Evaluation of the Corrosion Resistance and Cytocompatibility of a Bioactive Micro-Arc Oxidation Coating on AZ31 Mg Alloy
Abstract
:1. Introduction
2. Experimental
2.1. Preparation of Specimens
2.2. Characterization
2.3. Electrochemical Measurements
2.4. Animal Surgery and Implant Harvest
3. Results and Discussion
3.1. Determination of Optimal Level of Corrosion Resistance
3.1.1. Orthogonal Experiment and Analysis for MAO Process
3.1.2. Confirmation Runs for the Negative Duty Cycle
3.1.3. Confirmation Runs for the Treatment Time
3.2. Corrosion Resistance of Electrochemical Analysis
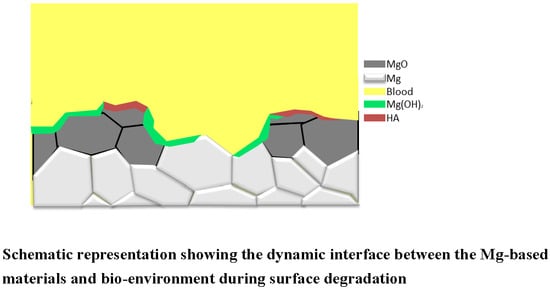
3.3. Bio-Degradable Implantation Test
4. Conclusions
- (1).
- This study used the Taguchi method to find out the optimal conditional combination to produce the micro arc oxidation coating on AZ31 Mg alloy with the greatest corrosion resistance. The corrosion resistance of different MAO-coated AZ31 substrates was evaluated by electrochemical measurements carried out in SBF solution. Accordingly, the MAO-coated substrate exhibited a better corrosion resistance than the uncoated one based on the electrochemical tests.
- (2).
- The optimal conditional combination obtained in this study is: current density of 200 mA/cm2, electrical frequency of 4000 Hz, duty cycle of 20%, and treatment time of 30 min.
- (3).
- The surface chemical components were analyzed by XRD. The results demonstrated that all the MAO coatings were mainly composed of MgO and MgSiO3. Then, an in vivo testing showed that the MAO-coated AZ31 had good cytocompatibility and anticorrosive properties.
- (4).
- In vivo tests showed that the degradation rate of the Mg alloy-based screw is critical to the success of its application. The well-adhered MAO layer on AZ31 Mg alloy efficiently reduces the degradation rate of the screw. This is beneficial to bone healing.
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ratner, B.D.; Hoffman, A.S.; Schoen, F.J.; Lemons, J.E. Biomaterials Science: An Introduction to Materials in Medicine, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Jacobsen, N.; Hensten-Pettersen, A. Occupational health problems and adverse patient reactions in orthodontics. Eur. J. Orthod. 1989, 11, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.L.; Wataha, J.C.; Hanks, C.T. Effects of metal ions on osteoblast-like cell metabolism and differentiation. J. Biomed. Mater. Res. 1997, 34, 29–37. [Google Scholar] [CrossRef]
- Vahey, J.W.; Simonian, P.T. Carcinogenicity and metallic implants. Am. J. Orthop. 1995, 24, 319–324. [Google Scholar] [PubMed]
- Zhao, D.; Witte, F.; Lu, F.; Wang, J.; Li, J.; Qin, L. Current status on clinical applications of magnesium-based orthopaedic implants: A review from clinical translational perspective. Biomaterials 2017, 112, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Huiskes, R.; Weinans, H.; Van Rietbergen, B. The relationship between stress shielding and bone resorption around total hip stems and the effects of flexible materials. Clin. Orthop. Relat. Res. 1992, 274, 124–134. [Google Scholar] [CrossRef]
