Chitosan Coating Applications in Probiotic Microencapsulation
Abstract
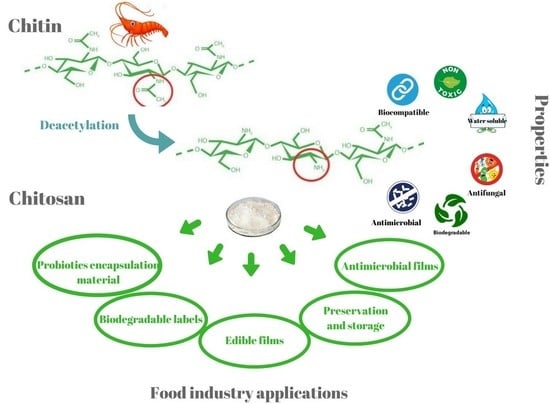
:1. Introduction
2. Coatings for Probiotic Microencapsulation
3. Chitosan-Based Coating Microencapsulation
3.1. Effectiveness of Improving Cell Survival
3.2. Microcapsules Size and Protection Performance
3.3. Chitosan Application According to the Technology and Bio-Based Matrices Used for the Microencapsulation
3.4. Food Applications of Probiotic Microencapsulated in Chitosan-Based Coatings
3.4.1. Dairy Products
3.4.2. Beverages
3.4.3. Other Food Products
4. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vodnar, D.C.; Călinoiu, L.F.; Dulf, F.V.; Ştefănescu, B.E.; Crişan, G.; Socaciu, C. Identification of the bioactive compounds and antioxidant, antimutagenic and antimicrobial activities of thermally processed agro-industrial waste. Food Chem. 2017, 231, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Calinoiu, L.-F.; Mitrea, L.; Precup, G.; Bindea, M.; Rusu, B.; Dulf, F.-V.; Stefanescu, B.-E.; Vodnar, D.-C. Characterization of Grape and Apple Peel Wastes’ Bioactive Compounds and Their Increased Bioavailability After Exposure to Thermal Process. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca-Food Sci. Technol. 2017, 74, 80–89. [Google Scholar] [CrossRef]
- Szabo, K.; Cătoi, A.-F.; Vodnar, D.C. Bioactive Compounds Extracted from Tomato Processing by-Products as a Source of Valuable Nutrients. Plant Foods Hum. Nutr. 2018. [Google Scholar] [CrossRef] [PubMed]
- Călinoiu, L.F.; Vodnar, D.C. Whole Grains and Phenolic Acids: A Review on Bioactivity, Functionality, Health Benefits and Bioavailability. Nutrients 2018, 10, 1615. [Google Scholar] [CrossRef] [PubMed]
- Călinoiu, L.F.; Mitrea, L.; Precup, G.; Bindea, M.; Rusu, B.; Szabo, K.; Dulf, F.V.; Ştefănescu, B.E.; Vodnar, D.C. Sustainable use of agro-industrial wastes for feeding 10 billion people by 2050. In Professionals in Food Chains; Wageningen Academic Publishers: Wageningen, The Netherlands, 2018; pp. 482–486. ISBN 978-90-8686-321-1. [Google Scholar]
- Mitrea, L.; Trif, M.; Catoi, A.-F.; Vodnar, D.-C. Utilization of biodiesel derived-glycerol for 1,3-PD and citric acid production. Microb. Cell Factories 2017, 16, 190. [Google Scholar] [CrossRef] [PubMed]
- Dulf, F.V.; Unguresan, M.L.; Vodnar, D.C.; Socaciu, C. Free and Esterified Sterol Distribution in Four Romanian Vegetable Oil. Not. Bot. Horti Agrobot. Cluj-Napoca 2010, 38, 91–97. [Google Scholar]
- Coldea, T.E.R.; Socaciu, C.; Parv, M.; Vodnar, D. Gas-Chromatographic Analysis of Major Volatile Compounds Found in Traditional Fruit Brandies from Transylvania, Romania. Not. Bot. Horti Agrobot. Cluj-Napoca 2011, 39, 109–116. [Google Scholar] [CrossRef] [Green Version]
- Andreicut, A.-D.; Parvu, A.E.; Mot, A.C.; Parvu, M.; Fodor, E.F.; Catoi, A.F.; Feldrihan, V.; Cecan, M.; Irimie, A. Phytochemical Analysis of Anti-Inflammatory and Antioxidant Effects of Mahonia aquifolium Flower and Fruit Extracts. Oxid. Med. Cell. Longev. 2018, 2879793. [Google Scholar] [CrossRef]
- Andreicu, A.-D.; Pârvu, A.E.; Pârvu, M.; Fischer-Fodor, E.; Feldrihan, V.; Florinela, A.; Cecan, M.; Irimie, A. Anti-Inflammatory and Antioxidant Effects of Mahonia Aquifolium Leaves and Bark Extracts. Farmacia 2018, 66, 49–58. [Google Scholar]
- Probiotics Market Research Report: Market size, Industry outlook, Market Forecast, Demand Analysis, Market Share, Market Report 2018–2023. Available online: https://industryarc.com/Report/7492/probiotics-market-analysis.html?gclid=EAIaIQobChMIm--8urnb4AIVyOF3Ch1E7AwNEAAYAiAAEgJlh_D_BwE (accessed on 27 February 2019).
