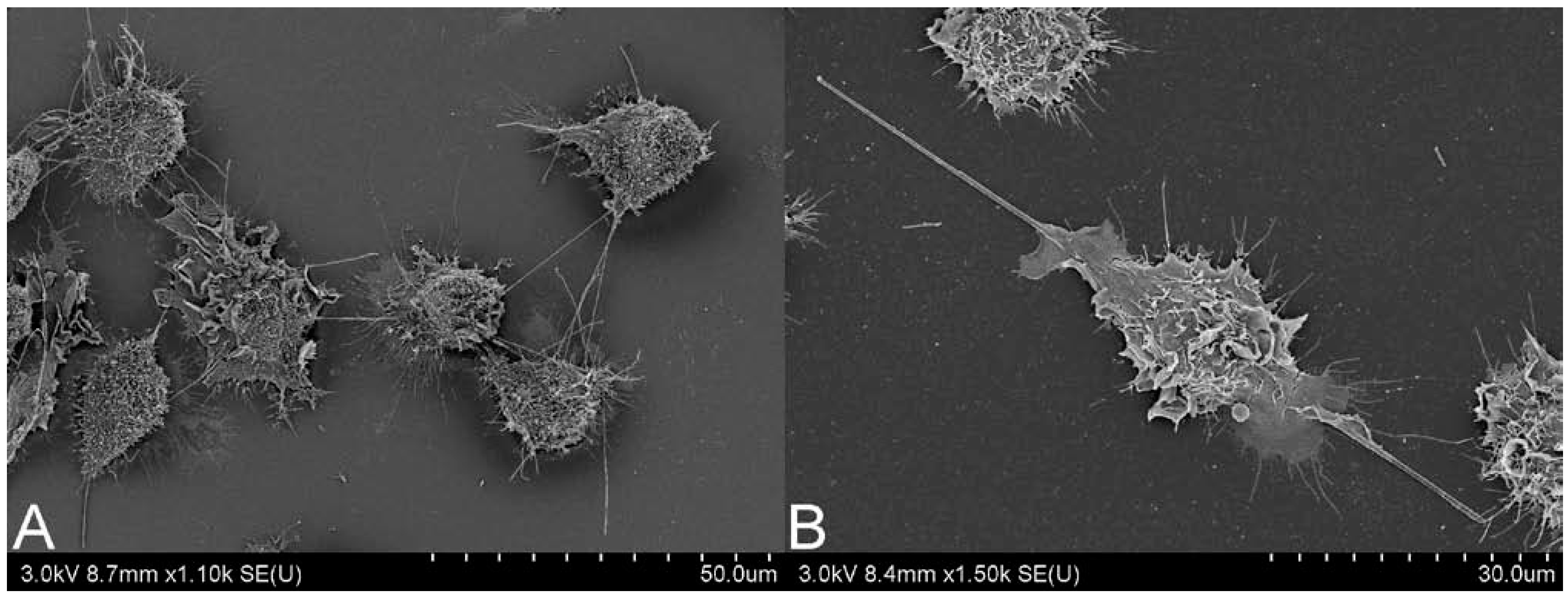
2.2. CNT Instillation and Aspiration

2.3. In vivo Determination of Responsible Characteristics and Significance of in Vitro Models
2.4. The Role of in Vitro Systems
3. Intentional Administration of CNTs—Nanomedical Applications
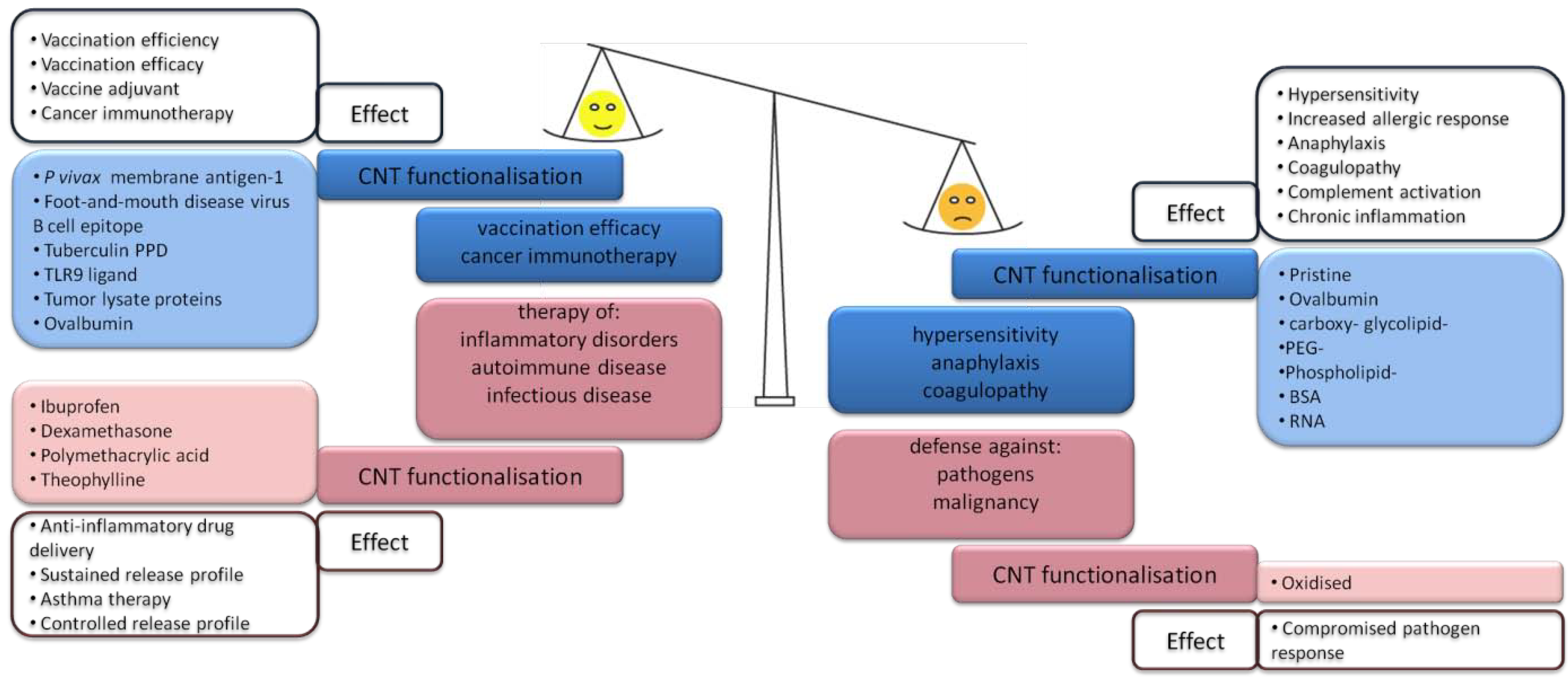
3.1. CNT-Induced Immune Responses upon Subcutaneous Administration
3.2. CNT Induced Immune Responses within the Venous System—Pro-Inflammatory Effects
3.3. CNT-Induced Immune Responses within the Venous System—Immunomodulatory Profile
4. Other Routes of Exposure—Gastrointestinal Tract
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Martin-Gullon, I.; Vera, J.; Conesa, J.A.; Gonzalez, J.L.; Merino, C. Differences between carbon nanofibers produced using Fe and Ni catalysts in a floating catalyst reactor. Carbon 2006, 44, 1572–1580. [Google Scholar] [CrossRef]
- Kisin, E.R.; Murray, A.R.; Sargent, L.; Lowry, D.; Chirila, M.; Siegrist, K.J.; Schwegler-Berry, D.; Leonard, S.; Castranova, V.; Fadeel, B.; et al. Genotoxicity of carbon nanofibers: Are they potentially more or less dangerous than carbon nanotubes or asbestos? Toxicol. Appl. Pharmacol. 2011, 252, 1–10. [Google Scholar] [CrossRef]
- Sanchez, V.C.; Pietruska, J.R.; Miselis, N.R.; Hurt, R.H.; Kane, A.B. Biopersistence and potential adverse health impacts of fibrous nanomaterials: What have we learned from asbestos? Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2009, 1, 511–529. [Google Scholar] [CrossRef]
- Price, R.L.; Waid, M.C.; Haberstroh, K.M.; Webster, T.J. Selective bone cell adhesion on formulations containing carbon nanofibers. Biomaterials 2003, 24, 1877–1887. [Google Scholar] [CrossRef]
- Price, R.L.; Haberstroh, K.M.; Webster, T.J. Improved osteoblast viability in the presence of smaller nanometre dimensioned carbon fibres. Nanotechnology 2004, 15, 892–900. [Google Scholar] [CrossRef]
- Elias, K.L.; Price, R.L.; Webster, T.J. Enhanced functions of osteoblasts on nanometer diameter carbon fibers. Biomaterials 2002, 23, 3279–3287. [Google Scholar] [CrossRef]
- Tran, C.L.; Hankin, S.M.; Ross, B.; Aitken, R.J.; Jones, A.D.; Donaldson, K.; Stone, V.; Trantra, R. An Outline Scoping Study to Determine whether High Aspect Ratio Nanoparticles (HARN) should Raise the Same Concerns as do Asbestos Fibres. Report on Project CB0406 2008. Available online: http://www.safenano.org/Portals/3/SN_Content/Documents/HARN.pdf (accessed on 1 November 2013).
