Study of a Nano-SiO2 Microsphere-Modified Basalt Flake Epoxy Resin Coating
Abstract
:1. Introduction
2. Experimental Procedures
2.1. Materials
2.2. Sample Preparation
2.3. Characterization Techniques
2.4. Chemical Durability and Water Absorption Tests
3. Results
3.1. Characterization and Properties of BFs
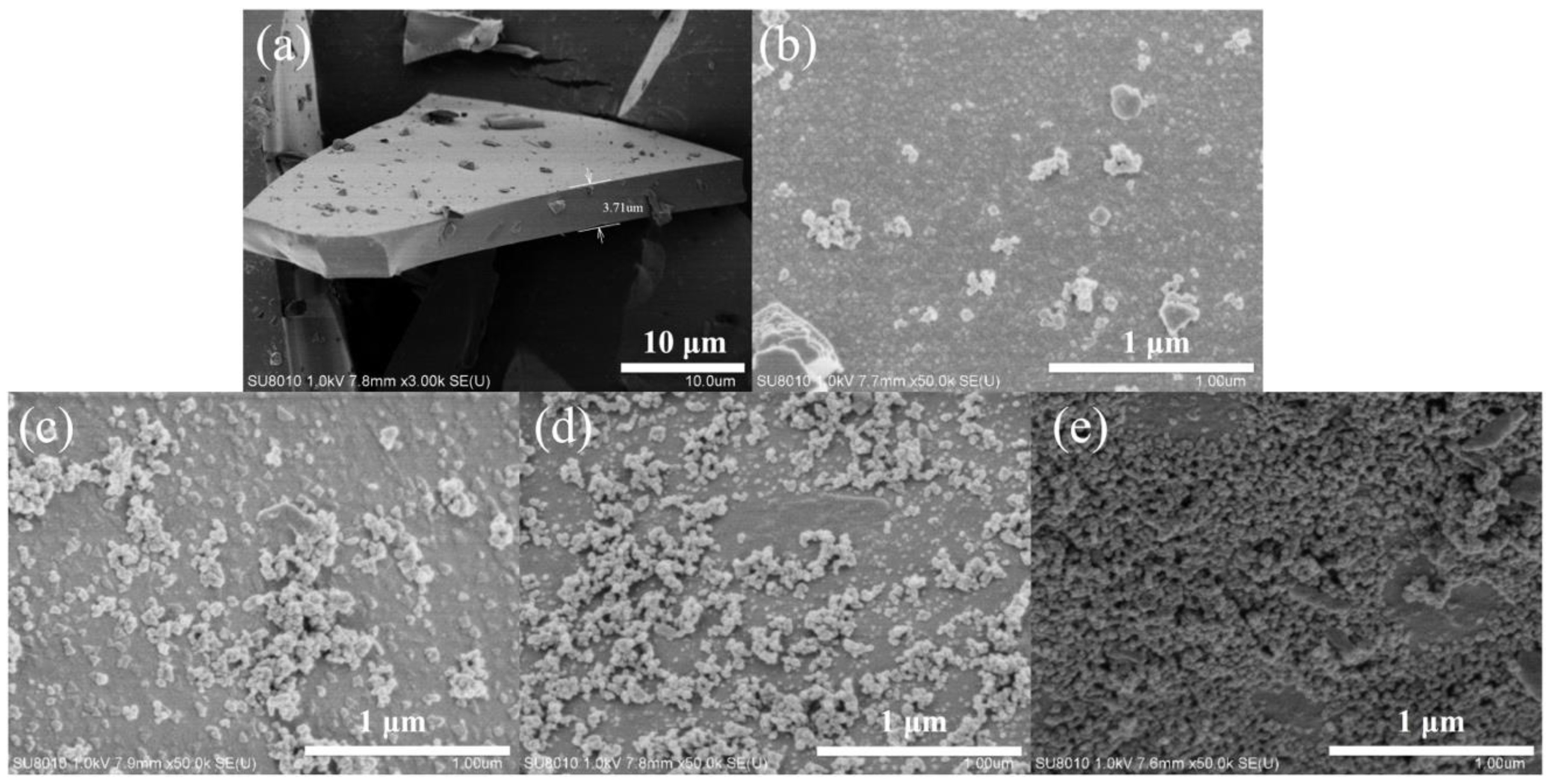
3.1.1. Surface Morphology Analysis of BFs
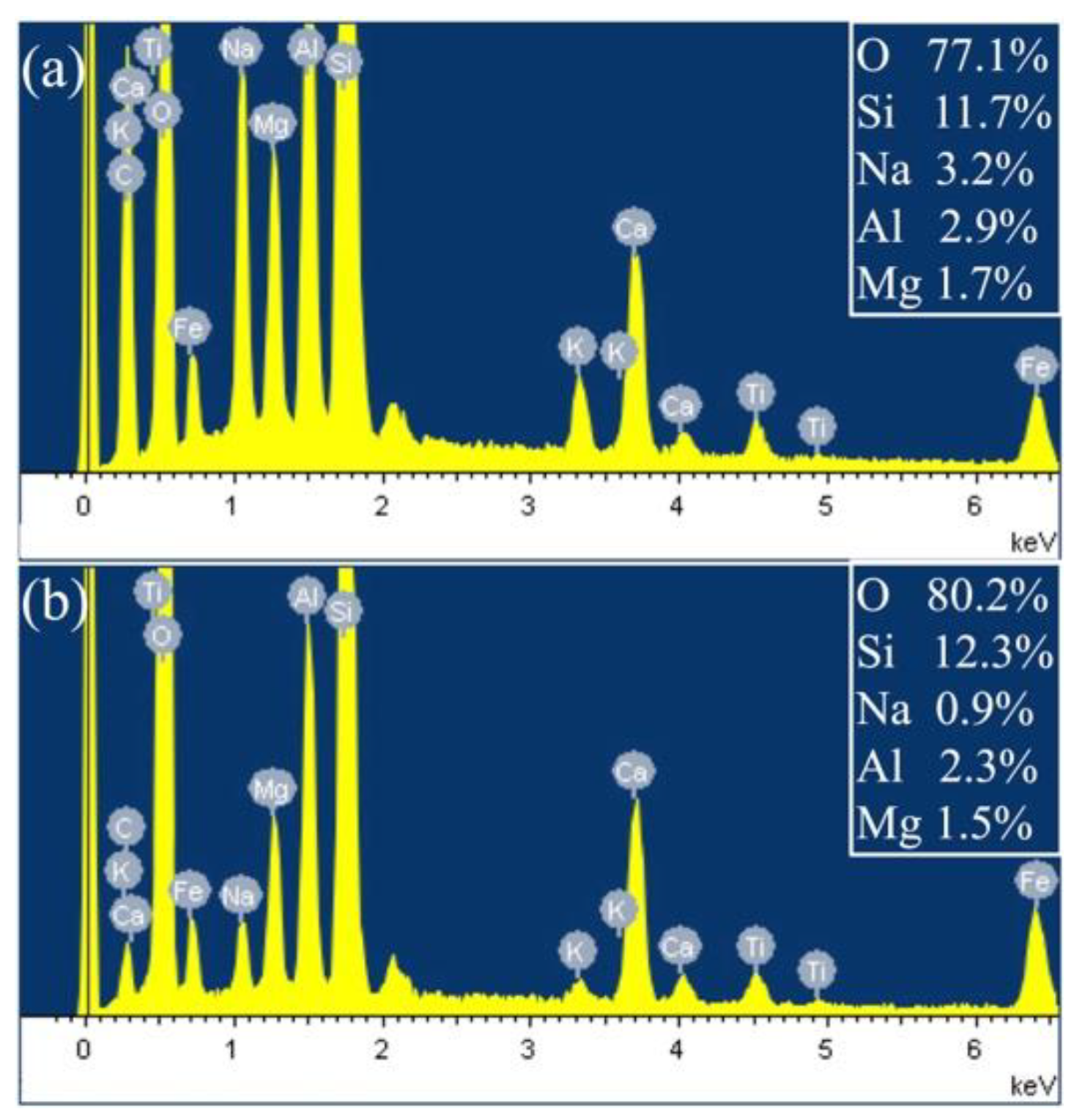
3.1.2. Surface Composition Analysis of BFs
3.2. Characterization and Properties of the Modified BF Epoxy Resin Coating
3.2.1. Surface Morphology Analysis of the Modified BF Epoxy Resin Coating
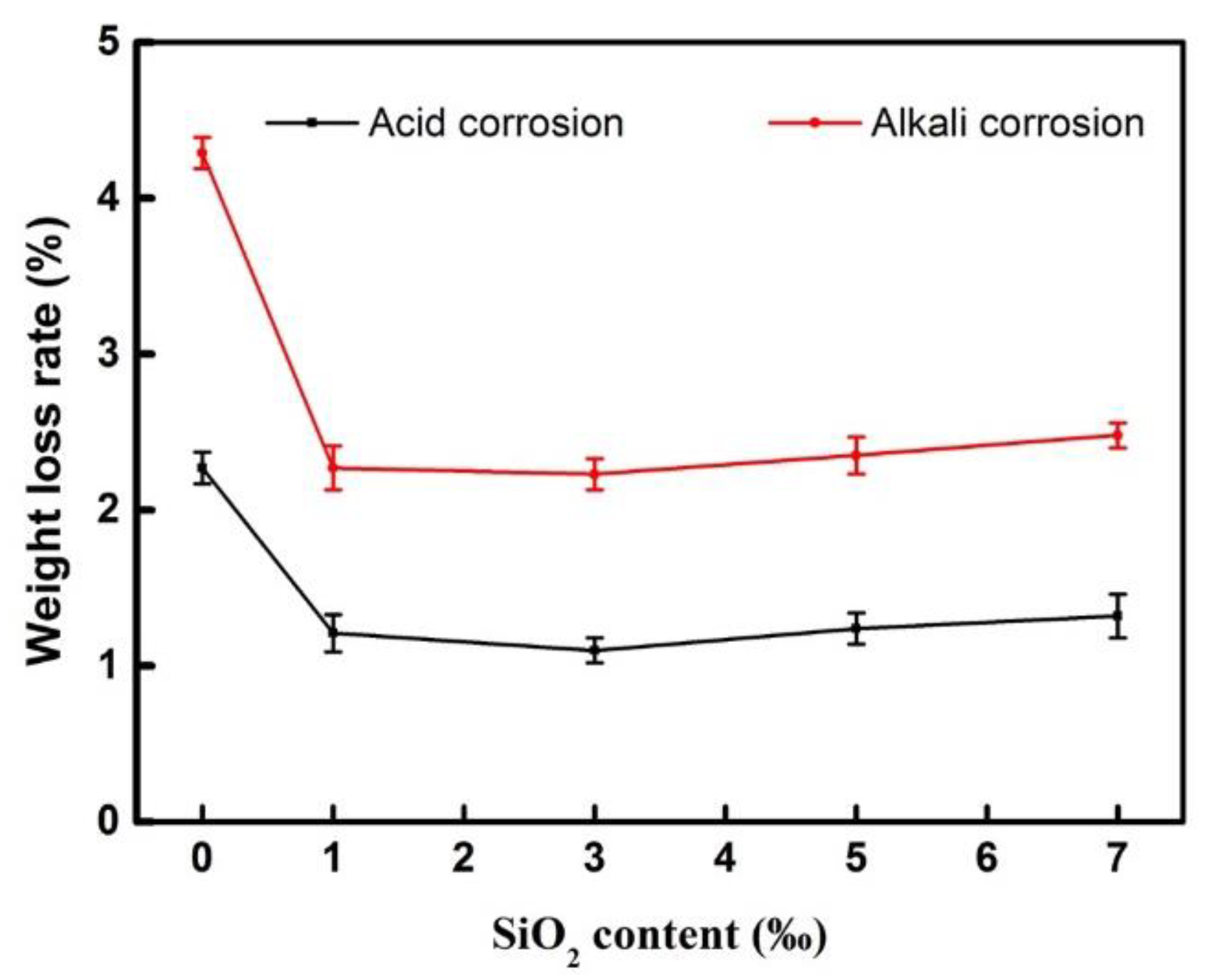
3.2.2. Chemical Durability of the Modified BF Epoxy Resin Coating
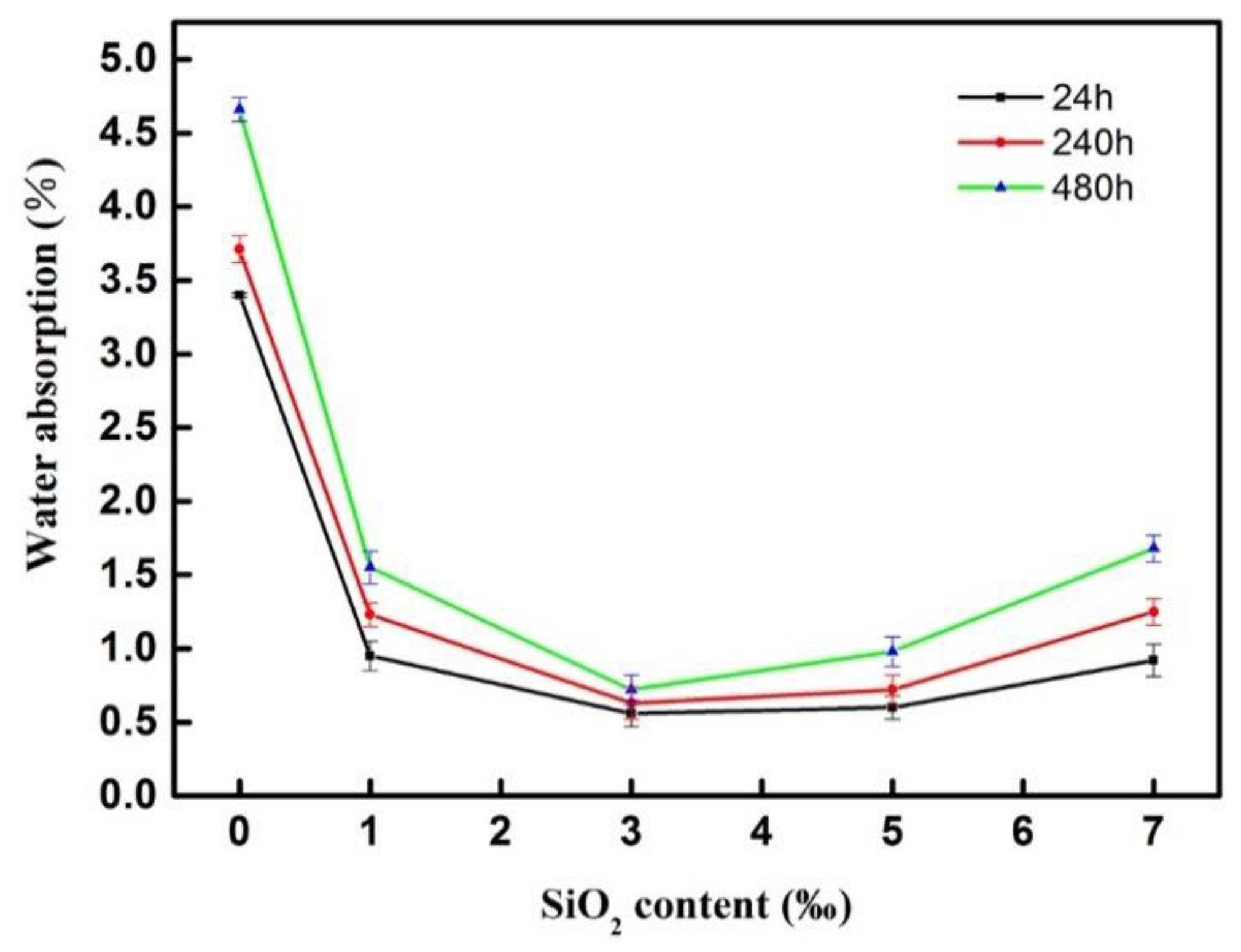
3.2.3. Water Absorption of the Modified BF Epoxy Resin Coating
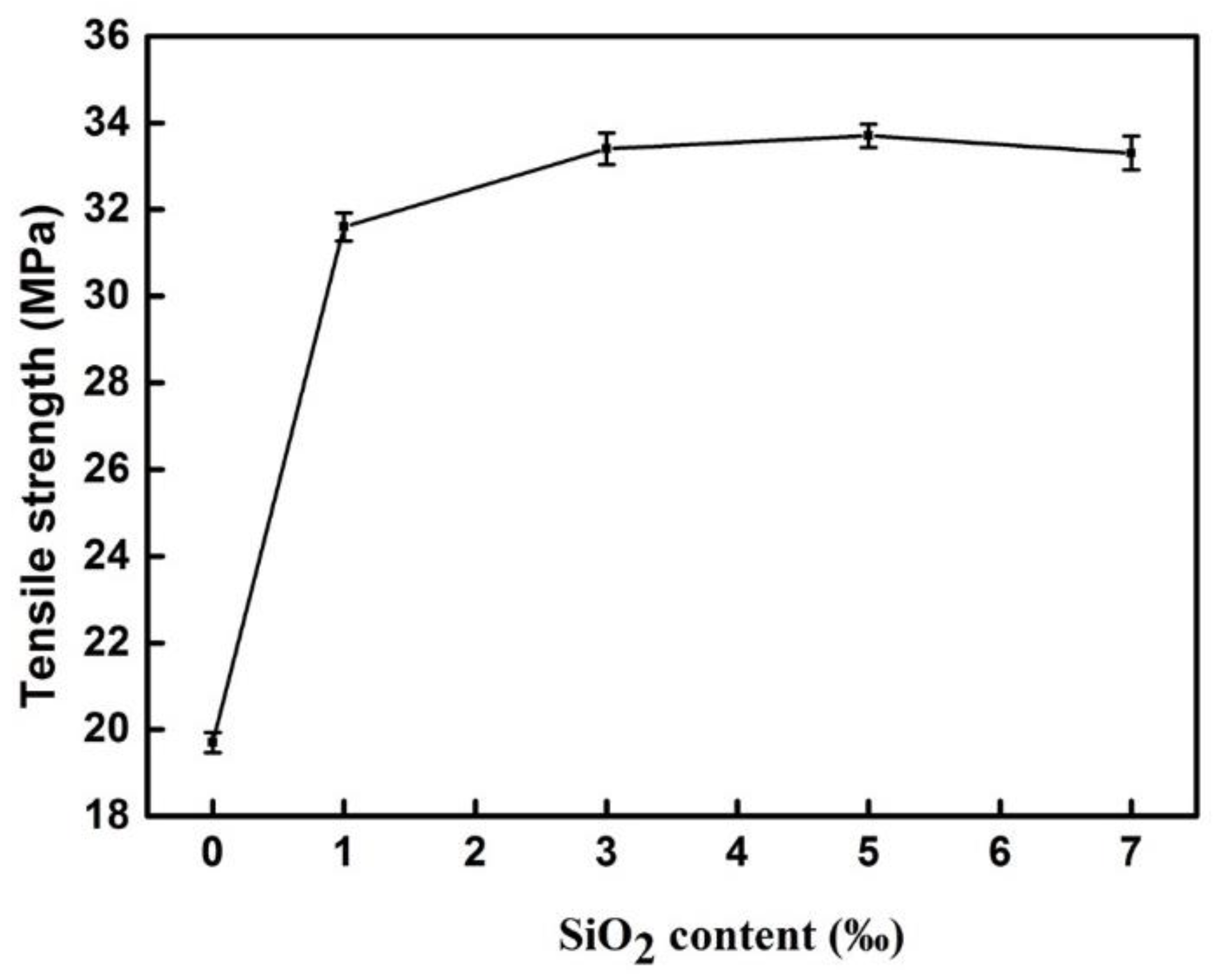
3.2.4. Tensile Strength the Modified BF Epoxy Resin Coating