- Food and Agriculture Organization of the United Nations; World Health Organization. Probiotics in Food: Health and Nutritional Properties and Guidelines for Evaluation; Food and Agriculture Organization of the United Nations, World Health Organization: Rome, Italy, 2006; ISBN 978-92-5-105513-7. [Google Scholar]
- Solanki, H.K.; Pawar, D.D.; Shah, D.A.; Prajapati, V.D.; Jani, G.K.; Mulla, A.M.; Thakar, P.M. Development of microencapsulation delivery system for long-term preservation of probiotics as biotherapeutics agent. BioMed Res. Int. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Dunne, C. Adaptation of bacteria to the intestinal niche: probiotics and gut disorder. Inflamm. Bowel Dis. 2001, 7, 136–145. [Google Scholar] [CrossRef]
- Ziemer, C.J.; Gibson, G.R. An overview of probiotics, prebiotics and synbiotics in the functional food concept: Perspectives and future strategies. Proc. Int. Dairy J. 1998, 8, 473–479. [Google Scholar] [CrossRef]
- Vodnar, D.C.; Venus, J.; Schneider, R.; Socaciu, C. Lactic Acid Production by Lactobacillus paracasei 168 in Discontinuous Fermentation Using Lucerne Green juice as Nutrient Substitute. Chem. Eng. Technol. 2010, 33, 468–474. [Google Scholar] [CrossRef]
- Pop, O.L.; Brandau, T.; Schwinn, J.; Vodnar, D.C.; Socaciu, C. The influence of different polymers on viability of Bifidobacterium lactis 300b during encapsulation, freeze-drying and storage. J. Food Sci. Technol.-Mysore 2015, 52, 4146–4155. [Google Scholar] [CrossRef]
- Rotar, A.M.; Vodnar, D.C.; Bunghez, F.; Catunescu, G.M.; Pop, C.R.; Jimborean, M.; Semeniuc, C.A. Effect of Goji Berries and Honey on Lactic Acid Bacteria Viability and Shelf Life Stability of Yoghurt. Not. Bot. Horti Agrobot. Cluj-Napoca 2015, 43, 196–203. [Google Scholar] [CrossRef]
- Rokka, S.; Rantamäki, P. Protecting probiotic bacteria by microencapsulation: Challenges for industrial applications. Eur. Food Res. Technol. 2010, 231, 1–12. [Google Scholar] [CrossRef]
- Capozzi, V.; Arena, M.P.; Crisetti, E.; Spano, G.; Fiocco, D. The hsp 16 Gene of the Probiotic Lactobacillus acidophilus Is Differently Regulated by Salt, High Temperature and Acidic Stresses, as Revealed by Reverse Transcription Quantitative PCR (qRT-PCR) Analysis. Int. J. Mol. Sci. 2011, 12, 5390–5405. [Google Scholar] [CrossRef]
- Taranu, I.; Marin, D.E.; Braicu, C.; Pistol, G.C.; Sorescu, I.; Pruteanu, L.L.; Berindan Neagoe, I.; Vodnar, D.C. In Vitro Transcriptome Response to a Mixture of Lactobacilli Strains in Intestinal Porcine Epithelial Cell Line. Int. J. Mol. Sci. 2018, 19, 1923. [Google Scholar] [CrossRef] [PubMed]
- Calinoiu, L.-F.; Vodnar, D.-C.; Precup, G. The Probiotic Bacteria Viability under Different Conditions. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca-Food Sci. Technol. 2016, 73, 55–60. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; Coppola, R.; Sada, A.; Orlando, P. Fermentative ability of alginate-prebiotic encapsulated Lactobacillus acidophilus and survival under simulated gastrointestinal conditions. J. Funct. Foods 2009, 1, 319–323. [Google Scholar] [CrossRef]
- Pop, O.L.; Dulf, F.V.; Cuibus, L.; Castro-Giráldez, M.; Fito, P.J.; Vodnar, D.C.; Coman, C.; Socaciu, C.; Suharoschi, R. Characterization of a Sea Buckthorn Extract and Its Effect on Free and Encapsulated Lactobacillus casei. Int. J. Mol. Sci. 2017, 18, 2513. [Google Scholar] [CrossRef] [PubMed]
- Anal, A.K.; Singh, H. Recent advances in microencapsulation of probiotics for industrial applications and targeted delivery. Trends Food Sci. Technol. 2007, 18, 240–251. [Google Scholar] [CrossRef]
- Dimitrellou, D.; Kandylis, P.; Petrović, T.; Dimitrijević-Branković, S.; Lević, S.; Nedović, V.; Kourkoutas, Y. Survival of spray dried microencapsulated Lactobacillus casei ATCC 393 in simulated gastrointestinal conditions and fermented milk. LWT Food Sci. Technol. 2016, 71, 169–174. [Google Scholar] [CrossRef]
- Arena, M.P.; Caggianiello, G.; Russo, P.; Albenzio, M.; Massa, S.; Fiocco, D.; Capozzi, V.; Spano, G. Functional Starters for Functional Yogurt. Foods 2015, 4, 15–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.Y.; Chen, X.G.; Cha, D.S.; Park, H.J.; Liu, C.S. Microencapsulation of a probiotic bacteria with alginategelatin and its properties. J. Microencapsul. 2009, 26, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Cho, S.Y.; Kim, S.H.; Song, O.J.; Shin, I.S.; Cha, D.S.; Park, H.J. Effect of microencapsulation on viability and other characteristics in Lactobacillus acidophilus ATCC 43121. LWT Food Sci. Technol. 2008, 41, 493–500. [Google Scholar] [CrossRef]
- Desai, K.G.H.; Jin Park, H. Recent Developments in Microencapsulation of Food Ingredients. Dry. Technol. 2005, 23, 1361–1394. [Google Scholar] [CrossRef]