- Coker, R.K.; Laurent, G.J. Pulmonary fibrosis: Cytokines in the balance. Eur. Respir. J. 1998, 11, 1218–1221. [Google Scholar] [CrossRef]
- Mossman, B.T.; Churg, A. Mechanisms in the pathogenesis of asbestosis and silicosis. Am. J. Respir. Crit. Care Med. 1998, 157, 1666–1680. [Google Scholar] [CrossRef]
- Cassel, S.L.; Eisenbarth, S.C.; Iyer, S.S.; Sadler, J.J.; Colegio, O.R.; Tephly, L.A.; Carter, A.B.; Rothman, P.B.; Flavell, R.A.; Sutterwala, F.S. The Nalp3 inflammasome is essential for the development of silicosis. Proc. Natl. Acad. Sci. USA 2008, 105, 9035–9040. [Google Scholar] [CrossRef]
- Dostert, C.; Petrilli, V.; Van Bruggen, R.; Steele, C.; Mossman, B.T.; Tschopp, J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science 2008, 320, 674–677. [Google Scholar] [CrossRef]
- Driscoll, K.E.; Carter, J.; Hassenbein, D.; Howard, B. Cytokines and particle-induced inflammatory cell recruitment. Environ. Health Perspect. 1997, 105, 1159–1164. [Google Scholar]
- Brown, D.M.; Hutchison, L.; Donaldson, K.; MacKenzie, S.J.; Dick, C.A.J.; Stone, V. The effect of oxidative stress on macrophages and lung epithelial cells: The role of phosphodiesterases 1 and 4. Toxicol. Lett. 2007, 168, 1–6. [Google Scholar] [CrossRef]
- Sen, C.K.; Roy, S.; Packer, L. Involvement of intracellular Ca2+ in oxidant-induced NF-[kappa]B activation. FEBS Lett. 1996, 385, 58–62. [Google Scholar] [CrossRef]
- Kannan, K.; Jain, S.K. Oxidative stress and apoptosis. Pathophysiology 2000, 7, 153–163. [Google Scholar] [CrossRef]
- Han, M.; Ye, S.; Wen, W.; Zhang, Q. Oxidative stress-mediated pro-inflammatory responses in lung epithelial cells exposed to multi-walled carbon nanotubes. In Proceedings of the 2010 4th International Conference on Bioinformatics and Biomedical Engineering (iCBBE), Chengdu, China, 18–20 June 2010; pp. 1–4.
- Hirano, S.; Fujitani, Y.; Furuyama, A.; Kanno, S. Uptake and cytotoxic effects of multi-walled carbon nanotubes in human bronchial epithelial cells. Toxicol. Appl. Pharmacol. 2010, 249, 8–15. [Google Scholar] [CrossRef]
- Pacurari, M.; Yin, X.J.; Zhao, J.; Ding, M.; Leonard, S.S.; Schwegler-Berry, D.; Ducatman, B.S.; Sbarra, D.; Hoover, M.D.; Castranova, V.; Vallyathan, V. Raw single-wall carbon nanotubes induce oxidative stress and activate MAPKs, AP-1, NF-kappaB, and Akt in normal and malignant human mesothelial cells. Environ. Health Perspect. 2008, 116, 1211–1217. [Google Scholar] [CrossRef]
- Donaldson, K.; Tran, C.L. An introduction to the short-term toxicology of respirable industrial fibres. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2004, 553, 5–9. [Google Scholar] [CrossRef]
- Dörger, M.; Münzing, S.; Allmeling, A.-M.; Messmer, K.; Krombach, F. Differential responses of rat alveolar and peritoneal macrophages to man-made vitreous fibers in vitro. Environ. Res. 2001, 85, 207–214. [Google Scholar] [CrossRef]
- Brown, D.M.; Kinloch, I.A.; Bangert, U.; Windle, A.H.; Walter, D.M.; Walker, G.S.; Scotchford, C.A.; Donaldson, K.; Stone, V. An in vitro study of the potential of carbon nanotubes and nanofibres to induce inflammatory mediators and frustrated phagocytosis. Carbon 2007, 45, 1743–1756. [Google Scholar] [CrossRef]
- Poland, C.A.; Duffin, R.; Kinloch, I.; Maynard, A.; Wallace, W.A.H.; Seaton, A.; Stone, V.; Brown, S.; MacNee, W.; Donaldson, K. Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nat. Nanotechnol. 2008, 3, 423–428. [Google Scholar] [CrossRef]
- Ye, J.; Shi, X.; Jones, W.; Rojanasakul, Y.; Cheng, N.; Schwegler-Berry, D.; Baron, P.; Deye, G.J.; Li, C.; Castranova, V. Critical role of glass fiber length in TNF-alpha production and transcription factor activation in macrophages. Am. J. Physiol. 1999, 276, L426–L434. [Google Scholar]
- Kagan, V.E.; Konduru, N.V.; Feng, W.; Allen, B.L.; Conroy, J.; Volkov, Y.; Vlasova, I.I.; Belikova, N.A.; Yanamala, N.; Kapralov, A.; et al. Carbon nanotubes degraded by neutrophil myeloperoxidase induce less pulmonary inflammation. Nat. Nanotechnol. 2010, 5, 354–359. [Google Scholar] [CrossRef]
- Shvedova, A.A.; Kapralov, A.A.; Feng, W.H.; Kisin, E.R.; Murray, A.R.; Mercer, R.R.; St. Croix, C.M.; Lang, M.A.; Watkins, S.C.; Konduru, N.V.; et al. Impaired clearance and enhanced pulmonary inflammatory/fibrotic response to carbon nanotubes in myeloperoxidase-deficient mice. PLoS One 2012, 7, e30923. [Google Scholar] [CrossRef] [Green Version]
- Andón, F.T.; Kapralov, A.A.; Yanamala, N.; Feng, W.; Baygan, A.; Chambers, B.J.; Hultenby, K.; Ye, F.; Toprak, M.S.; Brandner, B.D.; et al. Biodegradation of single-walled carbon nanotubes by eosinophil peroxidase. Small 2013, 9, 2721–2729. [Google Scholar] [CrossRef]
- Spurzem, J.R.; Saltini, C.; Rom, W.; Winchester, R.J.; Crystal, R.G. Mechanisms of macrophage accumulation in the lungs of asbestos-exposed subjects. Am. Rev. Respir. Dis. 1987, 136, 276–280. [Google Scholar] [CrossRef]
- Choe, N.; Tanaka, S.; Xia, W.; Hemenway, D.R.; Roggli, V.L.; Kagan, E. Pleural macrophage recruitment and activation in asbestos-induced pleural injury. Environ. Health Perspect. 1997, 105, 1257–1260. [Google Scholar]
- Liu, X.; Hurt, R.H.; Kane, A.B. Biodurability of single-walled carbon nanotubes depends on surface functionalization. Carbon 2010, 48, 1961–1969. [Google Scholar] [CrossRef]
- DeLorme, M.P.; Muro, Y.; Arai, T.; Banas, D.A.; Frame, S.R.; Reed, K.L.; Warheit, D.B. Ninety-day inhalation toxicity study with a vapor grown carbon nanofiber in rats. Toxicol. Sci. 2012, 128, 449–460. [Google Scholar] [CrossRef]
