Development of a Polyacrylate/Silica Nanoparticle Hybrid Emulsion for Delaying Nutrient Release in Coated Controlled-Release Urea
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Nutrient Release Behavior of Coated CRU
3.2. Fourier-Transform Infrared Photoacoustic Spectrum
3.3. Water Absorption Rate
3.4. Water Vapor Permeability
3.5. Glass Transition Temperature of Films
3.6. Mechanical Properties of Isolated Films
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Premarathna, L.; Mclaughlin, M.J.; Kirby, J.K.; Hettiarachchi, G.M.; Stacey, S.; Chittleborough, D.J. Selenate-enriched urea granules are a highly effective fertilizer for selenium biofortification of paddy rice grain. J. Agric. Food Chem. 2012, 60, 6037–6044. [Google Scholar] [CrossRef] [PubMed]
- Shaviv, A.; Mikkelsen, R.L. Controlled-release fertilizers to increase efficiency of nutrient use and minimize environmental degradation-A review. Fertil. Res. 1993, 35, 1–12. [Google Scholar] [CrossRef]
- Smith, L.E.D.; Siciliano, G. A comprehensive review of constraints to improved management of fertilizers in China and mitigation of diffuse water pollution from agriculture. Agric. Ecosyst. Environ. 2015, 209, 15–25. [Google Scholar] [CrossRef] [Green Version]
- Costa, M.M.E.; Cabralalbuquerque, E.C.M.; Alves, T.L.M.; Pinto, J.C.; Fialho, R.L. Use of polyhydroxybutyrate and ethyl cellulose for coating of urea granules. J. Agric. Food Chem. 2013, 61, 9984–9991. [Google Scholar] [CrossRef] [PubMed]
- Azeem, B.; Kushaari, K.; Man, Z.B.; Basit, A.; Thanh, T.H. Review on materials & methods to produce controlled release coated urea fertilizer. J. Control. Release 2014, 181, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, M.; Li, Y.; Fan, X.; Geng, Y. Controlled-release urea commingled with rice seeds reduced emission of ammonia and nitrous oxide in rice paddy soil. J. Environ. Qual. 2013, 42, 1661–1673. [Google Scholar] [CrossRef] [PubMed]
- Donida, M.W.; Rocha, S.C.S. Coating of urea with an aueous polymeric suspension in a two-dimensional spouted bed. Dry. Technol. 2002, 20, 685–704. [Google Scholar] [CrossRef]
- Shen, Y.; Du, C.; Zhou, J. Aqueous polyacrylate/poly(silicone-co-acrylate) emulsion coated fertilizers for slow nutrient-release application. J. Appl. Polym. Sci. 2014, 131, 469–474. [Google Scholar] [CrossRef]
- Zhou, Z.; Du, C.; Li, T.; Shen, Y.; Zeng, Y.; Du, J.; Zhou, J. Biodegradation of a biochar-modified waterborne polyacrylate membrane coating for controlled-release fertilizer and its effects on soil bacterial community profiles. Environ. Sci. Pollut. Res. Int. 2015, 22, 8672–8682. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Shen, Y.; Du, C.; Zhou, J.; Wang, H.; Chen, X. Evaluation of waterborne coating for controlled-release fertilizer using wurster fluidized bed. Ind. Eng. Chem. Res. 2010, 49, 9644–9647. [Google Scholar] [CrossRef]
- Shen, Y.; Du, C.; Zhou, J.; Ma, F. Application of nano FeⅢ-tannic acid complexes in modifying aqueous acrylic latex for controlled-release coated urea. J. Agric. Food Chem. 2017, 65, 1030–1036. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Shen, Y.; Ma, F.; Du, C. Application of graphene-oxide-modified polyacrylate polymer for controlled-release coated urea. Coatings 2018, 8, 64. [Google Scholar] [CrossRef]
