In Situ Preparation and Corrosion Resistance of a ZrO2 Film on a ZrB2 Ceramic
Abstract
:1. Introduction
2. Experimental Procedures
2.1. Materials
2.2. Film Characterization
2.3. Electrochemical Measurements
3. Results and Discussion
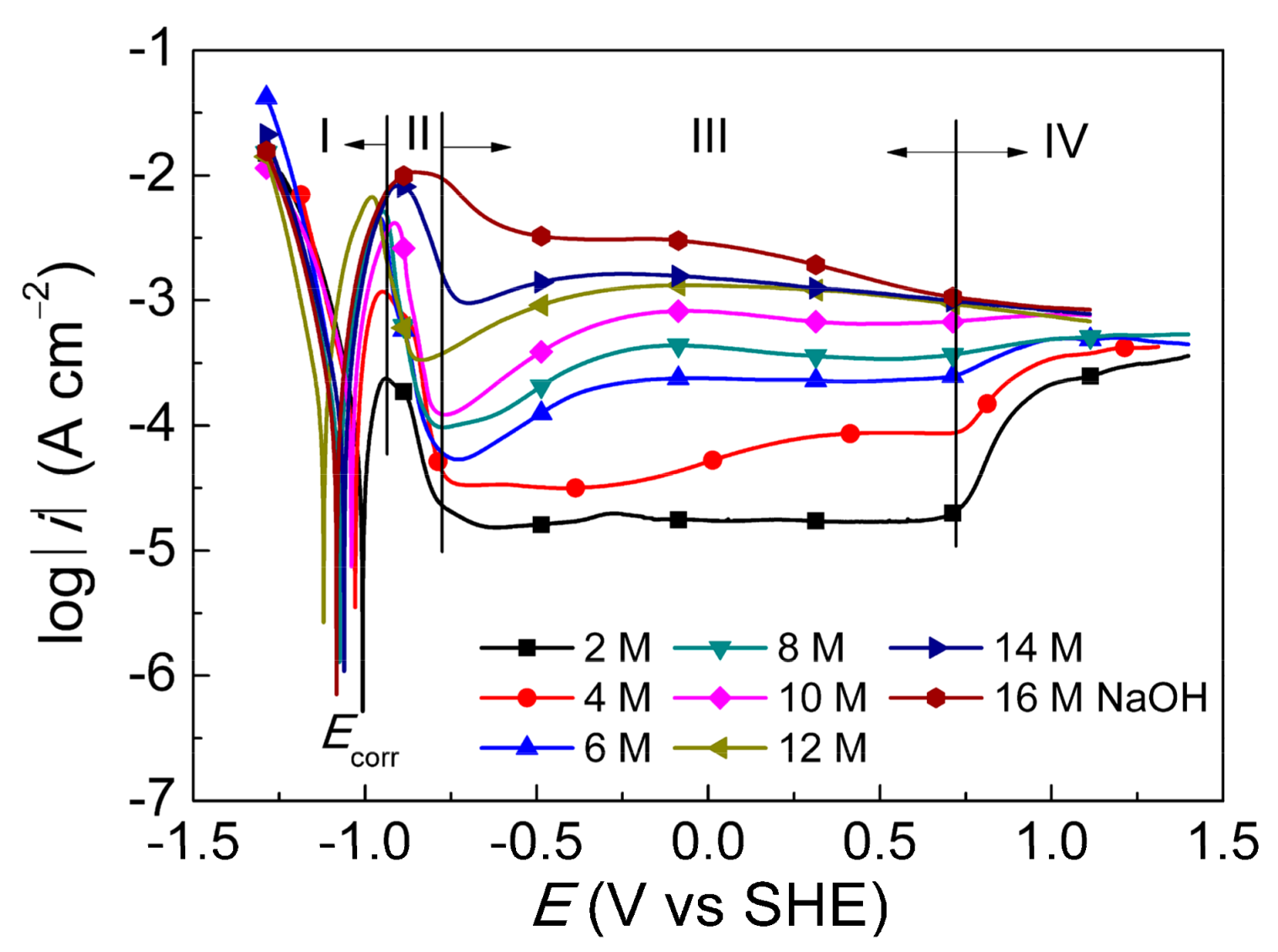
3.1. PDP of ZrB2 in NaOH Solutions