- Mitchell, L.A.; Gao, J.; Vander Wal, R.; Gigliotti, A.; Burchiel, S.W.; McDonald, J.D. Pulmonary and systemic immune response to inhaled multiwalled carbon nanotubes. Toxicol. Sci. 2007, 100, 203–214. [Google Scholar]
- Ryman-Rasmussen, J.P.; Tewksbury, E.W.; Moss, O.R.; Cesta, M.F.; Wong, B.A.; Bonner, J.C. Inhaled multiwalled carbon nanotubes potentiate airway fibrosis in murine allergic asthma. Am. J. Respir. Cell Mol. Biol. 2009, 40, 349–358. [Google Scholar] [CrossRef]
- Ma-Hock, L.; Treumann, S.; Strauss, V.; Brill, S.; Luizi, F.; Mertler, M.; Wiench, K.; Gamer, A.O.; van Ravenzwaay, B.; Landsiedel, R. Inhalation toxicity of multiwall carbon nanotubes in rats exposed for 3 months. Toxicol. Sci. 2009, 112, 468–481. [Google Scholar] [CrossRef]
- Ryman-Rasmussen, J.P.; Cesta, M.F.; Brody, A.R.; Shipley-Phillips, J.K.; Everitt, J.I.; Tewksbury, E.W.; Moss, O.R.; Wong, B.A.; Dodd, D.E.; Andersen, M.E.; et al. Inhaled carbon nanotubes reach the subpleural tissue in mice. Nat. Nanotechnol. 2009, 4, 747–751. [Google Scholar] [CrossRef]
- Mercer, R.R.; Scabilloni, J.; Wang, L.; Kisin, E.; Murray, A.R.; Schwegler-Berry, D.; Shvedova, A.A.; Castranova, V. Alteration of deposition pattern and pulmonary response as a result of improved dispersion of aspirated single-walled carbon nanotubes in a mouse model. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008, 294, L87–L97. [Google Scholar]
- Mercer, R.; Hubbs, A.; Scabilloni, J.; Wang, L.; Battelli, L.; Schwegler-Berry, D.; Castranova, V.; Porter, D. Distribution and persistence of pleural penetrations by multi-walled carbon nanotubes. Part. Fibre Toxicol. 2010, 7, 28. [Google Scholar] [CrossRef]
- Mercer, R.; Hubbs, A.; Scabilloni, J.; Wang, L.; Battelli, L.; Friend, S.; Castranova, V.; Porter, D. Pulmonary fibrotic response to aspiration of multi-walled carbon nanotubes. Part. Fibre Toxicol. 2011, 8, 21. [Google Scholar] [CrossRef]
- Mercer, R.; Scabilloni, J.; Hubbs, A.; Wang, L.; Battelli, L.; McKinney, W.; Castranova, V.; Porter, D. Extrapulmonary transport of MWCNT following inhalation exposure. Part. Fibre Toxicol. 2013, 10, 38. [Google Scholar] [CrossRef]
- Mercer, R.; Scabilloni, J.; Hubbs, A.; Battelli, L.; McKinney, W.; Friend, S.; Wolfarth, M.; Andrew, M.; Castranova, V.; Porter, D. Distribution and fibrotic response following inhalation exposure to multi-walled carbon nanotubes. Part. Fibre Toxicol. 2013, 10, 33. [Google Scholar] [CrossRef]
- Porter, D.W.; Hubbs, A.F.; Chen, B.T.; McKinney, W.; Mercer, R.R.; Wolfarth, M.G.; Battelli, L.; Wu, N.; Sriram, K.; Leonard, S.; et al. Acute pulmonary dose–responses to inhaled multi-walled carbon nanotubes. Nanotoxicology 2013, 7, 1179–1194. [Google Scholar] [CrossRef]
- Muller, J.; Huaux, F.; Moreau, N.; Misson, P.; Heilier, J.-F.; Delos, M.; Arras, M.; Fonseca, A.; Nagy, J.B.; Lison, D. Respiratory toxicity of multi-wall carbon nanotubes. Toxicol. Appl. Pharmacol. 2005, 207, 221–231. [Google Scholar] [CrossRef]
- Rothen-Rutishauser, B.; Brown, D.M.; Piallier-Boyles, M.; Kinloch, I.A.; Windle, A.H.; Gehr, P.; Stone, V. Relating the physicochemical characteristics and dispersion of multiwalled carbon nanotubes in different suspension media to their oxidative reactivity in vitro and inflammation in vivo. Nanotoxicology 2010, 4, 331–342. [Google Scholar] [CrossRef]
- Shvedova, A.A.; Kisin, E.R.; Mercer, R.; Murray, A.R.; Johnson, V.J.; Potapovich, A.I.; Tyurina, Y.Y.; Gorelik, O.; Arepalli, S.; Schwegler-Berry, D.; et al. Unusual inflammatory and fibrogenic pulmonary responses to single-walled carbon nanotubes in mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005, 289, L698–L708. [Google Scholar] [CrossRef]
- Lam, C.W.; James, J.T.; McCluskey, R.; Hunter, R.L. Pulmonary toxicity of single-wall carbon nanotubes in mice 7 and 90 days after intratracheal instillation. Toxicol. Sci. 2004, 77, 126–134. [Google Scholar]
- Shvedova, A.A.; Kisin, E.R.; Murray, A.R.; Gorelik, O.; Arepalli, S.; Castranova, V.; Young, S.-H.; Gao, F.; Tyurina, Y.Y.; Oury, T.D.; et al. Vitamin E deficiency enhances pulmonary inflammatory response and oxidative stress induced by single-walled carbon nanotubes in C57BL/6 mice. Toxicol. Appl. Pharmacol. 2007, 221, 339–348. [Google Scholar] [CrossRef]
- Wang, L.; Castranova, V.; Mishra, A.; Chen, B.; Mercer, R.; Schwegler-Berry, D.; Rojanasakul, Y. Dispersion of single-walled carbon nanotubes by a natural lung surfactant for pulmonary in vitro and in vivo toxicity studies. Part. Fibre Toxicol. 2010, 7, 31. [Google Scholar] [CrossRef]
- Murray, A.R.; Kisin, E.R.; Tkach, A.V.; Yanamala, N.; Mercer, R.; Young, S.H.; Fadeel, B.; Kagan, V.E.; Shvedova, A.A. Factoring-in agglomeration of carbon nanotubes and nanofibers for better prediction of their toxicity versus asbestos. Part. Fibre Toxicol. 2012, 9, 10. [Google Scholar] [CrossRef]
- Crouzier, D.; Follot, S.; Gentilhomme, E.; Flahaut, E.; Arnaud, R.; Dabouis, V.; Castellarin, C.; Debouzy, J.C. Carbon nanotubes induce inflammation but decrease the production of reactive oxygen species in lung. Toxicology 2010, 272, 39–45. [Google Scholar] [CrossRef] [Green Version]
- Jacobsen, N.; Moller, P.; Jensen, K.; Vogel, U.; Ladefoged, O.; Loft, S.; Wallin, H. Lung inflammation and genotoxicity following pulmonary exposure to nanoparticles in ApoE-/- mice. Part. Fibre Toxicol. 2009, 6, 2. [Google Scholar] [CrossRef] [Green Version]
