LDH Post-Treatment of Flash PEO Coatings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Specimens Preparation
2.3. Surface Treatment Based on PEO
2.4. Synthesis of Zn-Al-LDH Growth
2.5. Characterization
2.6. Electrochemical Behavior
3. Results and Discussion
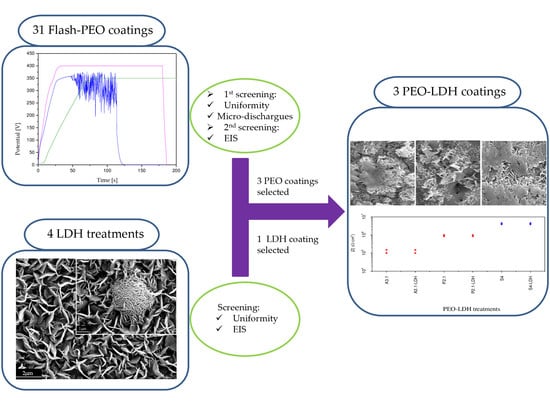
3.1. PEO Coating Screening
3.2. PEO Coatings Characterization
3.3. LDH Screening
3.4. PEO-LDH Coating Characterization
3.5. Corrosion Resistance of PEO + LDH Coatings
4. Conclusions
- Flash PEO coatings with ~1–2 µm thickness and ~2–5 KW·h·m−2·µm−1 energy consumption were genereated on a commercially pure aluminum alloy. Low energy consumption was ensured through relatively high electrolyte conductivity and a transition of the anodizing regime from constant current to constant voltage control.
- The first stage of the active protection system was successfully completed on flash PEO coatings via the development of an LDH layer. LDH coating is continuous and well defined when the PEO layer is thin (~1 μm), and the LDH formation is further facilitated when additional Al(OH)2+ cations are lixiviated from the coating.
- Corrosion resistance of inhibitor-free flash PEO/LDH coatings is mainly determined by the low porosity of the PEO layer. Formation of the LDH layer does not compromise the corrosion resistance of flash PEO coatings. Loading of the LDH scaffold with corrosion inhibitors is necessary in order to achieve an enhanced corrosion protection.
Author Contributions
Funding
Conflicts of Interest
References
- Matykina, E.; Arrabal, R.; Skeldon, P.; Thompson, G. Investigation of the growth processes of coatings formed by AC plasma electrolytic oxidation of aluminium. Electrochim. Acta 2009, 54, 6767–6778. [Google Scholar] [CrossRef]
- Arrabal, R.; Matykina, E.; Hashimoto, T.; Skeldon, P.; Thompson, G. Characterization of AC PEO coatings on magnesium alloys. Surf. Coat. Technol. 2009, 203, 2207–2220. [Google Scholar] [CrossRef]
- Matykina, E.; Skeldon, P.; Thompson, G.E. Fundamental and practical evaluations of PEO coatings of titanium. Int. Heat Surf. Eng. 2009, 3, 45–51. [Google Scholar] [CrossRef]
- Abrahami, S.T.; De Kok, J.M.M.; Mol, J.M.C.; Terryn, H. Towards Cr(VI)-free anodization of aluminum alloys for aerospace adhesive bonding applications: A review. Front. Chem. Sci. Eng. 2017, 11, 465–482. [Google Scholar] [CrossRef]
- Kulinich, S.; Akhtar, A.S. On conversion coating treatments to replace chromating for Al alloys: Recent developments and possible future directions. Russ. J. Non-Ferrous Met. 2012, 53, 176–203. [Google Scholar] [CrossRef]
- Gharbi, O.; Thomas, S.; Smith, C.; Birbilis, N. Chromate replacement: What does the future hold? npj Mater. Degrad. 2018, 2, 12. [Google Scholar] [CrossRef]
- Khan, R.; Yerokhin, A.; Li, X.; Dong, H.; Matthews, A. Surface characterisation of DC plasma electrolytic oxidation treated 6082 aluminium alloy: Effect of current density and electrolyte concentration. Surf. Coat. Technol. 2010, 205, 1679–1688. [Google Scholar] [CrossRef]