- Pavli, F.; Tassou, C.; Nychas, G.-J.E.; Chorianopoulos, N. Probiotic Incorporation in Edible Films and Coatings: Bioactive Solution for Functional Foods. Int. J. Mol. Sci. 2018, 19, 150. [Google Scholar] [CrossRef]
- Shori, A.B. Microencapsulation Improved Probiotics Survival During Gastric Transit. HAYATI J. Biosci. 2017, 24, 1–5. [Google Scholar] [CrossRef]
- Martín, M.J.; Lara-Villoslada, F.; Ruiz, M.A.; Morales, M.E. Microencapsulation of bacteria: A review of different technologies and their impact on the probiotic effects. Innov. Food Sci. Emerg. Technol. 2015, 27, 15–25. [Google Scholar] [CrossRef]
- Riaz, Q.U.A.; Masud, T. Recent Trends and Applications of Encapsulating Materials for Probiotic Stability. Crit. Rev. Food Sci. Nutr. 2013, 53, 231–244. [Google Scholar] [CrossRef] [PubMed]
- Heidebach, T.; Först, P.; Kulozik, U. Microencapsulation of Probiotic Cells for Food Applications. Crit. Rev. Food Sci. Nutr. 2012, 52, 291–311. [Google Scholar] [CrossRef]
- Cook, M.T.; Tzortzis, G.; Charalampopoulos, D.; Khutoryanskiy, V.V. Microencapsulation of probiotics for gastrointestinal delivery. J. Controlled Release 2012, 162, 56–67. [Google Scholar] [CrossRef] [Green Version]
- Morales, M.E.; Ruiz, M.A. 16—Microencapsulation of probiotic cells: applications in nutraceutic and food industry. In Nutraceuticals; Grumezescu, A.M., Ed.; Nanotechnology in the Agri-Food Industry; Academic Press: Cambridge, MA, USA, 2016; pp. 627–668. ISBN 978-0-12-804305-9. [Google Scholar]
- Ravi Kumar, M.N.V. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- De Prisco, A.; Mauriello, G. Probiotication of foods: A focus on microencapsulation tool. Trends Food Sci. Technol. 2016, 48, 27–39. [Google Scholar] [CrossRef]
- Nasui, L.; Vodnar, D.; Socaciu, C. Bioactive Labels for Fresh Fruits and Vegetables. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca Food Sci. Technol. 2013, 70, 75–83. [Google Scholar] [CrossRef]
- Dong, H.; Cheng, L.; Tan, J.; Zheng, K.; Jiang, Y. Effects of chitosan coating on quality and shelf life of peeled litchi fruit. J. Food Eng. 2004, 64, 355–358. [Google Scholar] [CrossRef]
- Ye, M.; Neetoo, H.; Chen, H. Control of Listeria monocytogenes on ham steaks by antimicrobials incorporated into chitosan-coated plastic films. Food Microbiol. 2008, 25, 260–268. [Google Scholar] [CrossRef]
- Vodnar, D.C. Inhibition of Listeria monocytogenes ATCC 19115 on ham steak by tea bioactive compounds incorporated into chitosan-coated plastic films. Chem. Cent. J. 2012, 6, 74. [Google Scholar] [CrossRef] [PubMed]
- Burgain, J.; Gaiani, C.; Linder, M.; Scher, J. Encapsulation of probiotic living cells: From laboratory scale to industrial applications. J. Food Eng. 2011, 104, 467–483. [Google Scholar] [CrossRef]
- Krasaekoopt, W.; Bhandari, B.; Deeth, H.C. Survival of probiotics encapsulated in chitosan-coated alginate beads in yoghurt from UHT- and conventionally treated milk during storage. LWT Food Sci. Technol. 2006, 39, 177–183. [Google Scholar] [CrossRef]
- Anselmo, A.C.; McHugh, K.J.; Webster, J.; Langer, R.; Jaklenec, A. Layer-by-Layer Encapsulation of Probiotics for Delivery to the Microbiome. Adv. Mater. 2016, 28, 9486–9490. [Google Scholar] [CrossRef] [Green Version]
- Arslan-Tontul, S.; Erbas, M. Single and double layered microencapsulation of probiotics by spray drying and spray chilling. LWT Food Sci. Technol. 2017, 81, 160–169. [Google Scholar] [CrossRef]
- Ashwar, B.A.; Gani, A.; Gani, A.; Shah, A.; Masoodi, F.A. Production of RS4 from rice starch and its utilization as an encapsulating agent for targeted delivery of probiotics. Food Chem. 2018, 239, 287–294. [Google Scholar] [CrossRef]
- Huq, T.; Khan, A.; Khan, R.A.; Riedl, B.; Lacroix, M. Encapsulation of Probiotic Bacteria in Biopolymeric System. Crit. Rev. Food Sci. Nutr. 2013, 53, 909–916. [Google Scholar] [CrossRef]
- Schönhoff, M. Layered polyelectrolyte complexes: physics of formation and molecular properties. J. Phys. Condens. Matter 2003, 15, R1781–R1808. [Google Scholar] [CrossRef]
- Valencia-Chamorro, S.A.; Palou, L.; del Río, M.A.; Pérez-Gago, M.B. Antimicrobial Edible Films and Coatings for Fresh and Minimally Processed Fruits and Vegetables: A Review. Crit. Rev. Food Sci. Nutr. 2011, 51, 872–900. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, M.A.; Bourbon, A.I.; Pinheiro, A.C.; Martins, J.T.; Souza, B.W.S.; Teixeira, J.A.; Vicente, A.A. Galactomannans use in the development of edible films/coatings for food applications. Trends Food Sci. Technol. 2011, 22, 662–671. [Google Scholar] [CrossRef] [Green Version]
- Šuput, D.Z.; Lazić, V.L.; Popović, S.Z.; Hromiš, N.M. Edible films and coatings: Sources, properties and application. Food Feed Res. 2015, 42, 11–22. [Google Scholar] [CrossRef] [Green Version]
- Ramos, P.E.; Cerqueira, M.A.; Teixeira, J.A.; Vicente, A.A. Physiological protection of probiotic microcapsules by coatings. Crit. Rev. Food Sci. Nutr. 2018, 58, 1864–1877. [Google Scholar] [CrossRef]