- Mangum, J.; Turpin, E.; Antao-Menezes, A.; Cesta, M.; Bermudez, E.; Bonner, J. Single-Walled Carbon Nanotube (SWCNT)-induced interstitial fibrosis in the lungs of rats is associated with increased levels of PDGF mRNA and the formation of unique intercellular carbon structures that bridge alveolar macrophages in situ. Part. Fibre Toxicol. 2006, 3, 15. [Google Scholar] [CrossRef]
- Shvedova, A.A.; Kisin, E.R.; Murray, A.R.; Kommineni, C.; Castranova, V.; Fadeel, B.; Kagan, V.E. Increased accumulation of neutrophils and decreased fibrosis in the lung of NADPH oxidase-deficient C57BL/6 mice exposed to carbon nanotubes. Toxicol. Appl. Pharmacol. 2008, 231, 235–240. [Google Scholar] [CrossRef]
- Cesta, M.F.; Ryman-Rasmussen, J.P.; Wallace, D.G.; Masinde, T.; Hurlburt, G.; Taylor, A.J.; Bonner, J.C. Bacterial lipopolysaccharide enhances PDGF signaling and pulmonary fibrosis in rats exposed to carbon nanotubes. Am. J. Respir. Cell Mol. Biol. 2010, 43, 142–151. [Google Scholar] [CrossRef]
- Nygaard, U.C.; Samuelsen, M.; Marioara, C.D.; Lovik, M. Carbon nanofibers have IgE adjuvant capacity but are less potent than nanotubes in promoting allergic airway responses. Biomed. Res. Int. 2013, 2013, 476010. [Google Scholar]
- Shvedova, A.A.; Kisin, E.; Murray, A.R.; Johnson, V.J.; Gorelik, O.; Arepalli, S.; Hubbs, A.F.; Mercer, R.R.; Keohavong, P.; Sussman, N.; et al. Inhalation vs. Aspiration of Single-Walled Carbon Nanotubes in C57BL/6 Mice: Inflammation, Fibrosis, Oxidative Stress, and Mutagenesis. Am J Physiol Lung Cell Mol Physiol. 2008, 295, L552–L565. [Google Scholar] [CrossRef]
- Kobayashi, N.; Naya, M.; Ema, M.; Endoh, S.; Maru, J.; Mizuno, K.; Nakanishi, J. Biological response and morphological assessment of individually dispersed multi-wall carbon nanotubes in the lung after intratracheal instillation in rats. Toxicology 2010, 276, 143–153. [Google Scholar] [CrossRef]
- Valko, M.; Morris, H.; Cronin, M.T.D. Metals, toxicity and oxidative stress. Curr. Med. Chem. 2005, 12, 1161–1208. [Google Scholar] [CrossRef]
- Hansen, K.; Mossman, B. Generation of superoxide (O2-.) from alveolar macrophages exposed to asbestiform and nonfibrous particles. Cancer Res. 1987, 47, 1681–1686. [Google Scholar]
- Hill, I.M.; Beswick, P.H.; Donaldson, K. Differential release of superoxide anions by macrophages treated with long and short fibre amosite asbestos is a consequence of differential affinity for opsonin. Occup. Environ. Med. 1995, 52, 92–96. [Google Scholar] [CrossRef]
- Boyles, M.S.P.; Young, L.; Brown, D.M.; MacCalman, L.; Cowie, H.; Moisala, A.; Smail, F.; Smith, P.J.W.; Proudfoot, L.; Windle, A.H.; et al. Length Dependent Effects of Multi-Walled Carbon Nanotubes on Macrophage Mediated Inflammation, Phagocytosis and Cytotoxicity—A Comparison with Asbestos and Nanoparticle Carbon Black, submitted.
- Muller, J.; Huaux, F.; Fonseca, A.; Nagy, J.B.; Moreau, N.; Delos, M.; Raymundo-Piñero, E.; Béguin, F.; Kirsch-Volders, M.; Fenoglio, I.; et al. Structural defects play a major role in the acute lung toxicity of multiwall carbon nanotubes: Toxicological aspects. Chem. Res. Toxicol. 2008, 21, 1698–1705. [Google Scholar] [CrossRef]
- Maynard, A.D.; Baron, P.A.; Foley, M.; Shvedova, A.A.; Kisin, E.R.; Castranova, V. Exposure to carbon nanotube material: Aerosol release during the handling of unrefined single-walled carbon nanotube material. J. Toxicol. Environ. Health Part A 2004, 67, 87–107. [Google Scholar] [CrossRef]
- Bernstein, D.; Castranova, V.; Donaldson, K.; Fubini, B.; Hadley, J.; Hesterberg, T.; Kane, A.; Lai, D.; McConnell, E.E.; Muhle, H.; et al. Testing of fibrous particles: Short-term assays and strategies. Inhal. Toxicol. 2005, 17, 497–537. [Google Scholar] [CrossRef]
- Brown, D.; Donaldson, K.; Stone, V. Nuclear translocation of Nrf2 and expression of antioxidant defence genes in THP-1 cells exposed to carbon nanotubes. J. Biomed. Nanotechnol. 2010, 6, 224–233. [Google Scholar] [CrossRef]
- Murphy, F.A.; Poland, C.A.; Duffin, R.; Al-Jamal, K.T.; Ali-Boucetta, H.; Nunes, A.; Byrne, F.; Prina-Mello, A.; Volkov, Y.; Li, S.; et al. Length-dependent retention of carbon nanotubes in the pleural space of mice initiates sustained inflammation and progressive fibrosis on the parietal pleura. Am. J. Pathol. 2011, 178, 2587–2600. [Google Scholar] [CrossRef]
- Snyder-Talkington, B.N.; Dymacek, J.; Porter, D.W.; Wolfarth, M.G.; Mercer, R.R.; Pacurari, M.; Denvir, J.; Castranova, V.; Qian, Y.; Guo, N.L. System-based identification of toxicity pathways associated with multi-walled carbon nanotube-induced pathological responses. Toxicol. Appl. Pharmacol. 2013, 272, 476–489. [Google Scholar] [CrossRef]
- Meunier, E.; Coste, A.; Olagnier, D.; Authier, H.; Lefèvre, L.; Dardenne, C.; Bernad, J.; Béraud, M.; Flahaut, E.; Pipy, B. Double-walled carbon nanotubes trigger IL-1β release in human monocytes through Nlrp3 inflammasome activation. Nanomedicine 2012, 8, 987–995. [Google Scholar] [CrossRef]
- Besnard, A.-G.; Togbe, D.; Couillin, I.; Tan, Z.; Zheng, S.G.; Erard, F.; le Bert, M.; Quesniaux, V.; Ryffel, B. Inflammasome–IL-1–Th17 response in allergic lung inflammation. J. Mol. Cell Biol. 2012, 4, 3–10. [Google Scholar] [CrossRef]
- Palomäki, J.; Välimäki, E.; Sund, J.; Vippola, M.; Clausen, P.A.; Jensen, K.A.; Savolainen, K.; Matikainen, S.; Alenius, H. Long, needle-like carbon nanotubes and asbestos activate the NLRP3 inflammasome through a similar mechanism. ACS Nano 2011, 5, 6861–6870. [Google Scholar] [CrossRef]