- Guan, Y.; Xia, Y.; Li, G. Growth mechanism and corrosion behavior of ceramic coatings on aluminum produced by autocontrol AC pulse PEO. Surf. Coat. Technol. 2008, 202, 4602–4612. [Google Scholar] [CrossRef]
- Han, I.; Choi, J.H.; Zhao, B.H.; Baik, H.K.; Lee, I.-S. Changes in anodized titanium surface morphology by virtue of different unipolar DC pulse waveform. Surf. Coat. Technol. 2007, 201, 5533–5536. [Google Scholar] [CrossRef]
- Yerokhin, A.; Shatrov, A.; Samsonov, V.; Shashkov, P.; Pilkington, A.; Leyland, A.; Matthews, A. Oxide ceramic coatings on aluminium alloys produced by a pulsed bipolar plasma electrolytic oxidation process. Surf. Coat. Technol. 2005, 199, 150–157. [Google Scholar] [CrossRef]
- Dunleavy, C.; Curran, J.; Clyne, T. Time dependent statistics of plasma discharge parameters during bulk AC plasma electrolytic oxidation of aluminium. Appl. Surf. Sci. 2013, 268, 397–409. [Google Scholar] [CrossRef]
- Matykina, E.; Arrabal, R.; Skeldon, P.; Thompson, G.; Belenguer, P. AC PEO of aluminium with porous alumina precursor films. Surf. Coat. Technol. 2010, 205, 1668–1678. [Google Scholar] [CrossRef]
- Curran, J.; Clyne, T. Thermo-physical properties of plasma electrolytic oxide coatings on aluminium. Surf. Coat. Technol. 2005, 199, 168–176. [Google Scholar] [CrossRef]
- Barik, R.; Wharton, J.; Wood, R.; Stokes, K.; Jones, R.; Wharton, J. Corrosion, erosion and erosion–corrosion performance of plasma electrolytic oxidation (PEO) deposited Al2O3 coatings. Surf. Coat. Technol. 2005, 199, 158–167. [Google Scholar] [CrossRef]
- Srinivasan, P.B.; Liang, J.; Blawert, C.; Stormer, M.; Dietzel, W. Effect of current density on the microstructure and corrosion behaviour of plasma electrolytic oxidation treated AM50 magnesium alloy. Appl. Surf. Sci. 2009, 255, 4212–4218. [Google Scholar] [CrossRef] [Green Version]
- Aliasghari, S. Plasma Electrolytic Oxidation of Titanium. Ph.D. Thesis, The University of Manchester, Manchester, UK, September 2014. [Google Scholar]
- Mohedano, M.; Lu, X.; Matykina, E.; Blawert, C.; Arrabal, R.; Zheludkevich, M.L. Plasma electrolytic oxidation (PEO) of metals and alloys. In Encyclopedia of Interfacial Chemistry, 1st ed.; Wandelt, K., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 423–438. [Google Scholar]
- Matykina, E.; Arrabal, R.; Pardo, A.; Mohedano, M.; Mingo, B.; Rodríguez, I.; González, J. Energy-efficient PEO process of aluminium alloys. Mater. Lett. 2014, 127, 13–16. [Google Scholar] [CrossRef] [Green Version]
- Sinko, J. Challenges of chromate inhibitor pigments replacement in organic coatings. Prog. Org. Coat. 2001, 42, 267–282. [Google Scholar] [CrossRef]
- Snizhko, L.; Yerokhin, A.; Gurevina, N.; Patalakha, V.; Matthews, A.; Snizhko, L. Excessive oxygen evolution during plasma electrolytic oxidation of aluminium. Thin Solid Films 2007, 516, 460–464. [Google Scholar] [CrossRef]