- Groboillot, A.F.; Champagne, C.P.; Darling, G.D.; Poncelet, D.; Neufeld, R.J. Membrane formation by interfacial cross-linking of chitosan for microencapsulation of Lactococcus lactis. Biotechnol. Bioeng. 1993, 42, 1157–1163. [Google Scholar] [CrossRef]
- Krasaekoopt, W.; Bhandari, B.; Deeth, H. Evaluation of encapsulation techniques of probiotics for yoghurt. Int. Dairy J. 2003, 13, 3–13. [Google Scholar] [CrossRef]
- Raafat, D.; Sahl, H.-G. Chitosan and its antimicrobial potential – a critical literature survey. Microb. Biotechnol. 2009, 2, 186–201. [Google Scholar] [CrossRef] [Green Version]
- de Araújo Etchepare, M.; Raddatz, G.C.; de Moraes Flores, É.M.; Zepka, L.Q.; Jacob-Lopes, E.; Barin, J.S.; Ferreira Grosso, C.R.; de Menezes, C.R. Effect of resistant starch and chitosan on survival of Lactobacillus acidophilus microencapsulated with sodium alginate. LWT Food Sci. Technol. 2016, 65, 511–517. [Google Scholar] [CrossRef] [Green Version]
- Shah, U.; Naqash, F.; Gani, A.; Masoodi, F.A. Art and Science behind Modified Starch Edible Films and Coatings: A Review. Compr. Rev. Food Sci. Food Saf. 2016, 15, 568–580. [Google Scholar] [CrossRef] [Green Version]
- Gharsallaoui, A.; Roudaut, G.; Chambin, O.; Voilley, A.; Saurel, R. Applications of spray-drying in microencapsulation of food ingredients: An overview. Food Res. Int. 2007, 40, 1107–1121. [Google Scholar] [CrossRef]
- Saravanan, M.; Rao, K.P. Pectin–gelatin and alginate–gelatin complex coacervation for controlled drug delivery: Influence of anionic polysaccharides and drugs being encapsulated on physicochemical properties of microcapsules. Carbohydr. Polym. 2010, 80, 808–816. [Google Scholar] [CrossRef]
- Weinbreck, F.; Bodnár, I.; Marco, M.L. Can encapsulation lengthen the shelf-life of probiotic bacteria in dry products? Int. J. Food Microbiol. 2010, 136, 364–367. [Google Scholar] [CrossRef]
- Doherty, S.B.; Gee, V.L.; Ross, R.P.; Stanton, C.; Fitzgerald, G.F.; Brodkorb, A. Development and characterisation of whey protein micro-beads as potential matrices for probiotic protection. Food Hydrocoll. 2011, 25, 1604–1617. [Google Scholar] [CrossRef]
- Gunasekaran, S.; Ko, S.; Xiao, L. Use of whey proteins for encapsulation and controlled delivery applications. J. Food Eng. 2007, 83, 31–40. [Google Scholar] [CrossRef]
- Iyer, C.; Kailasapathy, K. Effect of co-encapsulation of probiotics with prebiotics on increasing the viability of encapsulated bacteria under in vitro acidic and bile salt conditions and in yogurt. J. Food Sci. 2005. [Google Scholar]
- Zou, Q.; Zhao, J.; Liu, X.; Tian, F.; Zhang, H.; Zhang, H.; Chen, W. Microencapsulation of Bifidobacterium bifidum F-35 in reinforced alginate microspheres prepared by emulsification/internal gelation. Int. J. Food Sci. Technol. 2011, 46, 1672–1678. [Google Scholar] [CrossRef]
- Cui, J.-H.; Goh, J.-S.; Kim, P.-H.; Choi, S.-H.; Lee, B.-J. Survival and stability of bifidobacteria loaded in alginate poly-l-lysine microparticles. Int. J. Pharm. 2000, 210, 51–59. [Google Scholar] [CrossRef]
- Nualkaekul, S.; Cook, M.T.; Khutoryanskiy, V.V.; Charalampopoulos, D. Influence of encapsulation and coating materials on the survival of Lactobacillus plantarum and Bifidobacterium longum in fruit juices. Food Res. Int. 2013, 53, 304–311. [Google Scholar] [CrossRef]
- Woo, J.-W.; Roh, H.-J.; Park, H.-D.; Ji, C.-I.; Lee, Y.-B.; Kim, S.-B. Sphericity Optimization of Calcium Alginate Gel Beads and the Effects of Processing Conditions on Their Physical Properties. Food Sci. Biotechnol. 2007, 16, 715–721. [Google Scholar]
- Stummer, S.; Salar-Behzadi, S.; Unger, F.M.; Oelzant, S.; Penning, M.; Viernstein, H. Application of shellac for the development of probiotic formulations. Food Res. Int. 2010, 43, 1312–1320. [Google Scholar] [CrossRef]
- Buch, K.; Penning, M.; Wächtersbach, E.; Maskos, M.; Langguth, P. Investigation of various shellac grades: additional analysis for identity. Drug Dev. Ind. Pharm. 2009, 35, 694–703. [Google Scholar] [CrossRef]
- Shi, L.-E.; Li, Z.-H.; Zhang, Z.-L.; Zhang, T.-T.; Yu, W.-M.; Zhou, M.-L.; Tang, Z.-X. Encapsulation of Lactobacillus bulgaricus in carrageenan-locust bean gum coated milk microspheres with double layer structure. LWT Food Sci. Technol. 2013, 54, 147–151. [Google Scholar] [CrossRef]
- Krasaekoopt, W.; Bhandari, B.; Deeth, H. The influence of coating materials on some properties of alginate beads and survivability of microencapsulated probiotic bacteria. Int. Dairy J. 2004, 14, 737–743. [Google Scholar] [CrossRef]
- Vodnar, D.C.; Socaciu, C.; Rotar, A.M.; Stãnilã, A. Morphology, FTIR fingerprint and survivability of encapsulated lactic bacteria (Streptococcus thermophilus and Lactobacillus delbrueckii subsp. bulgaricus) in simulated gastric juice and intestinal juice. Int. J. Food Sci. Technol. 2010, 45, 2345–2351. [Google Scholar] [CrossRef]