- Clift, M.J.; Endes, C.; Vanhecke, D.; Wick, P.; Gehr, P.; Schins, R.P.; Petri-Fink, A.; Rothen-Rutishauser, B. A comparative study of different in vitro lung cell culture systems to assess the most beneficial tool for screening the potential adverse effects of carbon nanotubes. Toxicol. Sci. 2014, 137, 55–64. [Google Scholar] [CrossRef]
- Müller, L.; Riediker, M.; Wick, P.; Mohr, M.; Gehr, P.; Rothen-Rutishauser, B. Oxidative stress and inflammation response after nanoparticle exposure: Differences between human lung cell monocultures and an advanced three-dimensional model of the human epithelial airways. J. R. Soc. Interface 2010, 7, S27–S40. [Google Scholar] [CrossRef]
- Gasser, M.; Wick, P.; Clift, M.; Blank, F.; Diener, L.; Yan, B.; Gehr, P.; Krug, H.; Rothen-Rutishauser, B. Pulmonary surfactant coating of multi-walled carbon nanotubes (MWCNTs) influences their oxidative and pro-inflammatory potential in vitro. Part. Fibre Toxicol. 2012, 9, 1–13. [Google Scholar] [CrossRef]
- Chen, X.; Kis, A.; Zettl, A.; Bertozzi, C.R. A cell nanoinjector based on carbon nanotubes. Proc. Natl. Acad. Sci. USA 2007, 104, 8218–8222. [Google Scholar] [CrossRef]
- Fabbro, C.; Ali-Boucetta, H.; Da Ros, T.; Kostarelos, K.; Bianco, A.; Prato, M. Targeting carbon nanotubes against cancer. Chem. Commun. (Camb) 2012, 48, 3911–3926. [Google Scholar]
- Kam, N.W.; Dai, H. Carbon nanotubes as intracellular protein transporters: Generality and biological functionality. J. Am. Chem. Soc. 2005, 127, 6021–6026. [Google Scholar] [CrossRef]
- Pantarotto, D.; Briand, J.P.; Prato, M.; Bianco, A. Translocation of bioactive peptides across cell membranes by carbon nanotubes. Chem. Commun. (Camb) 2004, 1, 16–17. [Google Scholar] [CrossRef]
- Singh, R.; Pantarotto, D.; McCarthy, D.; Chaloin, O.; Hoebeke, J.; Partidos, C.D.; Briand, J.P.; Prato, M.; Bianco, A.; Kostarelos, K. Binding and condensation of plasmid DNA onto functionalized carbon nanotubes: Toward the construction of nanotube-based gene delivery vectors. J. Am. Chem. Soc. 2005, 127, 4388–4396. [Google Scholar] [CrossRef]
- Meng, J.; Meng, J.; Duan, J.; Kong, H.; Li, L.; Wang, C.; Xie, S.; Chen, S.; Gu, N.; Xu, H.; et al. Carbon nanotubes conjugated to tumor lysate protein enhance the efficacy of an antitumor immunotherapy. Small 2008, 4, 1364–1370. [Google Scholar] [CrossRef]
- Villa, C.H.; Dao, T.; Ahearn, I.; Fehrenbacher, N.; Casey, E.; Rey, D.A.; Korontsvit, T.; Zakhaleva, V.; Batt, C.A.; Philips, M.R.; et al. Single-walled carbon nanotubes deliver peptide antigen into dendritic cells and enhance IgG responses to tumor-associated antigens. ACS Nano 2011, 5, 5300–5311. [Google Scholar] [CrossRef]
- Zeinali, M.; Jammalan, M.; Ardestani, S.K.; Mosaveri, N. Immunological and cytotoxicological characterization of tuberculin purified protein derivative (PPD) conjugated to single-walled carbon nanotubes. Immunol. Lett. 2009, 126, 48–53. [Google Scholar] [CrossRef]
- Yandar, N.; Pastorin, G.; Prato, M.; Bianco, A.; Patarroyo, M.E.; Lozano, J.M. Immunological profile of a Plasmodium vivax AMA-1 N-terminus peptide-carbon nanotube conjugate in an infected Plasmodium berghei mouse model. Vaccine 2008, 26, 5864–5873. [Google Scholar] [CrossRef]
- Parra, J.; Abad-Somovilla, A.; Mercader, J.V.; Taton, T.A.; Abad-Fuentes, A. Carbon nanotube-protein carriers enhance size-dependent self-adjuvant antibody response to haptens. J. Control. Release 2013, 170, 242–251. [Google Scholar] [CrossRef]
- Klumpp, C.; Kostarelos, K.; Prato, M.; Bianco, A. Functionalized carbon nanotubes as emerging nanovectors for the delivery of therapeutics. Biochim. Biophys. Acta 2006, 1758, 404–412. [Google Scholar]
- Abe, S.; Itoh, S.; Hayashi, D.; Kobayashi, T.; Kiba, T.; Akasaka, T.; Uo, M.; Yawaka, Y.; Sato, S.; Watari, F.; et al. Biodistribution of aqueous suspensions of carbon nanotubes in mice and their biocompatibility. J. Nanosci. Nanotechnol. 2012, 12, 700–706. [Google Scholar] [CrossRef]
- Dobrovolskaia, M.A.; McNeil, S.E. Handbook of Immunological Properties of Engineered Nanomaterials; World Scientific: Singapore, Singapore, 2013; p. 692 S. [Google Scholar]
- Zolnik, B.S.; Gonzalez-Fernandez, A.; Sadrieh, N.; Dobrovolskaia, M.A. Nanoparticles and the immune system. Endocrinology 2010, 151, 458–465. [Google Scholar] [CrossRef]
- Radomski, A.; Jurasz, P.; Alonso-Escolano, D.; Drews, M.; Morandi, M.; Malinski, T.; Radomski, M.W. Nanoparticle-induced platelet aggregation and vascular thrombosis. Br. J. Pharmacol. 2005, 146, 882–893. [Google Scholar] [CrossRef]
- Lacerda, S.H.; Semberova, J.; Holada, K.; Simakova, O.; Hudson, S.D.; Simak, J. Carbon nanotubes activate store-operated calcium entry in human blood platelets. ACS Nano 2011, 5, 5808–5813. [Google Scholar] [CrossRef]
- Zhang, C.-H.; Luo, Y.-L.; Chen, Y.-S.; Wei, Q.-B.; Fan, L.-H. Preparation and theophylline delivery applications of novel PMAA/MWCNT-COOH nanohybrid hydrogels. J. Biomater. Sci. Polym. Ed. 2009, 20, 1119–1135. [Google Scholar] [CrossRef]
- Yamaguchi, A.; Fujitani, T.; Ohyama, K.; Nakae, D.; Hirose, A.; Nishimura, T.; Ogata, A. Effects of sustained stimulation with multi-wall carbon nanotubes on immune and inflammatory responses in mice. J. Toxicol. Sci. 2012, 37, 177–189. [Google Scholar] [CrossRef]