- Snizhko, L.; Yerokhin, A.; Pilkington, A.; Gurevina, N.; Misnyankin, D.; Leyland, A.; Matthews, A.; Snizhko, L. Anodic processes in plasma electrolytic oxidation of aluminium in alkaline solutions. Electrochim. Acta 2004, 49, 2085–2095. [Google Scholar] [CrossRef]
- Matykina, E.; Arrabal, R.; Mohedano, M.; Mingo, B.; Gonzalez, J.; Pardo, A.; Merino, M. Recent advances in energy efficient PEO processing of aluminium alloys. Trans. Nonferrous Met. Soc. China 2017, 27, 1439–1454. [Google Scholar] [CrossRef]
- Yasakau, K.; Tedim, J.; Zheludkevich, M.; Ferreira, M.; Yasakau, K.; Zheludkevich, M. Smart self-healing coatings for corrosion protection of aluminium alloys. In Handbook of Smart Coatings for Materials Protection, 1st ed.; Woodhead Publishing: Cambridge, UK, 2014; pp. 224–274. [Google Scholar]
- Mardel, J.; Garcia, S.; Corrigan, P.; Markley, T.; Hughes, A.; Muster, T.; Lau, D.; Harvey, T.; Glenn, A.; White, P.; et al. The characterisation and performance of Ce(dbp)3-inhibited epoxy coatings. Prog. Org. Coat. 2011, 70, 91–101. [Google Scholar] [CrossRef]
- Osborne, J.H.; Blohowiak, K.Y.; Taylor, S.; Hunter, C.; Bierwagon, G.; Carlson, B.; Bernard, D.; Donley, M.S. Testing and evaluation of nonchromated coating systems for aerospace applications. Prog. Org. Coat. 2001, 41, 217–225. [Google Scholar] [CrossRef]
- Guo, L.; Wu, W.; Zhou, Y.; Zhang, F.; Zeng, R.; Zeng, J. Layered double hydroxide coatings on magnesium alloys: A review. J. Mater. Sci. Technol. 2018, 34, 1455–1466. [Google Scholar] [CrossRef]
- Williams, G.; Geary, S.; McMurray, H.; McMurray, H. Smart release corrosion inhibitor pigments based on organic ion-exchange resins. Corros. Sci. 2012, 57, 139–147. [Google Scholar] [CrossRef]
- Cavani, F.; Trifirò, F.; Vaccari, A. Hydrotalcite-type anionic clays: Preparation, properties and applications. Catal. Today 1991, 11, 173–301. [Google Scholar] [CrossRef]
- Guo, X.; Xu, S.; Zhao, L.; Lu, W.; Zhang, F.; Evans, D.G.; Duan, X. One-step hydrothermal crystallization of a layered double hydroxide/alumina bilayer film on aluminum and its corrosion resistance properties. Langmuir 2009, 25, 9894–9897. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhang, C.-L.; Song, L.; Zeng, R.-C.; Liu, Z.-G.; Cui, H.-Z. Corrosion of in-situ grown MgAl-LDH coating on aluminum alloy. Trans. Nonferrous Met. Soc. China 2015, 25, 3498–3504. [Google Scholar] [CrossRef]
- Xu, Z.P.; Lu, G.Q. Hydrothermal synthesis of layered double hydroxides (LDHs) from mixed MgO and Al2O3: LDH formation mechanism. Chem. Mater. 2005, 17, 1055–1062. [Google Scholar] [CrossRef]