- Yuan, Y.; Chesnutt, B.M.; Haggard, W.O.; Bumgardner, J.D. Deacetylation of Chitosan: Material Characterization and in vitro Evaluation via Albumin Adsorption and Pre-Osteoblastic Cell Cultures. Materials 2011, 4, 1399–1416. [Google Scholar] [CrossRef] [Green Version]
- de Vos, P.; Faas, M.M.; Spasojevic, M.; Sikkema, J. Encapsulation for preservation of functionality and targeted delivery of bioactive food components. Int. Dairy J. 2010, 20, 292–302. [Google Scholar] [CrossRef]
- Aider, M. LWT Food Science and Technology Chitosan application for active bio-based films production and potential in the food industry: Review. LWT Food Sci. Technol. 2010, 43, 837–842. [Google Scholar] [CrossRef]
- Estevinho, B.N.; Rocha, F.; Santos, L.; Alves, A. Microencapsulation with chitosan by spray drying for industry applications—A review. Trends Food Sci. Technol. 2013, 31, 138–155. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. Oxf. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- George, A.; Roberts, F. Structure of chitin and chitosan. In Chitin Chemistry; Palgrave: London, UK, 1992; pp. 85–91. [Google Scholar]
- Mortazavian, A.; Razavi, S.H.; Ehsani, M.R.; Sohrabvandi, S. Principles and Methods of Microencapsulation of Probiotic Microorganisms. Iran. J. Biotechnol. 2007, 5, 1–18. [Google Scholar]
- Chávarri, M.; Marañón, I.; Ares, R.; Ibáñez, F.C.; Marzo, F.; del Carmen Villarán, M. Microencapsulation of a probiotic and prebiotic in alginate-chitosan capsules improves survival in simulated gastro-intestinal conditions. Int. J. Food Microbiol. 2010, 142, 185–189. [Google Scholar]
- Yeung, T.W.; Üçok, E.F.; Tiani, K.A.; McClements, D.J.; Sela, D.A. Microencapsulation in alginate and chitosan microgels to enhance viability of Bifidobacterium longum for oral delivery. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef]
- Vodnar, D.C.; Socaciu, C. Green tea increases the survival yield of Bifidobacteria in simulated gastrointestinal environment and during refrigerated conditions. Chem. Cent. J. 2012, 6, 61. [Google Scholar] [CrossRef]
- Koo, S.M.; Cho, Y.H.; Huh, C.S.; Baek, Y.J.; Park, J. Improvement of the stability of Lactobacillus casei YIT 9018 by microencapsulation using alginate and chitosan. J. Microbiol. Biotechnol. 2001, 11, 376–383. [Google Scholar]
- Vodnar, D.C.; Socaciu, C. Selenium enriched green tea increase stability of Lactobacillus casei and Lactobacillus plantarum in chitosan coated alginate microcapsules during exposure to simulated gastrointestinal and refrigerated conditions. LWT Food Sci. Technol. 2014, 57, 406–411. [Google Scholar] [CrossRef]
- Bepeyeva, A.; de Barros, J.M.S.; Albadran, H.; Kakimov, A.K.; Kakimova, Z.K.; Charalampopoulos, D.; Khutoryanskiy, V.V. Encapsulation of Lactobacillus casei into Calcium Pectinate-Chitosan Beads for Enteric Delivery. J. Food Sci. 2017, 82, 2954–2959. [Google Scholar] [CrossRef]
- Anekella, K.; Orsat, V. Optimization of microencapsulation of probiotics in raspberry juice by spray drying. LWT Food Sci. Technol. 2013, 50, 17–24. [Google Scholar] [CrossRef]
- Cook, M.T.; Tzortzis, G.; Charalampopoulos, D.; Khutoryanskiy, V.V. Microencapsulation of a synbiotic into PLGA/alginate multiparticulate gels. Int. J. Pharm. 2014, 466, 400–408. [Google Scholar] [CrossRef] [Green Version]
- Varankovich, N.; Martinez, M.F.; Nickerson, M.T.; Korber, D.R. Survival of probiotics in pea protein-alginate microcapsules with or without chitosan coating during storage and in a simulated gastrointestinal environment. Food Sci. Biotechnol. 2017, 26, 189–194. [Google Scholar] [CrossRef]
- Molan, A.L.; Flanagan, J.; Wei, W.; Moughan, P.J. Selenium-containing green tea has higher antioxidant and prebiotic activities than regular green tea. Food Chem. 2009, 114, 829–835. [Google Scholar] [CrossRef]
- Jantarathin, S.; Borompichaichartkul, C.; Sanguandeekul, R. Microencapsulation of probiotic and prebiotic in alginate-chitosan capsules and its effect on viability under heat process in shrimp feeding. Mater. Today Proc. 2017, 4, 6166–6172. [Google Scholar] [CrossRef]
- Darjani, P.; Hosseini Nezhad, M.; Kadkhodaee, R.; Milani, E. Influence of prebiotic and coating materials on morphology and survival of a probiotic strain of Lactobacillus casei exposed to simulated gastrointestinal conditions. LWT 2016, 73, 162–167. [Google Scholar] [CrossRef]
- Liserre, A.M.; Ré, M.I.; Franco, B.D.G.M. Microencapsulation of Bifidobacterium animalis subsp. lactis in Modified Alginate-chitosan Beads and Evaluation of Survival in Simulated Gastrointestinal Conditions. Food Biotechnol. 2007, 21, 1–16. [Google Scholar] [CrossRef]
- Khosravi Zanjani, M.A.; Tarzi, B.G.; Sharifan, A.; Mohammadi, N. Microencapsulation of probiotics by calcium alginate-gelatinized starch with chitosan coating and evaluation of survival in simulated human gastro-intestinal condition. Iran. J. Pharm. Res. 2014, 13, 843–852. [Google Scholar]