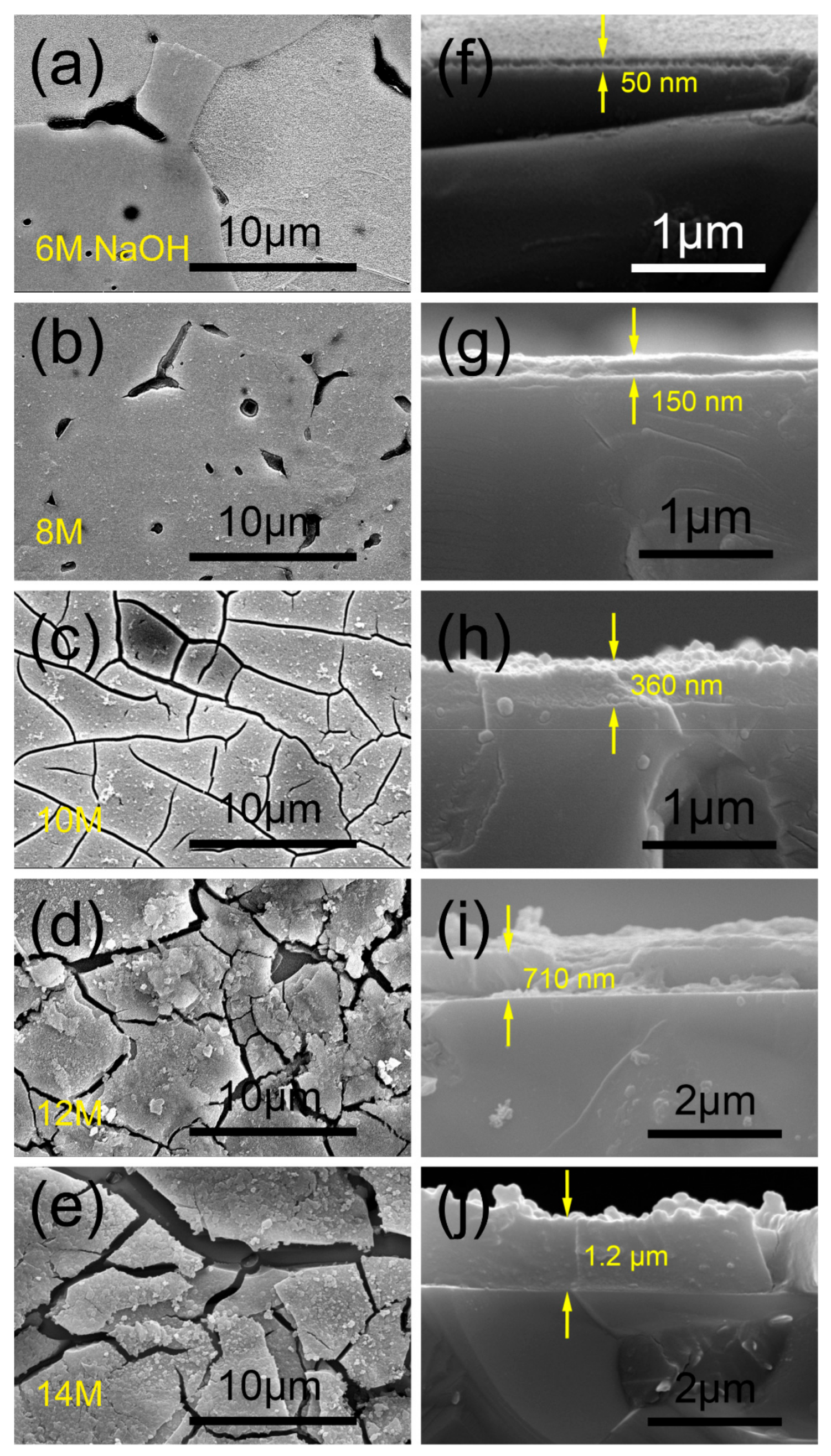
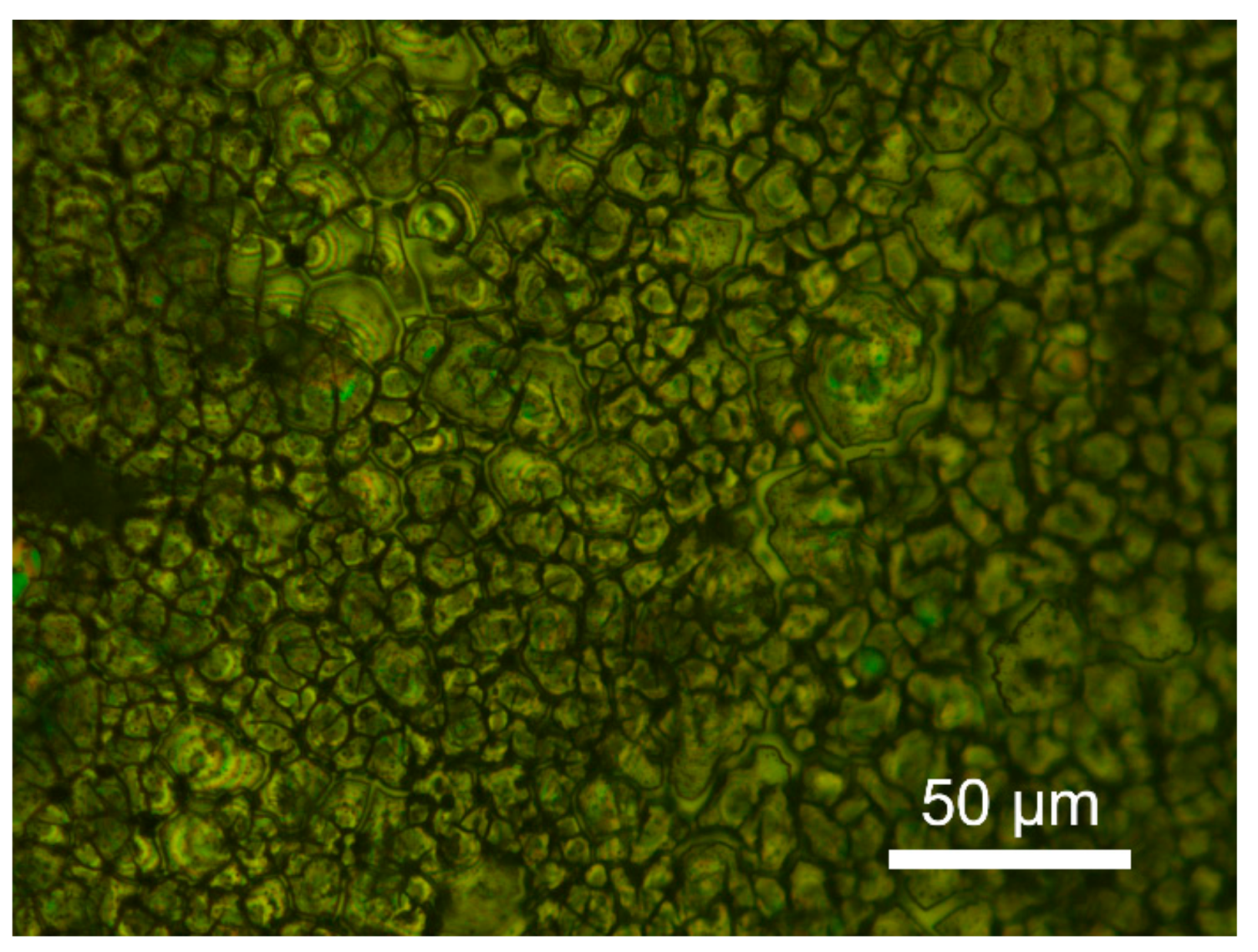
3.2. Morphology of the Anodic Film
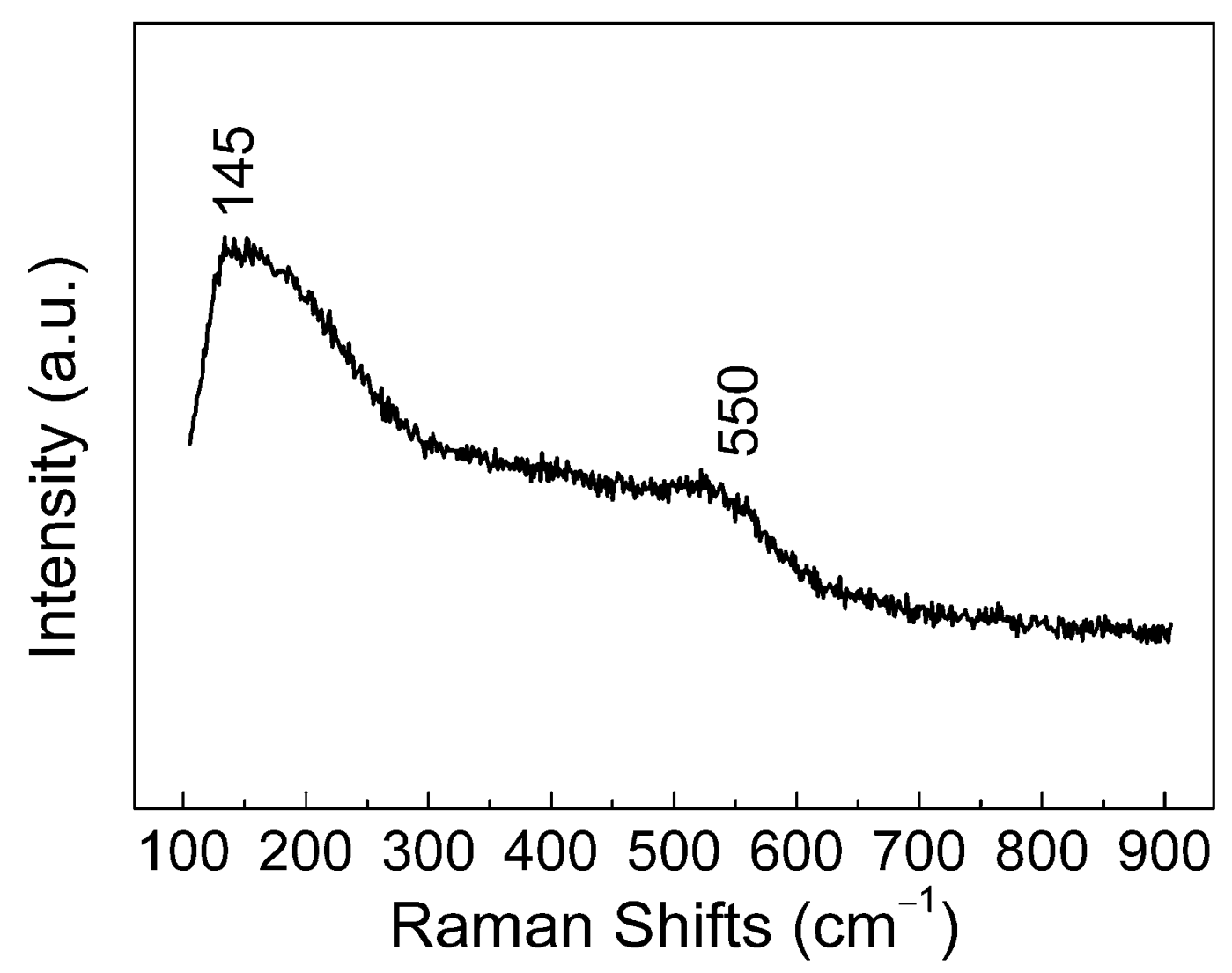
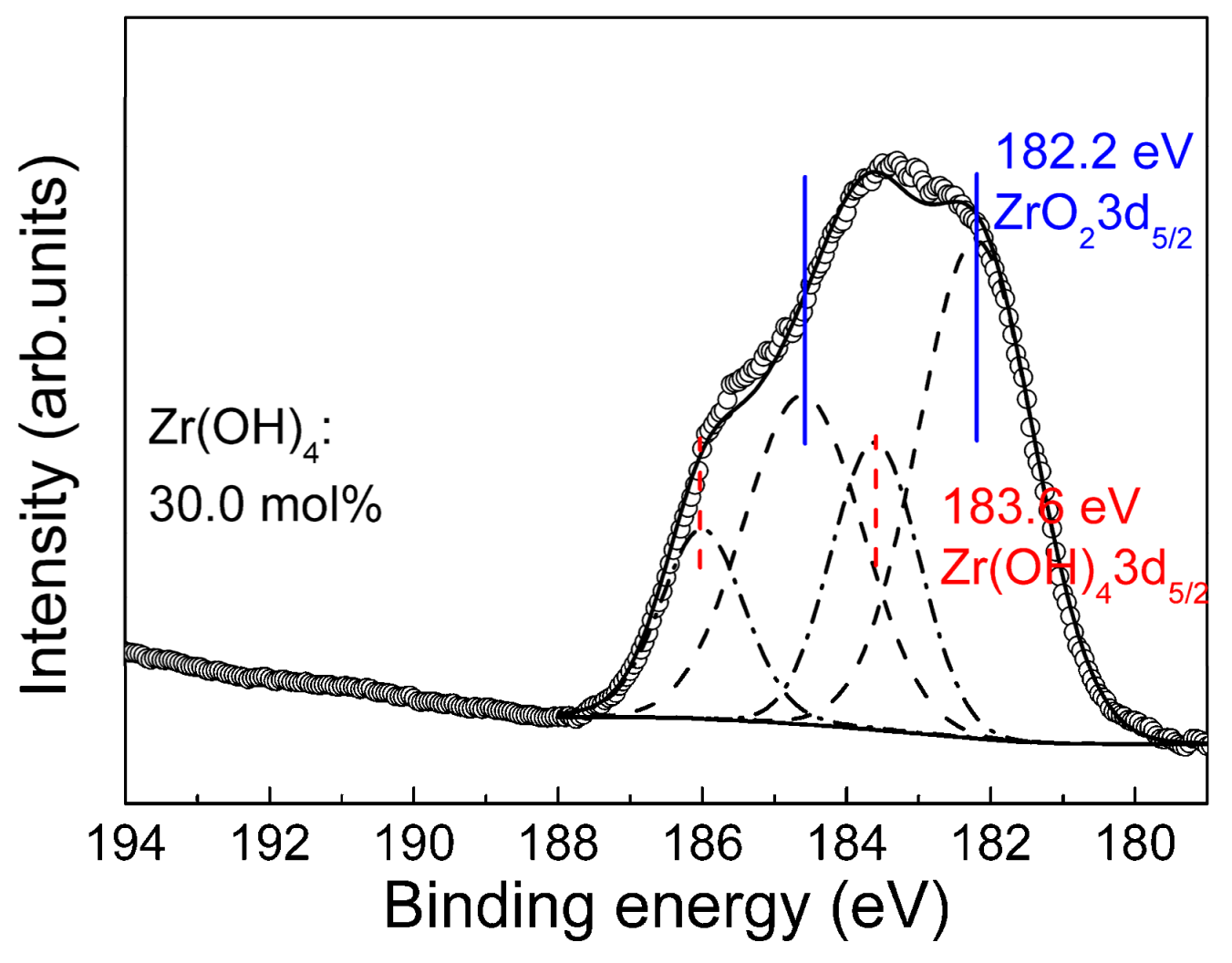
3.3. Composition of the Anodic Films
3.4. Corrosion Resistance of the Anodic Film When Put in a 0.1 M H2C2O4 Solution
4. Conclusions
- The anodic films could be prepared from −0.8 to 0.8 V (SHE). Furthermore, the passive current densities (10−5–10−3 A·cm−2) represented the film formation rate increase with the NaOH concentration.
- The amorphous ZrO2 was the major component of the films. During the anodization, the dehydration reaction of Zr(OH)4 to ZrO2 caused the volume shrinkage and tensile stress of the films. When the thickness exceeded a critical value, the mud-cracking morphology occurred. The critical thickness was found in the region of 150–360 nm.
- The films without cracks exhibited the inhibition effect and provided effective corrosion protection when put in a 0.1 M H2C2O4 solution, which had a positive correlation with the film thickness. The film obtained when put in a 8 M NaOH solution (~150 nm, near the critical thickness) performed best, almost one order of magnitude greater compared with the substrate.