- Rodriguez, L.L.; Gay, C.G. Development of vaccines toward the global control and eradication of foot-and-mouth disease. Expert Rev. Vaccines 2011, 10, 377–387. [Google Scholar] [CrossRef]
- Bianco, A.; Hoebeke, J.; Godefroy, S.; Chaloin, O.; Pantarotto, D.; Briand, J.-P.; Muller, S.; Prato, M.; Partidos, C.D. Cationic carbon nanotubes bind to CpG oligodeoxynucleotides and enhance their immunostimulatory properties. J. Am. Chem. Soc. 2005, 127, 58–59. [Google Scholar] [CrossRef]
- Zhao, D.; Alizadeh, D.; Zhang, L.; Liu, W.; Farrukh, O.; Manuel, E.; Diamond, D.J.; Badie, B. Carbon nanotubes enhance CpG uptake and potentiate antiglioma immunity. Clin. Cancer Res. 2011, 17, 771–782. [Google Scholar] [CrossRef]
- Park, E.J.; Cho, W.S.; Jeong, J.; Yi, J.; Choi, K.; Park, K. Pro-inflammatory and potential allergic responses resulting from B cell activation in mice treated with multi-walled carbon nanotubes by intratracheal instillation. Toxicology 2009, 259, 113–121. [Google Scholar] [CrossRef]
- Andersen, A.J.; Wibroe, P.P.; Moghimi, S.M. Perspectives on carbon nanotube-mediated adverse immune effects. Adv. Drug Deliv. Rev. 2012, 64, 1700–1705. [Google Scholar] [CrossRef]
- Bottini, M.; Bruckner, S.; Nika, K.; Bottini, N.; Bellucci, S.; Magrini, A.; Bergamaschi, A.; Mustelin, T. Multi-walled carbon nanotubes induce T lymphocyte apoptosis. Toxicol. Lett. 2006, 160, 121–126. [Google Scholar] [CrossRef]
- Inoue, K.; Koike, E.; Yanagisawa, R.; Hirano, S.; Nishikawa, M.; Takano, H. Effects of multi-walled carbon nanotubes on a murine allergic airway inflammation model. Toxicol. Appl. Pharmacol. 2009, 237, 306–316. [Google Scholar] [CrossRef]
- Nygaard, U.C.; Hansen, J.S.; Samuelsen, M.; Alberg, T.; Marioara, C.D.; Løvik, M. Single-walled and multi-walled carbon nanotubes promote allergic immune responses in mice. Toxicol. Sci. 2009, 109, 113–123. [Google Scholar] [CrossRef]
- Meng, J.; Yang, M.; Jia, F.; Xu, Z.; Kong, H.; Xu, H. Immune responses of BALB/c mice to subcutaneously injected multi-walled carbon nanotubes. Nanotoxicology 2011, 5, 583–591. [Google Scholar] [CrossRef]
- Meng, J.; Yang, M.; Jia, F.; Kong, H.; Zhang, W.; Wang, C.; Xing, J.; Xie, S.; Xu, H. Subcutaneous injection of water-soluble multi-walled carbon nanotubes in tumor-bearing mice boosts the host immune activity. Nanotechnology 2010, 21, 145104. [Google Scholar] [CrossRef]
- Yokoyama, A.; Sato, Y.; Nodasaka, Y.; Yamamoto, S.; Kawasaki, T.; Shindoh, M.; Kohgo, T.; Akasaka, T.; Uo, M.; Watari, F.; et al. Biological behavior of hat-stacked carbon nanofibers in the subcutaneous tissue in rats. Nano Lett. 2005, 5, 157–161. [Google Scholar] [CrossRef]
- Sato, Y.; Yokoyama, A.; Nodasaka, Y.; Kohgo, T.; Motomiya, K.; Matsumoto, H.; Nakazawa, E.; Numata, T.; Zhang, M.; Yudasaka, M.; et al. Long-term biopersistence of tangled oxidized carbon nanotubes inside and outside macrophages in rat subcutaneous tissue. Sci. Rep. 2013, 3, 2516. [Google Scholar]
- Cheng, W.W.; Lin, Z.Q.; Wei, B.F.; Zeng, Q.; Han, B.; Wei, C.X.; Fan, X.J.; Hu, C.L.; Liu, L.H.; Huang, J.H.; et al. Single-walled carbon nanotube induction of rat aortic endothelial cell apoptosis: Reactive oxygen species are involved in the mitochondrial pathway. Int. J. Biochem. Cell Biol. 2011, 43, 564–572. [Google Scholar] [CrossRef]
- Guo, X.; Jagannath, C.; Espitia, M.G.; Zhou, X. Uptake of silica and carbon nanotubes by human macrophages/monocytes induces activation of fibroblasts in vitro—Potential implication for pathogenesis of inflammation and fibrotic diseases. Int. J. Immunopathol. Pharmacol. 2012, 25, 713–719. [Google Scholar]
- Albini, A.; Mussi, V.; Parodi, A.; Ventura, A.; Principi, E.; Tegami, S.; Rocchia, M.; Francheschi, E.; Sogno, I.; Cammarota, R.; et al. Interactions of single-wall carbon nanotubes with endothelial cells. Nanomedicine 2010, 6, 277–288. [Google Scholar] [CrossRef]
- Pulskamp, K.; Diabate, S.; Krug, H.F. Carbon nanotubes show no sign of acute toxicity but induce intracellular reactive oxygen species in dependence on contaminants. Toxicol. Lett. 2007, 168, 58–74. [Google Scholar] [CrossRef]
- Vlasova, I.I.; Vakhrusheva, T.V.; Sokolov, A.V.; Kostevich, V.A.; Gusev, A.A.; Gusev, S.A.; Melnikova, V.I.; Lobach, A.S. PEGylated single-walled carbon nanotubes activate neutrophils to increase production of hypochlorous acid, the oxidant capable of degrading nanotubes. Toxicol. Appl. Pharmacol. 2012, 264, 131–142. [Google Scholar] [CrossRef]
- Sun, Z.; Liu, Z.; Meng, J.; Meng, J.; Duan, J.; Xie, S.; Lu, X.; Zhu, Z.; Wang, C.; Chen, S.; et al. Carbon nanotubes enhance cytotoxicity mediated by human lymphocytes in vitro. PLoS One 2011, 6, e21073. [Google Scholar]
- Clichici, S.; Biris, A.R.; Tabaran, F.; Filip, A. Transient oxidative stress and inflammation after intraperitoneal administration of multiwalled carbon nanotubes functionalized with single strand DNA in rats. Toxicol. Appl. Pharmacol. 2012, 259, 281–292. [Google Scholar] [CrossRef]
- Yuan, J.; Gao, H.; Sui, J.; Duan, H.; Chen, W.N.; Ching, C.B. Cytotoxicity evaluation of oxidized single-walled carbon nanotubes and graphene oxide on human hepatoma HepG2 cells: An iTRAQ-coupled 2D LC-MS/MS proteome analysis. Toxicol. Sci. 2012, 126, 149–161. [Google Scholar] [CrossRef]
- Kermanizadeh, A.; Gaiser, B.K.; Hutchison, G.R.; Stone, V. An in vitro liver model—Assessing oxidative stress and genotoxicity following exposure of hepatocytes to a panel of engineered nanomaterials. Part. Fibre Toxicol. 2012, 9, 28. [Google Scholar] [CrossRef]