- Long, M.; Rack, H.J. Titanium alloys in total joint replacement—A materials science perspective. Biomaterials 1998, 19, 1621–1639. [Google Scholar] [CrossRef]
- Agins, H.J.; Alcock, N.W.; Bansal, M.; Salvati, E.A.; Wilson, P.D.; Pellicci, P.M.; Bullough, P.G. Metallic wear in failed titanium-alloy total hip replacements. A histological and quantitative analysis. J. Bone Joint Surg. 1988, 70, 347–356. [Google Scholar] [CrossRef]
- Dearnley, P.A.; Dahm, K.L.; Çimenoǧlu, H. The corrosion—Wear behaviour of thermally oxidised CP-Ti and Ti–6Al–4V. Wear 2004, 256, 469–479. [Google Scholar] [CrossRef]
- Wu, G.; Ibrahim, J.M.; Chu, P.K. Surface design of biodegradable magnesium alloys—A review. Surf. Coat. Technol. 2013, 233, 2–12. [Google Scholar] [CrossRef]
- Minkowitz, R.B.; Bhadsavle, S.; Walsh, M.; Egol, K.A. Removal of painful orthopaedic implants after fracture union. J. Bone Joint Surg. Am. 2007, 89, 1906–1912. [Google Scholar]
- Zreiqat, H.; Howlett, C.R.; Zannettino, A.; Evans, P.; Schulze-Tanzil, G.; Knabe, C.; Shakibaei, M. Mechanisms of magnesium-stimulated adhesion of osteoblastic cells to commonly used orthopaedic implants. J. Biomed. Mater. Res. 2002, 62, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Kirkland, N.T.; Birbilis, N. Magnesium Biomaterials: Design, Testing, and Best Practice; Springer: Cham, Switzerland, 2014. [Google Scholar]
- Song, G.; Song, S.Z. A Possible biodegradable magnesium implant material. Adv. Eng. Mater. 2007, 9, 298–302. [Google Scholar] [CrossRef]
- Córdoba, L.C.; Montemor, M.F.; Coradin, T. Silane/TiO2 coating to control the corrosion rate of magnesium alloys in simulated body fluid. Corros. Sci. 2016, 104, 152–161. [Google Scholar] [CrossRef]
- Hiromoto, S. Self-healing property of hydroxyapatite and octacalcium phosphate coatings on pure magnesium and magnesium alloy. Corros. Sci. 2015, 100, 284–294. [Google Scholar] [CrossRef]
- Lei, T.; Ouyang, C.; Tang, W.; Li, L.F.; Zhou, L.S. Enhanced corrosion protection of MgO coatings on magnesium alloy deposited by an anodic electrodeposition process. Corros. Sci. 2010, 52, 3504–3508. [Google Scholar] [CrossRef]
- Cai, Q.; Wang, L.; Wei, B.; Liu, Q. Electrochemical performance of microarc oxidation films formed on AZ91D magnesium alloy in silicate and phosphate electrolytes. Surf. Coat. Technol. 2006, 200, 3727–3733. [Google Scholar] [CrossRef]
- Arrabal, R.; Matykina, E.; Viejo, F.; Skeldon, P.; Thompson, G.E. Corrosion resistance of WE43 and AZ91D magnesium alloys with phosphate PEO coatings. Corros. Sci. 2008, 50, 1744–1752. [Google Scholar] [CrossRef]
- Witte, F.; Kaese, V.; Haferkamp, H.; Switzer, E.; Meyer-Lindenberg, A.; Wirth, C.J.; Windhagen, H. In vivo corrosion of four magnesium alloys and the associated bone response. Biomaterials 2005, 26, 3557–3563. [Google Scholar] [CrossRef]
- Narayanan, T.S.; Park, I.S.; Lee, M.H. Strategies to improve the corrosion resistance of microarc oxidation (MAO) coated magnesium alloys for degradable implants: Prospects and challenges. Prog. Mater. Sci. 2014, 60, 1–71. [Google Scholar] [CrossRef]