- Corona-Hernandez, R.I.; Álvarez-Parrilla, E.; Lizardi-Mendoza, J.; Islas-Rubio, A.R.; de la Rosa, L.A.; Wall-Medrano, A. Structural Stability and Viability of Microencapsulated Probiotic Bacteria: A Review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 614–628. [Google Scholar] [CrossRef] [Green Version]
- Borges, J.; Mano, J.F. Molecular Interactions Driving the Layer-by-Layer Assembly of Multilayers. Chem. Rev. 2014, 114, 8883–8942. [Google Scholar] [CrossRef]
- Cook, M.T.; Tzortzis, G.; Charalampopoulos, D.; Khutoryanskiy, V.V. Production and evaluation of dry alginate-chitosan microcapsules as an enteric delivery vehicle for probiotic bacteria. Biomacromolecules 2011, 12, 2834–2840. [Google Scholar] [CrossRef]
- Pinto, G.L.D.; Campana-Filho, S.P.; de Almeida, L.A. Frontiers in Biomaterials: Chitosan Based Materials and its Applications; Bentham Science Publishers: Sharjah, UAE, 2017; ISBN 978-1-68108-485-5. [Google Scholar]
- Singh, P.; Medronho, B.; Alves, L.; da Silva, G.J.; Miguel, M.G.; Lindman, B. Development of carboxymethyl cellulose-chitosan hybrid micro- and macroparticles for encapsulation of probiotic bacteria. Carbohydr. Polym. 2017, 175, 87–95. [Google Scholar] [CrossRef]
- Li, X.Y.; Chen, X.G.; Sun, Z.W.; Park, H.J.; Cha, D.-S. Preparation of alginate/chitosan/carboxymethyl chitosan complex microcapsules and application in Lactobacillus casei ATCC 393. Carbohydr. Polym. 2011, 83, 1479–1485. [Google Scholar] [CrossRef]
- Cook, M.T.; Tzortzis, G.; Khutoryanskiy, V.V.; Charalampopoulos, D. Layer-by-layer coating of alginate matrices with chitosan–alginate for the improved survival and targeted delivery of probiotic bacteria after oral administration. J. Mater. Chem. B 2013, 1, 52–60. [Google Scholar] [CrossRef] [Green Version]
- Kamalian, N.; Mirhosseini, H.; Mustafa, S.; Manap, M.Y.A. Effect of alginate and chitosan on viability and release behavior of Bifidobacterium pseudocatenulatum G4 in simulated gastrointestinal fluid. Carbohydr. Polym. 2014, 111, 700–706. [Google Scholar] [CrossRef]
- Zhang, F.; Li, X.Y.; Park, H.J.; Zhao, M. Effect of microencapsulation methods on the survival of freeze-dried Bifidobacterium bifidum. J. Microencapsul. 2013, 30, 511–518. [Google Scholar] [CrossRef]
- Fareez, I.M.; Lim, S.M.; Lim, F.T.; Mishra, R.K.; Ramasamy, K. Microencapsulation of Lactobacillus SP. Using Chitosan-Alginate-Xanthan Gum-β-Cyclodextrin and Characterization of its Cholesterol Reducing Potential and Resistance Against pH, Temperature and Storage. J. Food Process Eng. 2017, 40, e12458. [Google Scholar] [CrossRef]
- Fareez, I.M.; Lim, S.M.; Mishra, R.K.; Ramasamy, K. Chitosan coated alginate–xanthan gum bead enhanced pH and thermotolerance of Lactobacillus plantarum LAB12. Int. J. Biol. Macromol. 2015, 72, 1419–1428. [Google Scholar] [CrossRef]
- Yucel Falco, C.; Sotres, J.; Rascón, A.; Risbo, J.; Cárdenas, M. Design of a potentially prebiotic and responsive encapsulation material for probiotic bacteria based on chitosan and sulfated β-glucan. J. Colloid Interface Sci. 2017, 487, 97–106. [Google Scholar] [CrossRef]
- Kanmani, P.; Satish Kumar, R.; Yuvaraj, N.; Paari, K.A.; Pattukumar, V.; Arul, V. Effect of cryopreservation and microencapsulation of lactic acid bacterium Enterococcus faecium MC13 for long-term storage. Biochem. Eng. J. 2011, 58–59, 140–147. [Google Scholar] [CrossRef]
- Trabelsi, I.; Bejar, W.; Ayadi, D.; Chouayekh, H.; Kammoun, R.; Bejar, S.; Ben Salah, R. Encapsulation in alginate and alginate coated-chitosan improved the survival of newly probiotic in oxgall and gastric juice. Int. J. Biol. Macromol. 2013, 61, 36–42. [Google Scholar] [CrossRef]
- Zaeim, D.; Sarabi-Jamab, M.; Ghorani, B.; Kadkhodaee, R.; Tromp, R.H. Electrospray assisted fabrication of hydrogel microcapsules by single- and double-stage procedures for encapsulation of probiotics. Food Bioprod. Process. 2017, 102, 250–259. [Google Scholar] [CrossRef]
- Krasaekoopt, W.; Watcharapoka, S. Effect of addition of inulin and galactooligosaccharide on the survival of microencapsulated probiotics in alginate beads coated with chitosan in simulated digestive system, yogurt and fruit juice. LWT Food Sci. Technol. 2014, 57, 761–766. [Google Scholar] [CrossRef]
- Prisco, A.D.; Maresca, D.; Ongeng, D.; Mauriello, G. Microencapsulation by vibrating technology of the probiotic strain Lactobacillus reuteri DSM 17938 to enhance its survival in foods and in gastrointestinal environment. LWT Food Sci. Technol. 2015, 2, 452–462. [Google Scholar] [CrossRef]
- Ramos, V.; Albertengo, L.; Agullo, E. Present and Future Role of Chitin and Chitosan in Food. Macromol. Biosci. 2003, 3, 521–530. [Google Scholar]