- Hao, L.; Yan, T.; Zhang, Y.; Zhao, X.; Lei, X.; Xu, S.; Zhang, F. Fabrication and anticorrosion properties of composite films of silica/layered double hydroxide. Surf. Coat. Technol. 2017, 326, 200–206. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Ren, Y.; Wang, H.; Chen, F. Double-doped LDH films on aluminum alloys for active protection. Mater. Lett. 2017, 192, 33–35. [Google Scholar] [CrossRef]
- Staal, L.B.; Pushparaj, S.S.C.; Forano, C.; Prevot, V.; Ravnsbæk, D.B.; Bjerring, M.; Nielsen, U.G. Competitive reactions during synthesis of zinc aluminum layered double hydroxides by thermal hydrolysis of urea. J. Mater. Chem. A 2017, 5, 21795–21806. [Google Scholar] [CrossRef]
- Dou, B.; Wang, Y.; Zhang, T.; Liu, B.; Shao, Y.; Meng, G.; Wang, F. Growth behaviors of layered double hydroxide on microarc oxidation film and anti-corrosion performances of the composite film. J. Electrochem. Soc. 2016, 163, C917–C927. [Google Scholar] [CrossRef]
- Zhang, Y. Investigating the growth behavior of LDH layers on MAO-coated aluminum alloy: Influence of microstructure and surface element. Int. J. Electrochem. Sci. 2018, 13, 610–620. [Google Scholar] [CrossRef]
- Mohedano, M.; Serdechnova, M.; Starykevich, M.; Karpushenkov, S.; Bouali, A.; Ferreira, M.; Zheludkevich, M. Active protective PEO coatings on AA2024: Role of voltage on in-situ LDH growth. Mater. Des. 2017, 120, 36–46. [Google Scholar] [CrossRef]
- Serdechnova, M.; Mohedano, M.; Kuznetsov, B.; Mendis, C.L.; Starykevich, M.; Karpushenkov, S.; Tedim, J.; Ferreira, M.G.S.; Blawert, C.; Zheludkevich, M.L. PEO coatings with active protection based on in-situ formed LDH-nanocontainers. J. Electrochem. Soc. 2017, 164, C36–C45. [Google Scholar] [CrossRef]
- Chen, F.; Yu, P.; Zhang, Y. Healing effects of LDHs nanoplatelets on MAO ceramic layer of aluminum alloy. J. Alloy. Compd. 2017, 711, 342–348. [Google Scholar] [CrossRef]
- Tedim, J.; Kuznetsova, A.; Salak, A.; Montemor, F.; Snihirova, D.; Pilz, M.; Zheludkevich, M.; Ferreira, M.; Salak, A.; Zheludkevich, M. Zn–Al layered double hydroxides as chloride nanotraps in active protective coatings. Corros. Sci. 2012, 55, 1–4. [Google Scholar] [CrossRef]
- Liu, Y.; Yin, X.; Zhang, J.; Yu, S.; Han, Z.; Ren, L. A electro-deposition process for fabrication of biomimetic super-hydrophobic surface and its corrosion resistance on magnesium alloy. Electrochim. Acta 2014, 125, 395–403. [Google Scholar] [CrossRef]
- Tsunekawa, S.; Aoki, Y.; Habazaki, H. Two-step plasma electrolytic oxidation of Ti–15V–3Al–3Cr–3Sn for wear-resistant and adhesive coating. Surf. Coat. Technol. 2011, 205, 4732–4740. [Google Scholar] [CrossRef]
- Baron-Wiechec, A.; Burke, M.; Hashimoto, T.; Liu, H.; Skeldon, P.; Thompson, G.; Habazaki, H.; Ganem, J.-J.; Vickridge, I. Tracer study of pore initiation in anodic alumina formed in phosphoric acid. Electrochim. Acta 2013, 113, 302–312. [Google Scholar] [CrossRef]
- Cheng, Y.; Cao, J.; Mao, M.; Xie, H.; Skeldon, P. Key factors determining the development of two morphologies of plasma electrolytic coatings on an Al–Cu–Li alloy in aluminate electrolytes. Surf. Coat. Technol. 2016, 291, 239–249. [Google Scholar] [CrossRef]