- Mori, Y.; Koshi, A.; Liao, J.; Asoh, H.; Ono, S. Characteristics and corrosion resistance of plasma electrolytic oxidation coatings on AZ31B Mg alloy formed in phosphate—Silicate mixture electrolytes. Corros. Sci. 2014, 88, 254–262. [Google Scholar] [CrossRef]
- Yue, Y.A.N.G.; Hua, W.U. Effect of current density on corrosion resistance of micro-arc oxide coatings on magnesium alloy. T. Nonferr. Metal. Soc. China 2010, 20, s688–s692. [Google Scholar]
- Liu, J.; Zhang, W.; Zhang, H.; Hu, X.; Zhang, J. Effect of microarc oxidation time on electrochemical behaviors of coated bio-compatible magnesium alloy. Strategies to improve the corrosion resistance of microarc oxidation (MAO) coated magnesium alloys for degradable implants: Prospects and challenges. Mater. Today Proc. 2014, 1, 70–81. [Google Scholar] [CrossRef]
- Gu, Y.; Chen, C.F.; Bandopadhyay, S.; Ning, C.; Zhang, Y.; Guo, Y. Corrosion mechanism and model of pulsed DC microarc oxidation treated AZ31 alloy in simulated body fluid. Appl. Surf. Sci. 2012, 258, 6116–6126. [Google Scholar] [CrossRef]
- Hussein, R.O.; Zhang, P.; Nie, X.; Xia, Y.; Northwood, D.O. The effect of current mode and discharge type on the corrosion resistance of plasma electrolytic oxidation PEO) coated magnesium alloy AJ62. Surf. Coat. Technol. 2011, 206, 1990–1997. [Google Scholar] [CrossRef]
- Hussein, R.O.; Nie, X.; Northwood, D.O. Influence of process parameters on electrolytic plasma discharging behaviour and aluminum oxide coating microstructure. Surf. Coat. Technol. 2010, 205, 1659–1667. [Google Scholar] [CrossRef]
- Ben-Arfa, B.A.; Salvado, I.M.M.; Frade, J.R.; Pullar, R.C. Fast route for synthesis of stoichiometric hydroxyapatite by employing the Taguchi method. Mater. Des. 2016, 109, 547–555. [Google Scholar] [CrossRef]
- Song, G. Control of biodegradation of biocompatable magnesium alloys. Corros. Sci. 2007, 49, 1696–1701. [Google Scholar] [CrossRef]
- Atrens, A.; Liu, M.; Abidin, N.I.Z. Corrosion mechanism applicable to biodegradable magnesium implants. Mater. Sci. Eng. B 2011, 176, 1609–1636. [Google Scholar] [CrossRef]
- Neil, W.C.; Forsyth, M.; Howlett, P.C.; Hutchinson, C.R.; Hinton, B.R.W. Corrosion of heat treated magnesium alloy ZE41. Corros. Sci. 2011, 53, 3299–3308. [Google Scholar] [CrossRef]
- ASTM D-3359. Standard Test Methods for Rating Adhesion by Tape Test; ASTM International: West Conshohocken, PA, USA, 2017. [Google Scholar]
- Lee, S.J.; Do Toan, L.H.; Lee, J.L.; Chen, C.Y.; Peng, H.C. Effects of pulsed unipolar and bipolar current regimes on the characteristics of micro-arc oxidation coating on LZ91 magnesium-lithium alloy. Int. J. Electrochem. Sci. 2018, 13, 2705–2717. [Google Scholar] [CrossRef]
- Sah, S.P.; Tsuji, E.; Aoki, Y.; Habazaki, H. Cathodic pulse breakdown of anodic films on aluminium in alkaline silicate electrolyte—Understanding the role of cathodic half-cycle in AC plasma electrolytic oxidation. Corros. Sci. 2012, 55, 90–96. [Google Scholar] [CrossRef]
- Yerokhin, A.L.; Nie, X.; Leyland, A.; Matthews, A. Characterisation of oxide films produced by plasma electrolytic oxidation of a Ti–6Al–4V alloy. Surf. Coat. Technol. 2000, 130, 195–206. [Google Scholar] [CrossRef]