- Gandomi, H.; Abbaszadeh, S.; Misaghi, A.; Bokaie, S.; Noori, N. Effect of chitosan-alginate encapsulation with inulin on survival of Lactobacillus rhamnosus GG during apple juice storage and under simulated gastrointestinal conditions. LWT Food Sci. Technol. 2016. [Google Scholar] [CrossRef]
- Malmo, C.; La Storia, A.; Mauriello, G. Microencapsulation of Lactobacillus reuteri DSM 17938 Cells Coated in Alginate Beads with Chitosan by Spray Drying to Use as a Probiotic Cell in a Chocolate Soufflé. Food Bioprocess Technol. 2013, 6, 795–805. [Google Scholar] [CrossRef]
- Kailasapathy, K. Survival of free and encapsulated probiotic bacteria and their effect on the sensory properties of yoghurt. LWT Food Sci. Technol. 2006, 39, 1221–1227. [Google Scholar] [CrossRef]
- Champagne, C.P.; Fustier, P. Microencapsulation for the improved delivery of bioactive compounds into foods. Curr. Opin. Biotechnol. 2007, 18, 184–190. [Google Scholar] [CrossRef]
- Le-Tien, C.; Millette, M.; Mateescu, M.-A.; Lacroix, M. Modified alginate and chitosan for lactic acid bacteria immobilization. Biotechnol. Appl. Biochem. 2004, 39, 347–354. [Google Scholar] [CrossRef]
- Lee, J.S.; Cha, D.S.; Park, H.J. Survival of freeze-dried Lactobacillus bulgaricus KFRI 673 in chitosan-coated calcium alginate microparticles. J. Agric. Food Chem. 2004, 52, 7300–7305. [Google Scholar] [CrossRef]
- Yu, W.-K.; Yim, T.-B.; Lee, K.-Y.; Heo, T.-R. Effect of skim milk-alginate beads on survival rate of bifidobacteria. Biotechnol. Bioprocess Eng. 2001, 6, 133–138. [Google Scholar] [CrossRef]
- Urbanska, A.M.; Bhathena, J.; Prakash, S. Live encapsulated Lactobacillus acidophilus cells in yogurt for therapeutic oral delivery: preparation and in vitro analysis of alginate–chitosan microcapsules. Can. J. Physiol. Pharmacol. 2007, 85, 884–893. [Google Scholar]
- Brinques, G.B.; Ayub, M.A.Z. Effect of microencapsulation on survival of Lactobacillus plantarum in simulated gastrointestinal conditions, refrigeration, and yogurt. J. Food Eng. 2011, 103, 123–128. [Google Scholar] [CrossRef]
- Obradović, N.S.; Krunić, T.Ž.; Trifković, K.T.; Bulatović, M.L.; Rakin, M.P.; Rakin, M.B.; Bugarski, B.M. Influence of Chitosan Coating on Mechanical Stability of Biopolymer Carriers with Probiotic Starter Culture in Fermented Whey Beverages. Available online: https://www.hindawi.com/journals/ijps/2015/732858/abs/ (accessed on 28 February 2019).
- García-Ceja, A.; Mani-López, E.; Palou, E.; López-Malo, A. Viability during refrigerated storage in selected food products and during simulated gastrointestinal conditions of individual and combined lactobacilli encapsulated in alginate or alginate-chitosan. LWT Food Sci. Technol. 2015, 63, 482–489. [Google Scholar] [CrossRef]
- Chen, L.; Yang, T.; Song, Y.; Shu, G.; Chen, H. Effect of xanthan-chitosan-xanthan double layer encapsulation on survival of Bifidobacterium BB01 in simulated gastrointestinal conditions, bile salt solution and yogurt. LWT Food Sci. Technol. 2017, 81, 274–280. [Google Scholar] [CrossRef]
- Nualkaekul, S.; Lenton, D.; Cook, M.T.; Khutoryanskiy, V.V.; Charalampopoulos, D. Chitosan coated alginate beads for the survival of microencapsulated Lactobacillus plantarum in pomegranate juice. Carbohydr. Polym. 2012, 90, 1281–1287. [Google Scholar] [CrossRef]
- Talebzadeh, S.; Sharifan, A. Developing Probiotic Jelly Desserts with Lactobacillus Acidophilus. J. Food Process. Preserv. 2017, 41, e13026. [Google Scholar] [CrossRef]
- Seyedain-Ardabili, M.; Sharifan, A.; Ghiassi Tarzi, B. The Production of Synbiotic Bread by Microencapsulation. Food Technol. Biotechnol. 2016, 54, 52–59. [Google Scholar] [CrossRef]
- de Farias, T.G.S.; Ladislau, H.F.L.; Stamford, T.C.M.; Medeiros, J.A.C.; Soares, B.L.M.; Stamford Arnaud, T.M.; Stamford, T.L.M. Viabilities of Lactobacillus rhamnosus ASCC 290 and Lactobacillus casei ATCC 334 (in free form or encapsulated with calcium alginate-chitosan) in yellow mombin ice cream. LWT 2019, 100, 391–396. [Google Scholar] [CrossRef]
- Zanjani, M.A.K.; Ehsani, M.R.; Tarzi, B.G.; Sharifan, A. Promoting Lactobacillus casei and Bifidobacterium adolescentis survival by microencapsulation with different starches and chitosan and poly L-lysine coatings in ice cream. J. Food Process. Preserv. 2018, 42. [Google Scholar] [CrossRef]



| Coating | Core | Technique | Pros | Cons | Ref. |
|---|---|---|---|---|---|
| Chitosan | Alginate, pectin | Extrusion, layer-by-layer (LbL), Emulsion | unique cationic property and high resistance to acidic environment; excellent film-forming abilities; high biocompatibility with living cells and broad antimicrobial activity; tolerance against the deteriorative effects of calcium chelating and anti-gelling agent; dens and strong beads | increases the excretion of sterols and produces a reduction in the digestibility of ileal fats; reported to have inhibitory effects on lactic acid bacteria (LAB) as core material | [31,37,45,55,56,57] |