- Sykes, J.; Thompson, G.E.; Mayo, D.; Skeldon, P. Anodic film formation on high strength aluminium alloy FVS0812. J. Mater. Sci. 1997, 32, 4909–4916. [Google Scholar] [CrossRef]
- Fratila-Apachitei, L.; Tichelaar, F.; Thompson, G.; Terryn, H.; Skeldon, P.; Duszczyk, J.; Katgerman, L. A transmission electron microscopy study of hard anodic oxide layers on AlSi(Cu) alloys. Electrochim. Acta 2004, 49, 3169–3177. [Google Scholar] [CrossRef]
- Guo-Hua, L.; Wei-Chao, G.; Huan, C.; Li, L.; Er-Wu, N.; Si-Ze, Y. Microstructure and corrosion performance of oxide coatings on aluminium by plasma electrolytic oxidation in silicate and phosphate electrolytes. Chin. Phys. Lett. 2006, 23, 3331–3333. [Google Scholar] [CrossRef]
- Cao, Y.; Zheng, D.; Li, X.; Lin, J.; Wang, C.; Dong, S.; Lin, C. Enhanced corrosion resistance of superhydrophobic layered double hydroxide (LDH) films with long-term stability on Al substrate. ACS Appl. Mater. Interfaces 2018, 10, 15150–15162. [Google Scholar] [CrossRef]
- Zhang, M.; Ma, L.; Sun, Y.; Liu, Y.; Wang, L.-L. Insights into the use of metal-organic framework as high performance anti-corrosion coatings. ACS Appl. Mater. Interfaces 2018, 10, 2259–2263. [Google Scholar] [CrossRef]
- Ay, A.N.; Mafra, L.; Zümreoglu-Karan, B.; Zümreoglu-Karan, B.; Zümreoglu-Karan, B. A simple mechanochemical route to layered double hydroxides: Synthesis of hydrotalcite-like Mg-Al-NO3-LDH by manual grinding in a mortar. Zeitschrift für anorganische und allgemeine Chemie 2009, 635, 1470–1475. [Google Scholar] [CrossRef]
- Sertsova, A.; Subcheva, E.N.; Yurtov, E. Synthesis and study of structure formation of layered double hydroxides based on Mg, Zn, Cu, and Al. Russ. J. Inorg. Chem. 2015, 60, 23–32. [Google Scholar] [CrossRef]
- Tedim, J.; Zheludkevich, M.; Bastos, A.; Salak, A.; Lisenkov, A.; Ferreira, M.; Zheludkevich, M.; Bastos, A.; Lisenkov, A. Influence of preparation conditions of layered double hydroxide conversion films on corrosion protection. Electrochim. Acta 2014, 117, 164–171. [Google Scholar] [CrossRef]
- Evans, D.G.; Slade, R.C. Structural aspects of layered double hydroxides. In Layered Double Hydroxides; Duan, X., Evans, D.G., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 1–87. [Google Scholar]
- Kuznetsov, B.; Serdechnova, M.; Tedim, J.; Starykevich, M.; Kallip, S.; Oliveira, M.P.; Hack, T.; Nixon, S.; Ferreira, M.G.S.; Zheludkevich, M. Sealing of tartaric sulfuric (TSA) anodized AA2024 with nanostructured LDH layers. RSC Adv. 2016, 6, 13942–13952. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Li, S.; Zhang, Y.; Yu, M.; Liu, J. Enhanced protective Zn–Al layered double hydroxide film fabricated on anodized 2198 aluminum alloy. J. Alloy. Compd. 2015, 630, 29–36. [Google Scholar] [CrossRef]