- An, D.; Liu, B.; Yang, L.; Wang, T.J.; Kan, C. Fabrication of graphene oxide/polymer latex composite film coated on KNO3 fertilizer to extend its release duration. Chem. Eng. J. 2017, 311, 318–325. [Google Scholar] [CrossRef]
- Du, X.; He, J. A Self-templated etching route to surface-rough silica nanoparticles for superhydrophobic coatings. ACS Appl. Mater. Interface 2011, 3, 1269–1276. [Google Scholar] [CrossRef]
- Ma, J.Z.; Hu, J.; Zhang, Z.J. Polyacrylate/silica nanocomposite materials prepared by sol–gel process. Eur. Polym. J. 2007, 43, 4169–4177. [Google Scholar] [CrossRef]
- Liu, J.L.; Ma, J.Z.; Bao, Y.; Zhu, Z.F.; Liu, Y.H.; Zheng, Y. Preparation of polyacrylate/ZnO nanocomposite. Mater. Sci. Forum 2011, 694, 430–434. [Google Scholar] [CrossRef]
- Sheibatothman, N.; Bourgeatlami, E. Use of silica particles for the formation of organic−inorganic particles by surfactant-free emulsion polymerization. Langmuir 2009, 25, 10121–10133. [Google Scholar] [CrossRef]
- Chau, J.L.H.; Hsieh, C.C.; Lin, Y.M.; Li, A.K. Preparation of transparent silica–PMMA nanocomposite hard coatings. Prog. Org. Coat. 2008, 62, 436–439. [Google Scholar] [CrossRef]
- An, Q.; Wei, X.; Hao, L.; Fu, Y.; Huang, L. Fabrication of superhydrophobic fabric coating using microphase-separated dodecafluoroheptyl-containing polyacrylate and nanosilica. J. Appl. Polym. Sci. 2013, 128, 3050–3056. [Google Scholar] [CrossRef]
- Schmidt, D.F.; Giannelis, E.P. Silicate dispersion and mechanical reinforcement in polysiloxane/layered silicate nanocomposites. Chem. Mater. 2010, 22, 167–174. [Google Scholar] [CrossRef]
- Li, L.; Sun, Y.; Cao, B.; Song, H.; Xiao, Q.; Yi, W. Preparation and performance of polyurethane/mesoporous silica composites for coated urea. Mater. Des. 2016, 99, 21–25. [Google Scholar] [CrossRef]
- Huang, Y.; Hu, M.; Yi, S.; Liu, X.; Li, H.; Huang, C.; Luo, Y.; Li, Y. Preparation and characterization of silica/fluorinated acrylate copolymers hybrid films and the investigation of their icephobicity. Thin Solid Films 2012, 520, 5644–5651. [Google Scholar] [CrossRef]
- Choudalakis, G.; Gotsis, A.D. Free volume and mass transport in polymer nanocomposites. Curr. Opin. Colloid Interface Sci. 2012, 17, 132–140. [Google Scholar] [CrossRef]
- Zhou, J.; Xin, C.; Hao, D.; Ma, J.; Ma, Y. Synthesis and characterization of nano-SiO2 modified fluorine-containing polyacrylate emulsifier-free emulsion. Appl. Surf. Sci. 2015, 331, 504–511. [Google Scholar] [CrossRef]
- Bao, Y.; Yang, Y.; Ma, J. Fabrication of monodisperse hollow silica spheres and effect on water vapor permeability of polyacrylate membrane. J. Colloid Interface Sci. 2013, 407, 155–163. [Google Scholar] [CrossRef] [PubMed]
- ASTM D638-03 Standard Test Method for Tensile Properties of Plastics; ASTM International: West Conshohocken, PA, USA, 2003.
- ASTM D2240-03 Standard Test Method for Rubber Property—Durometer Hardness; ASTM International: West Conshohocken, PA, USA, 2003.