Author Contributions
Funding
Conflicts of Interest
References
- Chen, F.; Jin, D.; Tyeb, K.; Wang, B.; Han, Y.-H.; Kim, S.; Schoenung, J.M.; Shen, Q.; Zhang, L. Field assisted sintering of graphene reinforced zirconia ceramics. Ceram. Int. 2015, 41, 6113–6116. [Google Scholar] [CrossRef]
- Eltayeb, K.; Hong, W.; Chen, F.; Han, Y.-H.; Shen, Q.; Zhang, L. Field-assisted sintering of multiphase toughening zirconia ceramics. J. Ceram. Process. Res. 2017, 18, 1–9. [Google Scholar]
- Catauro, M.; Bollino, F.; Tranquillo, E.; Tuffi, R.; Dell’Era, A.; Ciprioti, S.V. Morphological and thermal characterization of zirconia/hydroxyapatite composites prepared via sol-gel for biomedical applications. Ceram. Int. 2019, 45, 2835–2845. [Google Scholar] [CrossRef]
- Dixit, V.K.; Marathe, A.; Bhatt, G.; Khamari, S.K.; Rajiv, K.; Kumar, R.; Mukherjee, C.; Panchal, C.J.; Sharma, T.K.; Oak, S.M. Evaluation of structural and microscopic properties of tetragonal ZrO2 for the facet coating of 980 nm semiconductor laser diodes. J. Phys. D. Appl. Phys. 2015, 48, 105102. [Google Scholar] [CrossRef]
- Han, H.; Qiu, F.; Liu, Z.; Han, X.-E. ZrO2-coated Li3V2(PO4)3/C nanocomposite: A high-voltage cathode for rechargeable lithium-ion batteries with remarkable cycling performance. Ceram. Int. 2015, 41, 8779–8784. [Google Scholar] [CrossRef]
- Garg, N.; Bera, S.; Velmurugan, S. Effect of coating thickness and grain size on the electrochemical properties of hydrothermally deposited nano-ZrO2coatings on stainless steel surface. Thin Solid Films 2019, 670, 60–67. [Google Scholar] [CrossRef]
- Yu, J.; Ji, G.; Shi, Z.; Wang, X. Corrosion resistance of ZrO2 films under different humidity coal gas conditions at high temperature. J. Alloy. Comp. 2019, 783, 371–378. [Google Scholar] [CrossRef]
- Jang, B.-K.; Sun, J.; Kim, S.; Oh, Y.-S.; Lee, S.-M.; Kim, H.-T. Thermal conductivity of ZrO2-4mol%Y2O3 thin coatings by pulsed thermal imaging method. Surf. Coat. Technol. 2015, 284, 57–62. [Google Scholar] [CrossRef]
- Setare, E.; Raeissi, K.; Golozar, M.A.; Fathi, M.H. The structure and corrosion barrier performance of nanocrystalline zirconia electrodeposited coating. Corros. Sci. 2009, 51, 1802–1808. [Google Scholar] [CrossRef]
- Khan, M.; Zeng, Y.; Lan, Z.; Wang, Y. Reduced thermal conductivity of solid solution of 20% CeO2 + ZrO2 and 8% Y2O3 + ZrO2 prepared by atmospheric plasma spray technique. Ceram. Int. 2019, 45, 839–842. [Google Scholar] [CrossRef]
- Zhang, X.T.; Zhang, R.H.; Hu, S.M.; Wang, Y. Development of Zr/ZrO2 high temperature and high pressure sensors for in-situ measuring chemical parameters of deep-sea water. Sci. China. Ser. E Technol. Sci. 2009, 52, 2466–2473. [Google Scholar] [CrossRef]
- Park, Y.J.; Kim, J.W.; Ali, G.; Cho, S.O. Enhancement of oxidation resistance of zirconium alloy with anodic nanoporous oxide layer in high-temperature air/steam environments. Corros. Sci. 2018, 140, 217–222. [Google Scholar] [CrossRef]
- Ortiz, A.L.; Zamora, V.; Rodríguez-Rojas, F. A study of the oxidation of ZrB2 powders during high-energy ball-milling in air. Ceram. Int. 2012, 38, 2857–2863. [Google Scholar] [CrossRef]