- Galvão, T.L.; Neves, C.S.; Caetano, A.P.; Maia, F.; Mata, D.; Malheiro, E.; Ferreira, M.J.; Bastos, A.C.; Salak, A.N.; Gomes, J.R.; et al. Control of crystallite and particle size in the synthesis of layered double hydroxides: Macromolecular insights and a complementary modeling tool. J. Colloid Interface Sci. 2016, 468, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Cussler, E.L. Diffusion: Mass Transfer in Fluid Systems, 3rd ed.; Cambridge University Press: Cambridge, UK, 2009. [Google Scholar]
- Xiang, N.; Song, R.-G.; Zhuang, J.-J.; Song, R.-X.; Lu, X.-Y.; Su, X.-P. Effects of current density on microstructure and properties of plasma electrolytic oxidation ceramic coatings formed on 6063 aluminum alloy. Trans. Nonferrous Met. Soc. China 2016, 26, 806–813. [Google Scholar] [CrossRef]









| Coating | Electrolyte (g/L) | Coating | Electrolyte (g/L) | Coating | Electrolyte (g/L) | |||
|---|---|---|---|---|---|---|---|---|
| – | NaAlO2 | KOH | – | (Na3P3O6)3 | KOH | – | Na2SiO3 ** | KOH |
| A1.1 | 4 | 1.0 | P1.1 | 30 | 1 | S1.1 | 10.5 | 2.8 |
| A1.2 | 4 | 3.3 | P1.2 | 30 | 3 | S1.2 | 10.5 | 3.5 |
| A1.3 | 4 | 5.6 | P1.3 | 30 | 5 | S1.3 | 10.5 | 4.6 |
| A2.1 | 7 | 1.0 | P2.1 | 40 | 1 | S2.1 | 15.0 | 2.8 |
| A2.2 | 7 | 3.3 | P2.2 | 40 | 3 | S2.2 | 15.0 | 3.5 |
| A2.3 | 7 | 5.6 | P2.3 | 40 | 5 | S2.3 | 15.0 | 4.6 |
| A3.1 | 10 | 1.0 | P3.1 | 50 | 1 | S2.4 | 15.0 | 6.0 |
| A3.2 | 10 | 3.3 | P3.2 | 50 | 3 | S2.5 | 15.0 | 8.0 |
| A3.3 | 10 | 5.6 | P3.3 | 50 | 5 | S2.6 | 15.0 | 10.0 |
| – | – | – | P4 * | Na4P2O7: 20 | 2.8 | S4 * | Na2SiO3·5H2O: 25 | 2.8 |
| – | – | – | P5 * | Na3PO4·12H2O: 20 | 2.8 | S5 * | Na2SiO3·5H2O: 5 | 8.4 |
| LDH Treatment | Chemical Composition (M) | Exposure Time (min) |
|---|---|---|
| 1 | Zn(NO3)2·6H2O: 0.01 NH4NO3: 0.06 | 30 |
| 2 | Zn(NO3)2·6H2O: 0.01 NH4NO3: 0.06 | 60 |
| 3 | Zn(NO3)2·6H2O: 0.01 NaNO3: 0.06 | 30 |
| 4 | Zn(NO3)2·6H2O: 0.01 NaNO3: 0.06 | 60 |
| Coating | Electrolyte Composition (g/L) | σ (mS/cm) | pH | Ubd (V) | Thickness (µm) | Growth Rate (µm·min−1) | Energy Consumption (KW·h·m−2·µm−1) |
|---|---|---|---|---|---|---|---|
| A3.1 | NaAlO2: 10 KOH: 1 | 13.9 | 12.70 | 320 | 1.1 ± 0.3 | 0.37 | 4.98 |
| P2.1 | (Na3P3O6)3: 40 KOH: 1 | 11.1 | 12.5 | 257 | 2.4 ± 0.4 | 1.31 | 4.70 |
| S4 | Na2SiO3·5H2O: 25 KOH: 2.8 | 32.9 | 12.7 | 108 | 1.3 ± 0.2 | 0.39 | 2.20 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
del Olmo, R.; Mohedano, M.; Mingo, B.; Arrabal, R.; Matykina, E. LDH Post-Treatment of Flash PEO Coatings. Coatings 2019, 9, 354. https://doi.org/10.3390/coatings9060354
del Olmo R, Mohedano M, Mingo B, Arrabal R, Matykina E. LDH Post-Treatment of Flash PEO Coatings. Coatings. 2019; 9(6):354. https://doi.org/10.3390/coatings9060354
Chicago/Turabian Styledel Olmo, Rubén, Marta Mohedano, Beatriz Mingo, Raúl Arrabal, and Endzhe Matykina. 2019. "LDH Post-Treatment of Flash PEO Coatings" Coatings 9, no. 6: 354. https://doi.org/10.3390/coatings9060354