- Yang, Y.C.; Zhang, M.; Li, Y.; Fan, X.H.; Geng, Y.Q. Improving the quality of polymer-coated urea with recycled plastic, proper additives, and large tablets. J. Agric. Food Chem. 2012, 60, 11229–11237. [Google Scholar] [CrossRef]
- Shaviv, A.; Raban, S.; Zaidel, E. Modeling controlled nutrient release from polymer coated fertilizers: Diffusion release from single granules. Environ. Sci. Technol. 2003, 37, 2251–2256. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Park, H.B.; Rhim, J.W.; Lee, Y.M. Preparation and characterization of crosslinked PVA/SiO2 hybrid membranes containing sulfonic acid groups for direct methanol fuel cell applications. J. Membr. Sci. 2004, 240, 37–48. [Google Scholar] [CrossRef]
- Torabi, B.; Ameri, E. Methyl acetate production by coupled esterification-reaction process using synthesized cross-linked PVA/silica nanocomposite membranes. Chem. Eng. J. 2016, 288, 461–472. [Google Scholar] [CrossRef]
- Bryan, S.; Pivovar, B.S.; Wang, Y.; Cussler, E.L. Pervaporation membranes in direct methanol fuel cells. J. Membr. Sci. 1999, 154, 155–162. [Google Scholar] [CrossRef]