- Lopes, N.I.A.; Freire, N.H.J.; Resende, P.D.; Santos, L.A.; Buono, V.T.L. Electrochemical deposition and characterization of ZrO2 ceramic nanocoatings on superelastic NiTi alloy. Appl. Surf. Sci. 2018, 450, 21–30. [Google Scholar] [CrossRef]
- Wang, H.; Chen, D.; Wang, C.-A.; Zhang, R.; Fang, D. Preparation and characterization of high-toughness ZrB2/Mo composites by hot-pressing process. Int. J. Refract. Met. Hard Mater. 2009, 27, 1024–1026. [Google Scholar] [CrossRef]
- Sun, X.; Han, W.; Liu, Q.; Hu, P.; Hong, C. ZrB2-ceramic toughened by refractory metal Nb prepared by hot-pressing. Mater. Des. 2010, 31, 4427–4431. [Google Scholar] [CrossRef]
- Wang, B.; Li, J.; Yang, H.; Shen, Q.; Zhang, L. Effect of ZrC thin film on preventing reactions between Nb and ZrB2-SiC. J. Synth. Cryst 2015, 44, 2762–2766. (In Chinese) [Google Scholar]
- Monticelli, C.; Bellosi, A.; Dal Colle, M. Electrochemical behavior of ZrB2 in aqueous solutions. J. Electrochem. Soc. 2004, 151, B331–B339. [Google Scholar] [CrossRef]
- Yang, H.T.; Zhang, J.; Li, J.G.; Chen, F.; Shen, Q.; Zhang, L.M. Electrochemical corrosion behavior of zirconium diboride ceramic in concentrated alkaline solutions. Mater. Res. Express 2018, 5, 126303. [Google Scholar] [CrossRef]
- Inzelt, G.; Lewenstam, A.; Scholz, F. Handbook of Reference Electrodes; Springer: Berlin, Germany, 2013. [Google Scholar]
- Cao, C.N. Principles of Electrochemistry of Corrosion; Chemical Industrial Press: Beijing, China, 2008. [Google Scholar]
- Kelly, R.G.; Scully, J.R.; Shoesmith, D.; Buchheit, R.G. Electrochemical Techniques in Corrosion Science and Engineering; CRC Press: Boca Raton, FL, USA, 2002; pp. 55–58. [Google Scholar]
- Human, A.M.; Roebuck, B.; Exner, H.E. Electrochemical polarisation and corrosion behaviour of cobalt and Co(W, C) alloys in 1 N sulphuric acid. Mater. Sci. Eng. A 1998, 241, 202–210. [Google Scholar] [CrossRef]
- Hu, M.; Evans, A. The cracking and decohesion of thin films on ductile substrates. Acta Metall. 1989, 37, 917–925. [Google Scholar] [CrossRef]
- Krishnamurthy, S.; Reimanis, I. Multiple cracking in CrN and Cr2N films on brass. Surf. Coat. Technol. 2005, 192, 291–298. [Google Scholar] [CrossRef]
- Singh, K.B.; Tirumkudulu, M.S. Cracking in drying colloidal films. Phys. Rev. Lett. 2007, 98, 218302. [Google Scholar] [CrossRef] [PubMed]
- Li, M.J.; Feng, Z.C.; Ying, P.L.; Xin, Q.; Li, C. Phase transformation in the surface region of zirconia and doped zirconia detected by UV Raman spectroscopy. Phys. Chem. Chem. Phys. 2003, 5, 5326–5332. [Google Scholar] [CrossRef]
- Zhou, Y.; Fang, C.; Fang, Y.; Zhu, F. Polyborates in aqueous borate solution: A Raman and DFT theory investigation. Spectrochim. Acta A. 2011, 83, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Barr, T.L. An ESCA study of the termination of the passivation of elemental metals. J. Phys. Chem. 1978, 82, 1801–1810. [Google Scholar] [CrossRef]
- Huang, C.; Tang, Z.; Zhang, Z. Differences between zirconium hydroxide (Zr(OH)4·nH2O) and hydrous zirconia (ZrO2·nH2O). J. Am. Ceram. Soc. 2001, 84, 1637–1638. [Google Scholar] [CrossRef]
- Yüce, A.O.; Mert, B.D.; Kardaş, G.; Yazıcı, B. Electrochemical and quantum chemical studies of 2-amino-4-methyl-thiazole as corrosion inhibitor for mild steel in HCl solution. Corros. Sci. 2014, 83, 310–316. [Google Scholar] [CrossRef]