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gao, X.; Guo, L.Z.; Xiong, J.B. Corrosion protection technology of steel structure in marine environment. Appl. Mech. Mater. 2002, 744–746, 29–32. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, Y. Comparisons of clear coating degradation in NaCl solution and pure water. Prog. Org. Coat. 2013, 76, 1674–1682. [Google Scholar] [CrossRef]
- Liu, H.W.; Xie, K.F.; Yang, Z.B. The advantages of basalt glass flake coating for marine anticorrosion. Adv. Mater. Res. 2011, 332–334, 1619–1622. [Google Scholar] [CrossRef]
- Nematollahi, M.; Heidarian, M.; Peikari, M. Comparison between the effect of nanoglass flake and montmorillonite organoclay on corrosion performance of epoxy coating. Corros. Sci. 2010, 52, 1809–1817. [Google Scholar] [CrossRef]
- Alhumade, H.; Nogueira, R.P.; Yu, A. Role of surface functionalization on corrosion resistance and thermal stability of epoxy/glass flake composite coating on cold rolled steel. Prog. Org. Coat. 2018, 122, 180–188. [Google Scholar] [CrossRef]
- Broughton, W.R.; Lodeiro, M.J.; Pilkington, G.D. Influence of coupling agents on material behaviour of glass flake reinforced polypropylene. Compos. Part A: Appl. Sci. Manuf. 2010, 41, 506–514. [Google Scholar] [CrossRef]
- Yan, J.; Shi, J.; Zhang, P. Preparation and properties of epoxy/BFs anticorrosive coatings. Mater. Corros. 2018, 69, 1669–1675. [Google Scholar] [CrossRef]
- Pavlovski, D.; Mislavsky, B.; Antonov, A. CNG cylinder manufacturers test basalt fibre. Reinf. Plast. 2007, 51, 36–39. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, C.; Chu, P.K. Mechanical and thermal properties of basalt fiber reinforced poly (butylene succinate) composites. Mater. Chem. Phys. 2012, 133, 845–849. [Google Scholar] [CrossRef]
- Colombo, C.; Vergani, L.; Burman, M. Static and fatigue characterisation of new basalt fibre reinforced composites. Compos. Struct. 2012, 94, 1165–1174. [Google Scholar] [CrossRef]