| Alginate | Pectin | Extrusion | simplicity, non-toxicity, biocompatibility and low cost | sensitive in acidic environment; low stability in the presence of chelating agents | [37,45,54,56] |
| Resistant starch (corn, potato, cassava etc.) | Alginate | Extrusion, emulsion | inexpensive, abundant, biodegradable and easy to use; transparent, odorless, tasteless and colorless; low permeability to oxygen at low-to-intermediate relative humidity; resistant to pancreatic enzymes (amylases), therefore provides good enteric delivery characteristic; is an ideal surface for the adherence of the probiotic cells to the starch granules and this can enhance probiotic delivery | too high viscosity in solution for most of the encapsulation processes | [58,59,60] |
| Gelatin | Alginate, pectin | Extrusion | able to form complexes with anionic polymers, such as pectin and alginate | very soluble in aqueous systems | [31,37,61] |
| Whey protein | Pectin, alginate | Fluidized bed, extrusion | great gelation properties; biocompatible with probiotics; high nutritional value; improvement in the survival of probiotic after exposure to gastric conditions. | difficult to master, longer duration; do not confer additional protection to probiotics when exposed to simulated intestinal conditions | [62,63,64] |
| Poly-l-lysine (PLL) | Alginate | food-grade status, active properties and charged behavior | high porosity; does not have a strong capacity to be used as a microcapsule coating for probiotics protection against harsh media | [65,66,67] | |
| Glucomannan | Sodium alginate | is abundant in nature; is not hydrolyzed by human digestive enzymes | is a non-ionic polymer, so any coating would have to be the result of a non-ionic interaction, such as hydrogen bonding; there is very little work using glucomannan as a coating material for beads | [68,69] | |
| Shellac | Sodium alginate | Fluid bed | natural origin, therefore acceptable as coating material for food supplement products; good resistance to gastric fluid | the low solubility of shellac in the intestinal fluid, especially in the case of enteric coating of hydrophobic substances | [70,71] |
| Cellulose acetate phthalate (CAP) | Alginate | Emulsion | insoluble in acid media (pH ≤ 5) but it is soluble when the pH is ≥6 as a result of the presence of phthalate groups resulting in an effective way of delivering large numbers of viable bacterial cells to the colon | [44] | |
| k-carrageenan | Milk, alginate | Extrusion, emulsion | low susceptibility to the organic acids, good efficiency in lactic fermented products (such as yogurt); natural products | dissolves only at high temperatures (60–80 °C) for 2%–5% concentration; irregular shapes and poor mechanical characteristics; the produced gels are not able to withstand stresses | [33,37,72] |
| Microencapsulation Technique | Encapsulation Material | Chitosan-Coating | Probiotic Bacteria | Capsule Size (μm) | Application | Survivability (G: gastric; I: intestinal; colony) | Ref. |
|---|---|---|---|---|---|---|---|
| Extrusion; layer-by-layer (LbL) | Alginate (2%)+ 0.05 M CaCl2 | Chitosan (0.4%) | Bifidobacterium breve NCIMB 8807 | n.a. | In vitro GI exposure | (log colony forming units (CFU)/mL) (G; I) 7.3; 6.8 | [108] |
| Extrusion; LbL | Alginate (2%)+ 0.5 M CaCl2 + galactooligosaccharides and inulin | Chitosan (0.4%) | Lactobacillus acidophilus 5 and Lactobacillus casei 01 | 1830–1850 | In vitro GI exposure | (log CFU/mL) 2.7 and 2.3 > 107 CFU/g−1 | [111] |
| Refrigerated storage for 4 weeks in yogurt and juice | |||||||
| Extrusion; LbL | Alginate (1.8%) + 0.1 M CaCl2 + Hi–maize concentration of up to 1.0% (w/v) | Chitosan Poly-l-lysine (PLL) Alginate | L. acidophilus CSCC 2400 or CSCC 2409 | 500 | In vitro GI exposure | (log CFU, app) Chitosan: 9.1 PLL: 7.3 Alginate: 6 | [65] |
| Extrusion; LbL | Alginate (2%) + 0.5 M CaCl2 | Chitosan (0.7%) | Lactobacillus reuteri DSM 17938 | 110 ± 5 | 8 days storage in different solutions at 4 and 20 °C | log CFU/mL) (G; I) 9.15; 9.3 | [112] |
| In vitro GI exposure | |||||||
| Osmotic stress conditions |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Călinoiu, L.-F.; Ştefănescu, B.E.; Pop, I.D.; Muntean, L.; Vodnar, D.C. Chitosan Coating Applications in Probiotic Microencapsulation. Coatings 2019, 9, 194. https://doi.org/10.3390/coatings9030194
Călinoiu L-F, Ştefănescu BE, Pop ID, Muntean L, Vodnar DC. Chitosan Coating Applications in Probiotic Microencapsulation. Coatings. 2019; 9(3):194. https://doi.org/10.3390/coatings9030194
Chicago/Turabian StyleCălinoiu, Lavinia-Florina, Bianca Eugenia Ştefănescu, Ioana Delia Pop, Leon Muntean, and Dan Cristian Vodnar. 2019. "Chitosan Coating Applications in Probiotic Microencapsulation" Coatings 9, no. 3: 194. https://doi.org/10.3390/coatings9030194