- Cristea, M.V.; Riedl, B.; Blanchet, P. Effect of addition of nanosized UV absorbers on the physico-mechanical and thermal properties of an exterior waterborne stain for wood. Prog. Org. Coat. 2011, 72, 755–762. [Google Scholar] [CrossRef]
- Wel, G.K.V.D.; Adan, O.C.G. Moisture in organic coatings—A review. Prog. Org. Coat. 1999, 37, 1–14. [Google Scholar] [CrossRef]
- Pishvaei, M.; Tabrizi, F.F. Synthesis of high solid content polyacrylate/nanosilica latexes via miniemulsion polymerization. Iran. Polym. J. 2010, 19, 707–716. [Google Scholar]
- Rittigstein, P.; Torkelson, J.M. Polymer-nanoparticle interfacial interactions in polymer nanocomposites: Confinement effects on glass transition temperature and suppression of physical aging. J. Polym. Sci. Polym. Phys. 2010, 44, 2935–2943. [Google Scholar] [CrossRef]
- Pluta, M.; Jeszka, J.K.; Boiteux, G. Polylactide/montmorillonite nanocomposites: Structure, dielectric, viscoelastic and thermal properties. Eur. Polym. J. 2007, 43, 2819–2835. [Google Scholar] [CrossRef]
- Zhou, C.; Xu, S.; Pi, P.; Cheng, J.; Wang, L.; Yang, J.; Wen, X. Polyacrylate/silica nanoparticles hybrid emulsion coating with high silica content for high hardness and dry-wear-resistant. Prog. Org. Coat. 2018, 121, 30–37. [Google Scholar] [CrossRef]