- Hussein, R.O.; Nie, X.; Northwood, D.O. An investigation of ceramic coating growth mechanisms in plasma electrolytic oxidation (PEO) processing. Electrochim. Acta 2013, 112, 111–119. [Google Scholar] [CrossRef]
- ISO 10993-1. Standard Test Methods for Biological Evaluation of Medical Devices-Part 1: Evaluation and Testing within a Risk Management Process; ISO: Geneva, Switzerland, 2018. [Google Scholar]
- Wilke, B.M.; Zhang, L.; Li, W.; Ning, C.; Chen, C.F.; Gu, Y. Corrosion performance of MAO coatings on AZ31 Mg alloy in simulated body fluid vs. Earle’s balance salt solution. Appl. Surf. Sci. 2016, 363, 328–337. [Google Scholar] [CrossRef]
- Waizy, H.; Diekmann, J.; Weizbauer, A.; Reifenrath, J.; Bartsch, I.; Neubert, V.; Schavan, R.; Windhagen, H. In vivo study of a biodegradable orthopedic screw (MgYREZralloy) in a rabbit model for up to 12 months. J. Biomater. Appl. 2014, 28, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Zhao, D.; Liu, N.; Wu, Y.; Yang, J.; Wang, Y.; Xie, H.; Ji, Y.; Zhou, C.; Zhuang, J.; et al. Assessment of the degradation rates and effectiveness of different coated Mg-Zn-Ca alloy scaffolds for in vivo repair of critical-size bone defects. J. Mater. Sci. Mater. Med. 2018, 29, 138. [Google Scholar] [CrossRef]












| Duty Cycle (%) | Thickness (µm) | Surface Roughness (µm) | Porosity (%) | Current Density (Icorr A/cm2) | Hydrogen Volumes (mL/14 days) |
|---|---|---|---|---|---|
| 10 | 24.2 | 0.45 | 4.65 | 2.23 × 10−6 | 12 |
| 20 | 7.65 | 0.49 | 2.52 | 8.05 × 10−7 | 6 |
| 30 | 7.35 | 1.10 | 3.57 | 1.2 × 10−6 | 16 |
| Duty Cycle (%) | Elements (wt %) | |||||
|---|---|---|---|---|---|---|
| O | Mg | Al | Si | P | Ca | |
| 10 | 46.42 | 40.09 | 1.11 | 10.36 | 1.62 | 0.39 |
| 20 | 38.29 | 52.31 | 1.63 | 6.45 | 1.16 | 0.16 |
| 30 | 47.88 | 39.78 | 1.11 | 9.72 | 1.39 | 0.12 |
| Treatment Time (min) | Thickness (µm) | Surface Roughness (µm) | Porosity (%) | Corrosion Current Density (Icorr A/cm2) | Hydrogen Volumes (mL/14 days) |
|---|---|---|---|---|---|
| 20 | 7.50 | 1.21 | 3.88 | 3.06 × 10−6 | 10 |
| 30 | 7.65 | 0.49 | 2.52 | 8.05 × 10−7 | 6 |
| 40 | 10.8 | 0.68 | 3.65 | 1.13 × 10−6 | 7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jian, S.-Y.; Ho, M.-L.; Shih, B.-C.; Wang, Y.-J.; Weng, L.-W.; Wang, M.-W.; Tseng, C.-C. Evaluation of the Corrosion Resistance and Cytocompatibility of a Bioactive Micro-Arc Oxidation Coating on AZ31 Mg Alloy. Coatings 2019, 9, 396. https://doi.org/10.3390/coatings9060396
Jian S-Y, Ho M-L, Shih B-C, Wang Y-J, Weng L-W, Wang M-W, Tseng C-C. Evaluation of the Corrosion Resistance and Cytocompatibility of a Bioactive Micro-Arc Oxidation Coating on AZ31 Mg Alloy. Coatings. 2019; 9(6):396. https://doi.org/10.3390/coatings9060396
Chicago/Turabian StyleJian, Shun-Yi, Mei-Ling Ho, Bing-Ci Shih, Yue-Jun Wang, Li-Wen Weng, Min-Wen Wang, and Chun-Chieh Tseng. 2019. "Evaluation of the Corrosion Resistance and Cytocompatibility of a Bioactive Micro-Arc Oxidation Coating on AZ31 Mg Alloy" Coatings 9, no. 6: 396. https://doi.org/10.3390/coatings9060396