- Zeng, Z.P.; Lillard, R.S.; Cong, H.B. Effect of salt concentration on the corrosion behavior of carbon steel in CO2 environment. Corrosion 2016, 72, 805–823. [Google Scholar] [CrossRef]
- Zhang, G.A.; Cheng, Y.F. Corrosion of X65 steel in CO2-saturated oilfield formation water in the absence and presence omf acetic acid. Corros. Sci. 2009, 51, 1589–1595. [Google Scholar] [CrossRef]
- Rekha, M.Y.; Kamboj, A.; Srivastava, C. Electrochemical behavior of SnNi-graphene oxide composite coatings. Thin Solid Films 2018, 653, 82–92. [Google Scholar] [CrossRef]
- Chen, Z.; Yang, W.; Xu, B.; Chen, Y.; Qian, M.; Su, X.; Li, Z.; Yin, X.; Liu, Y. Corrosion protection of carbon steels by electrochemically synthesized V-TiO2/polypyrrole composite coatings in 0.1 M HCl solution. J. Alloy. Comp. 2019, 771, 857–868. [Google Scholar] [CrossRef]
- Hong, S.; Wu, Y.; Gao, W.; Zhang, J.; Zheng, Y.; Zheng, Y. Slurry erosion-corrosion resistance and microbial corrosion electrochemical characteristics of HVOF sprayed WC-10Co-4Cr coating for offshore hydraulic machinery. Int. J. Refract. Met. Hard Mater. 2018, 74, 7–13. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, J.; Claypool, J.B.; Castano, C.E.; O’Keefe, M.J. Structure and corrosion behavior of sputter deposited cerium oxide based coatings with various thickness on Al 2024-T3 alloy substrates. Appl. Surf. Sci. 2015, 355, 805–813. [Google Scholar] [CrossRef]
- Maeng, S.; Axe, L.; Tyson, T.A.; Gladczuk, L.; Sosnowski, M. Corrosion behaviour of magnetron sputtered α- and β-Ta coatings on AISI 4340 steel as a function of coating thickness. Corros. Sci. 2006, 48, 2154–2171. [Google Scholar] [CrossRef]
- Gu, Y.; Bandopadhyay, S.; Chen, C.F.; Ning, C.; Guo, Y. Long-term corrosion inhibition mechanism of microarc oxidation coated AZ31 Mg alloys for biomedical applications. Mater. Des. 2013, 46, 66–75. [Google Scholar] [CrossRef]




| Sample | Rs (Ω·cm2) | Rt (Ω·cm2) | Rf (Ω·cm2) | RL (Ω·cm2) | Qf (S·secn1·cm−2) | n1 | Qdl (S·secn2·cm−2) | n2 | L (H·cm−2) |
|---|---|---|---|---|---|---|---|---|---|
| Substrate | 12.9 | 349.3 | – | 4481.0 | – | – | 2.2 × 10−5 | 0.79 | 3532.5 |
| 2 M | 14.9 | 592.9 | 58.9 | 2959.2 | 5.5 × 10−6 | 0.96 | 1.2 × 10−5 | 0.70 | 6169.3 |
| 4 M | 13.6 | 1327.1 | 107.9 | 5974.8 | 4.0 × 10−6 | 0.87 | 3.6 × 10−7 | 0.98 | 1373.7 |
| 6 M | 14.7 | 2551.2 | 431.7 | 7386.5 | 4.3 × 10−6 | 0.87 | 2.2 × 10−7 | 0.96 | 1570.0 |
| 8 M | 17.4 | 3787.6 | 510.2 | 13320.5 | 2.9 × 10−6 | 0.91 | 1.7 × 10−7 | 0.94 | 4710 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Zhang, J.; Li, J.; Shen, Q.; Zhang, L. In Situ Preparation and Corrosion Resistance of a ZrO2 Film on a ZrB2 Ceramic. Coatings 2019, 9, 455. https://doi.org/10.3390/coatings9070455
Yang H, Zhang J, Li J, Shen Q, Zhang L. In Situ Preparation and Corrosion Resistance of a ZrO2 Film on a ZrB2 Ceramic. Coatings. 2019; 9(7):455. https://doi.org/10.3390/coatings9070455
Chicago/Turabian StyleYang, Haitao, Jian Zhang, Junguo Li, Qiang Shen, and Lianmeng Zhang. 2019. "In Situ Preparation and Corrosion Resistance of a ZrO2 Film on a ZrB2 Ceramic" Coatings 9, no. 7: 455. https://doi.org/10.3390/coatings9070455