- Kermanizadeh, A.; Pojana, G.; Gaiser, B.K.; Birkedal, R.; Bilanicová, D.; Wallin, H.; Jensen, K.A.; Sellergren, B.; Hutchison, G.R.; Marcomini, A.; et al. In vitro assessment of engineered nanomaterials using a hepatocyte cell line: Cytotoxicity, pro-inflammatory cytokines and functional markers. Nanotoxicology 2013, 7, 301–313. [Google Scholar] [CrossRef]
- Demento, S.L.; Eisenbarth, S.C.; Foellmer, H.G.; Platt, C.; Caplan, M.J.; Mark Saltzman, W.; Mellman, I.; Ledizet, M.; Fikrig, E.; Flavell, R.A.; et al. Inflammasome-activating nanoparticles as modular systems for optimizing vaccine efficacy. Vaccine 2009, 27, 3013–3021. [Google Scholar] [CrossRef]
- Boraschi, D.; Duschl, A. Nanoparticles and the Immune System—Safety and Effects; Adademic Press: Oxford, UK, 2013. [Google Scholar]
- Gao, N.; Zhang, Q.; Mu, Q.; Bai, Y.; Li, L.; Zhou, H.; Butch, E.R.; Powell, T.B.; Snyder, S.E.; Jiang, G.; et al. Steering carbon nanotubes to scavenger receptor recognition by nanotube surface chemistry modification partially alleviates NFkappaB activation and reduces its immunotoxicity. ACS Nano 2011, 5, 4581–4591. [Google Scholar] [CrossRef]
- Moghimi, S.M.; Andersen, A.J.; Ahmadvand, D.; Wibroe, P.P.; Andresen, T.L.; Hunter, A.C. Material properties in complement activation. Adv. Drug Deliv. Rev. 2011, 63, 1000–1007. [Google Scholar] [CrossRef]
- Salvador-Morales, C.; Flahaut, E.; Sim, E.; Sloan, J.; Green, M.L.; Sim, R.B. Complement activation and protein adsorption by carbon nanotubes. Mol. Immunol. 2006, 43, 193–201. [Google Scholar] [CrossRef] [Green Version]
- Rybak-Smith, M.J.; Sim, R.B. Complement activation by carbon nanotubes. Adv. Drug Deliv. Rev. 2011, 63, 1031–1041. [Google Scholar] [CrossRef]
- Carroll, M.V.; Sim, R.B. Complement in health and disease. Adv. Drug Deliv. Rev. 2011, 63, 965–975. [Google Scholar] [CrossRef]
- Ling, W.L.; Biro, A.; Bally, I.; Tacnet, P.; Deniaud, A.; Doris, E.; Frachet, P.; Schoehn, G.; Pebay-Peyroula, E.; Arlaud, G.J. Proteins of the innate immune system crystallize on carbon nanotubes but are not activated. ACS Nano 2011, 5, 730–737. [Google Scholar] [CrossRef]
- Andersen, A.J.; Robinson, J.T.; Dai, H.; Hunter, A.C.; Andresen, T.L.; Moghimi, S.M. Single-walled carbon nanotube surface control of complement recognition and activation. ACS Nano 2013, 7, 1108–1119. [Google Scholar] [CrossRef]
- Rybak-Smith, M.J.; Tripisciano, C.; Borowiak-Palen, E.; Lamprecht, C.; Sim, R.B. Effect of functionalization of carbon nanotubes with psychosine on complement activation and protein adsorption. J. Biomed. Nanotechnol. 2011, 7, 830–839. [Google Scholar] [CrossRef]
- Čolić, M.; Džopalić, T.; Tomić, S.; Rajković, J.; Rudolf, R.; Vuković, G.; Marinković, A.; Uskoković, P. Immunomodulatory effects of carbon nanotubes functionalized with a Toll-like receptor 7 agonist on human dendritic cells. Carbon 2014, 67, 273–287. [Google Scholar] [CrossRef]
- Gottardi, R.; Douradinha, B. Carbon nanotubes as a novel tool for vaccination against infectious diseases and cancer. J. Nanobiotechnology 2013, 11, 30. [Google Scholar] [CrossRef]
- Casals, E.; Pfaller, T.; Duschl, A.; Oostingh, G.J.; Puntes, V. Time evolution of the nanoparticle protein corona. ACS Nano 2010, 4, 3623–3632. [Google Scholar] [CrossRef]
- Monopoli, M.P.; Aberg, C.; Salvati, A.; Dawson, K.A. Biomolecular coronas provide the biological identity of nanosized materials. Nat. Nanotechnol. 2012, 7, 779–786. [Google Scholar] [CrossRef]
- Shannahan, J.H.; Brown, J.M.; Chen, R.; Ke, P.C.; Lai, X.; Mitra, S.; Witzmann, F.A. Comparison of nanotube-protein corona composition in cell culture media. Small 2013, 9, 2171–2181. [Google Scholar] [CrossRef]
- Tenzer, S.; Docter, D.; Kuharev, J.; Musyanovych, A.; Fetz, V.; Hecht, R.; Schlenk, F.; Fischer, D.; Kiouptsi, K.; Reinhardt, C.; et al. Rapid formation of plasma protein corona critically affects nanoparticle pathophysiology. Nat. Nanotechnol. 2013, 8, 772–781. [Google Scholar] [CrossRef]
- Laverny, G.; Casset, A.; Purohit, A.; Schaeffer, E.; Spiegelhalter, C.; de Blay, F.; Pons, F. Immunomodulatory properties of multi-walled carbon nanotubes in peripheral blood mononuclear cells from healthy subjects and allergic patients. Toxicol. Lett. 2013, 217, 91–101. [Google Scholar] [CrossRef]
- Palomaki, J.; Karisola, P.; Pylkkanen, L.; Savolainen, K.; Alenius, H. Engineered nanomaterials cause cytotoxicity and activation on mouse antigen presenting cells. Toxicology 2010, 267, 125–131. [Google Scholar] [CrossRef]
- Aldinucci, A.; Turco, A.; Biagioli, T.; Toma, F.M.; Bani, D.; Guasti, D.; Manuelli, C.; Rizzetto, L.; Cavalieri, D.; Massacesi, L.; et al. Carbon nanotube scaffolds instruct human dendritic cells: Modulating immune responses by contacts at the nanoscale. Nano Lett. 2013, 13, 6098–6105. [Google Scholar] [CrossRef]
- Thurnherr, T.; Brandenberger, C.; Fischer, K.; Diener, L.; Manser, P.; Maeder-Althaus, X.; Kaiser, J.P.; Krug, H.F.; Rothen-Rutishauser, B.; Wick, P. A comparison of acute and long-term effects of industrial multiwalled carbon nanotubes on human lung and immune cells in vitro. Toxicol. Lett. 2011, 200, 176–186. [Google Scholar] [CrossRef]
- Alam, A.; Sachar, S.; Puri, N.; Saxena, R.K. Interactions of polydispersed single-walled carbon nanotubes with T cells resulting in downregulation of allogeneic CTL responses in vitro and in vivo. Nanotoxicology 2013, 7, 1351–1360. [Google Scholar] [CrossRef]