- Deak, T.; Czigany, T. Chemical composition and mechanical properties of basalt and glass fibers: A Comparison. Text. Res. J. 2009, 79, 645–651. [Google Scholar] [CrossRef]
- He, P.; Wang, J.; Lu, F. Synergistic effect of polyaniline grafted basalt plates for enhanced corrosion protective performance of epoxy coatings. Prog. Org. Coat. 2017, 110, 1–9. [Google Scholar] [CrossRef]
- Arukalam, I.O.; Meng, M.; Xiao, H. Effect of perfluorodecyltrichlorosilane on the surface properties and anti-corrosion behavior of poly(dimethylsiloxane)-ZnO coatings. Appl. Surf. Sci. 2018, 433, 1113–1127. [Google Scholar] [CrossRef]
- Hu, C.; Chen, W.; Li, T. Constructing non-fluorinated porous superhydrophobic SiO2-based films with robust mechanical properties. Colloids Surf. A Physicochem. Eng. Asp. 2018, 551, 65–73. [Google Scholar] [CrossRef]
- Fei, J.; Luo, D.; Wang, H. Effect of nano-SiO2 particles on the carbon fabric/resin friction materials by microwave-hydrothermal treatment. J. Compos. Mater. 2018, 52, 245–252. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, G.; Xie, F. Mechanical properties of carbon fiber composites modified with nano-SiO2 in the interphase. J. Adhes. Sci. Technol. 2014, 28, 2154–2166. [Google Scholar] [CrossRef]
- Kim, J.W.; Kim, L.U.; Kim, C.K. Size Control of Silica Nanoparticles and Their Surface Treatment for Fabrication of Dental Nanocomposites. Biomacromolecules 2007, 8, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Ding, L.; Wang, Q. Preparation and characterization of continuous fly ash derived glass fibers with improved tensile strength. Mater. Lett. 2018, 231, 119–121. [Google Scholar] [CrossRef]
- Ghanbari, A.; Attar, M.M. A study on the anticorrosion performance of epoxy nanocomposite coatings containing epoxy-silane treated nano-silica on mild steel substrate. J. Ind. Eng. Chem. 2015, 23, 145–153. [Google Scholar] [CrossRef]
- Patwardhan, S.V.; Emami, F.S.; Berry, R.J. Chemistry of aqueous silica nanoparticle surfaces and the mechanism of selective peptide adsorption. J. Am. Chem. Soc. 2012, 134, 6244–6256. [Google Scholar] [CrossRef] [PubMed]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, L.; Ma, Q.; Wang, Q.; Ding, L.; Gong, Z.; Jiang, W. Study of a Nano-SiO2 Microsphere-Modified Basalt Flake Epoxy Resin Coating. Coatings 2019, 9, 154. https://doi.org/10.3390/coatings9030154
Luo L, Ma Q, Wang Q, Ding L, Gong Z, Jiang W. Study of a Nano-SiO2 Microsphere-Modified Basalt Flake Epoxy Resin Coating. Coatings. 2019; 9(3):154. https://doi.org/10.3390/coatings9030154
Chicago/Turabian StyleLuo, Lida, Qian Ma, Qingwei Wang, Linfeng Ding, Ziyan Gong, and Weizhong Jiang. 2019. "Study of a Nano-SiO2 Microsphere-Modified Basalt Flake Epoxy Resin Coating" Coatings 9, no. 3: 154. https://doi.org/10.3390/coatings9030154