- Erlanger, B.F.; Chen, B.; Zhu, M.; Brus, L. Binding of an anti-fullerene IgG monoclonal antibody to single wall carbon nanotubes. Nano Lett. 2001, 1, 465–467. [Google Scholar] [CrossRef]
- Pantarotto, D.; Partidos, C.D.; Hoebeke, J.; Brown, F.; Kramer, E.; Briand, J.P.; Muller, S.; Prato, M.; Bianco, A. Immunization with peptide-functionalized carbon nanotubes enhances virus-specific neutralizing antibody responses. Chem. Biol. 2003, 10, 961–966. [Google Scholar] [CrossRef]
- Delogu, L.G.; Venturelli, E.; Manetti, R.; Pinna, G.A.; Carru, C.; Madeddu, R.; Murgia, L.; Sgarrella, F.; Dumortier, H.; Bianco, A. Ex vivo impact of functionalized carbon nanotubes on human immune cells. Nanomedicine (Lond) 2012, 7, 231–243. [Google Scholar] [CrossRef]
- Dumortier, H.; Lacotte, S.; Pastorin, G.; Marega, R.; Wu, W.; Bonifazi, D.; Briand, J.P.; Prato, M.; Muller, S.; Bianco, A. Functionalized carbon nanotubes are non-cytotoxic and preserve the functionality of primary immune cells. Nano Lett. 2006, 6, 1522–1528. [Google Scholar] [CrossRef]
- Medepalli, K.; Alphenaar, B.; Raj, A.; Sethu, P. Evaluation of the direct and indirect response of blood leukocytes to carbon nanotubes (CNTs). Nanomedicine 2011, 7, 983–991. [Google Scholar] [CrossRef]
- Pescatori, M.; Bedognetti, D.; Venturelli, E.; Menard-Moyon, C.; Bernardini, C.; Muresu, E.; Piana, A.; Maida, G.; Manetti, R.; Sgarrella, F.; et al. Functionalized carbon nanotubes as immunomodulator systems. Biomaterials 2013, 34, 4395–4403. [Google Scholar] [CrossRef]
- Grecco, A.C.; Paula, R.F.; Mizutani, E.; Sartorelli, J.C.; Milani, A.M.; Longhini, A.L.; Oliveira, E.C.; Pradella, F.; Silva, V.D.; Moraes, A.S.; et al. Up-regulation of T lymphocyte and antibody production by inflammatory cytokines released by macrophage exposure to multi-walled carbon nanotubes. Nanotechnology 2011, 22, 265103. [Google Scholar] [CrossRef]
- Bergin, I.L.; Witzmann, F.A. Nanoparticle toxicity by the gastrointestinal route: Evidence and knowledge gaps. Int. J. Biomed. Nanosci. Nanotechnol. 2013, 3. [Google Scholar] [CrossRef]
- Upadhyayula, V.K.; Deng, S.G.; Mitchell, M.C.; Smith, G.B. Application of carbon nanotube technology for removal of contaminants in drinking water: A review. Sci. Total Environ. 2009, 408, 1–13. [Google Scholar] [CrossRef]
- Brody, A.L.; Bugusu, B.; Han, J.H.; Sand, C.K.; Mchugh, T.H. Innovative food packaging solutions. J. Food Sci. 2008, 73, R107–R116. [Google Scholar] [CrossRef]
- Prajapati, V.K.; Awasthi, K.; Yadav, T.P.; Rai, M.; Srivastava, O.N.; Sundar, S. An oral formulation of amphotericin b attached to functionalized carbon nanotubes is an effective treatment for experimental visceral leishmaniasis. J. Infect. Dis. 2012, 205, 333–336. [Google Scholar] [CrossRef]
- Kou, W.; Akasaka, T.; Watari, F.; Sjogren, G. An in vitro evaluation of the biological effects of carbon nanotube-coated dental zirconia. ISRN Dent. 2013, 2013, 296727. [Google Scholar]
- Wang, Z.Y.; Zhao, J.; Song, L.; Mashayekhi, H.; Chefetz, B.; Xing, B.S. Adsorption and desorption of phenanthrene on carbon nanotubes in simulated gastrointestinal fluids. Environ. Sci. Technol. 2011, 45, 6018–6024. [Google Scholar] [CrossRef]
- Szendi, K.; Varga, C. Lack of genotoxicity of carbon nanotubes in a pilot study. Anticancer Res. 2008, 28, 349–352. [Google Scholar]
- Folkmann, J.K.; Risom, L.; Jacobsen, N.R.; Wallin, H.; Loft, S.; Moller, P. Oxidatively damaged DNA in rats exposed by oral gavage to C60 fullerenes and single-walled carbon nanotubes. Environ. Health Perspect. 2009, 117, 703–708. [Google Scholar]
- Lim, J.-H.; Kim, S.-H.; Shin, I.-S.; Park, N.-H.; Moon, C.; Kang, S.-S.; Kim, S.-H.; Park, S.-C.; Kim, J.-C. Maternal exposure to multi-wall carbon nanotubes does not induce embryo–fetal developmental toxicity in rats. Birth Defects Res. Part B Dev. Reprod. Toxicol. 2011, 92, 69–76. [Google Scholar] [CrossRef]
- Philbrook, N.A.; Walker, V.K.; Afrooz, A.R.; Saleh, N.B.; Winn, L.M. Investigating the effects of functionalized carbon nanotubes on reproduction and development in Drosophila melanogaster and CD-1 mice. Reprod. Toxicol. 2011, 32, 442–448. [Google Scholar] [CrossRef]
- Matsumoto, M.; Serizawa, H.; Sunaga, M.; Kato, H.; Takahashi, M.; Hirata-Koizumi, M.; Ono, A.; Kamata, E.; Hirose, A. No toxicological effects on acute and repeated oral gavage doses of single-wall or multi-wall carbon nanotube in rats. J. Toxicol. Sci. 2012, 37, 463–474. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Boyles, M.S.P.; Stoehr, L.C.; Schlinkert, P.; Himly, M.; Duschl, A. The Significance and Insignificance of Carbon Nanotube-Induced Inflammation. Fibers 2014, 2, 45-74. https://doi.org/10.3390/fib2010045
Boyles MSP, Stoehr LC, Schlinkert P, Himly M, Duschl A. The Significance and Insignificance of Carbon Nanotube-Induced Inflammation. Fibers. 2014; 2(1):45-74. https://doi.org/10.3390/fib2010045
Chicago/Turabian StyleBoyles, Matthew S.P., Linda C. Stoehr, Paul Schlinkert, Martin Himly, and Albert Duschl. 2014. "The Significance and Insignificance of Carbon Nanotube-Induced Inflammation" Fibers 2, no. 1: 45-74. https://doi.org/10.3390/fib2010045
APA StyleBoyles, M. S. P., Stoehr, L. C., Schlinkert, P., Himly, M., & Duschl, A. (2014). The Significance and Insignificance of Carbon Nanotube-Induced Inflammation. Fibers, 2(1), 45-74. https://doi.org/10.3390/fib2010045