- Mizutani, T.; Arai, K.; Miyamoto, M.; Kimura, Y. Preparation of spherical nanocomposites consisting of silica core and polyacrylate shell by emulsion polymerization. J. Appl. Polym. Sci. 2006, 99, 659–669. [Google Scholar] [CrossRef]
- Guyard, A.; Persello, J.; Boisvert, J.P.; Cabane, B. Relationship between the polymer/silica interaction and properties of silica composite materials. J. Polym. Sci. Polym. Phys. 2010, 44, 1134–1146. [Google Scholar] [CrossRef]
- Bhattacharya, M.; Chaudhry, S. High-performance silica nanoparticle reinforced poly (vinyl alcohol) as templates for bioactive nanocomposites. Mater. Sci. Eng. C Mater. 2013, 33, 2601–2610. [Google Scholar] [CrossRef] [PubMed]
- Khoonsap, S.; Supanchaiyamat, N.; Hunt, A.J.; Klinsrisuk, S.; Amnuaypanich, S. Improving water selectivity of poly(vinyl alcohol) (PVA)–Fumed silica (FS) nanocomposite membranes by grafting of poly (2-hydroxyethyl methacrylate) (PHEMA) on fumed silica particles. Chem. Eng. Sci. 2015, 122, 373–383. [Google Scholar] [CrossRef]
- Li, J.; Suo, J.; Deng, R. Structure, Mechanical, and swelling behaviors of poly(vinyl alcohol)/SiO2 hybrid membranes. J. Reinf. Plast. Comp. 2010, 29, 618–629. [Google Scholar] [CrossRef]
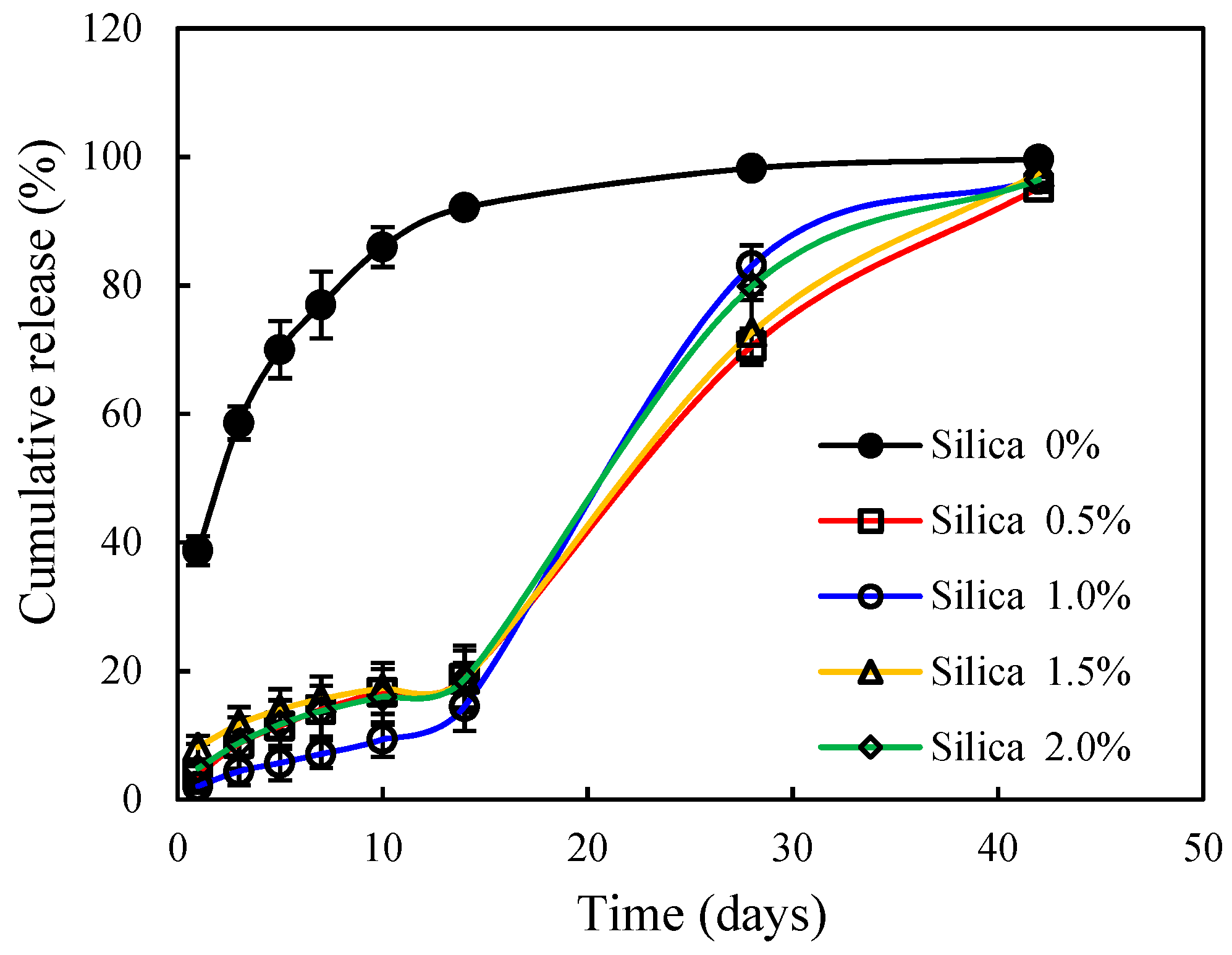

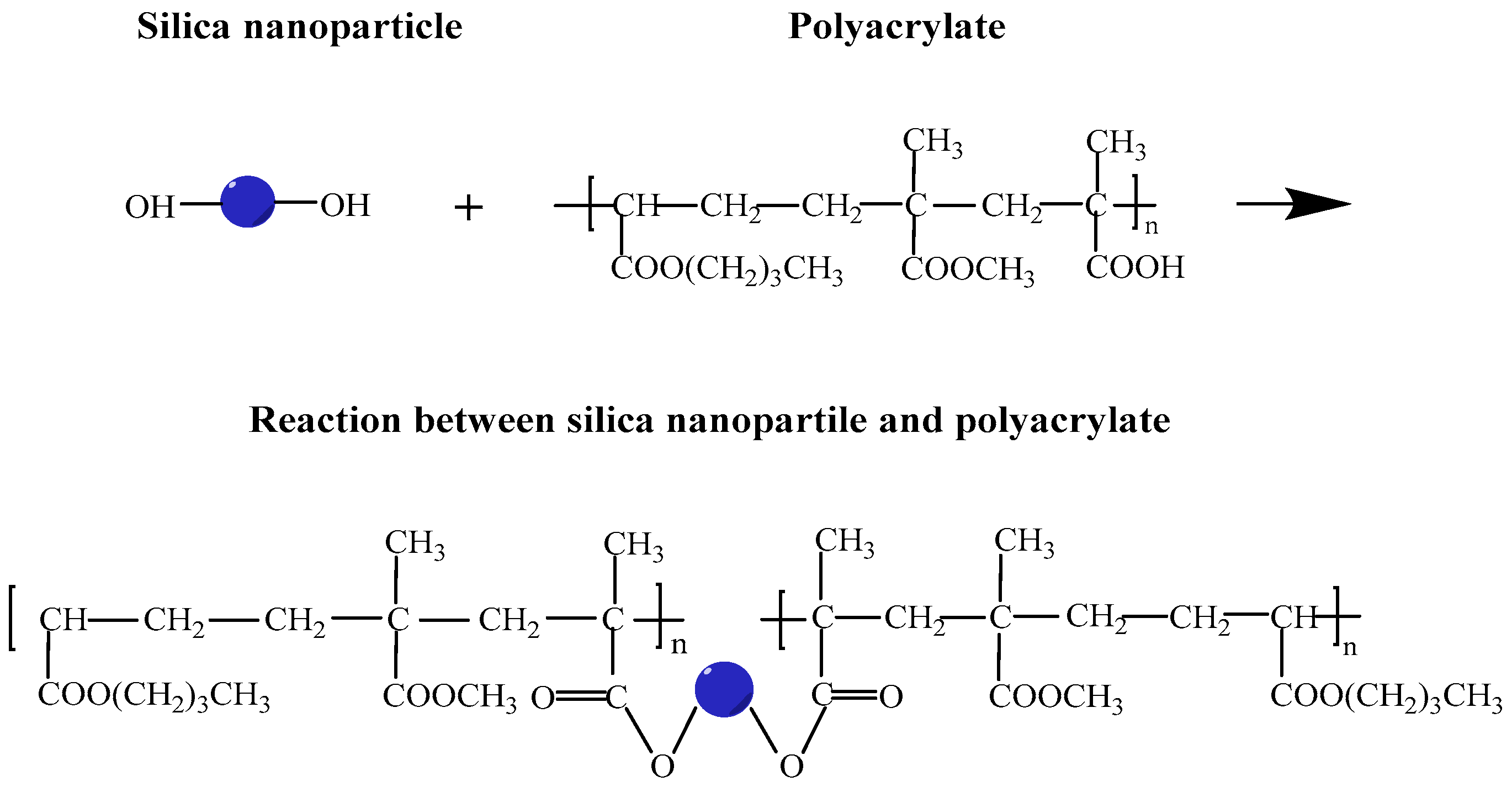

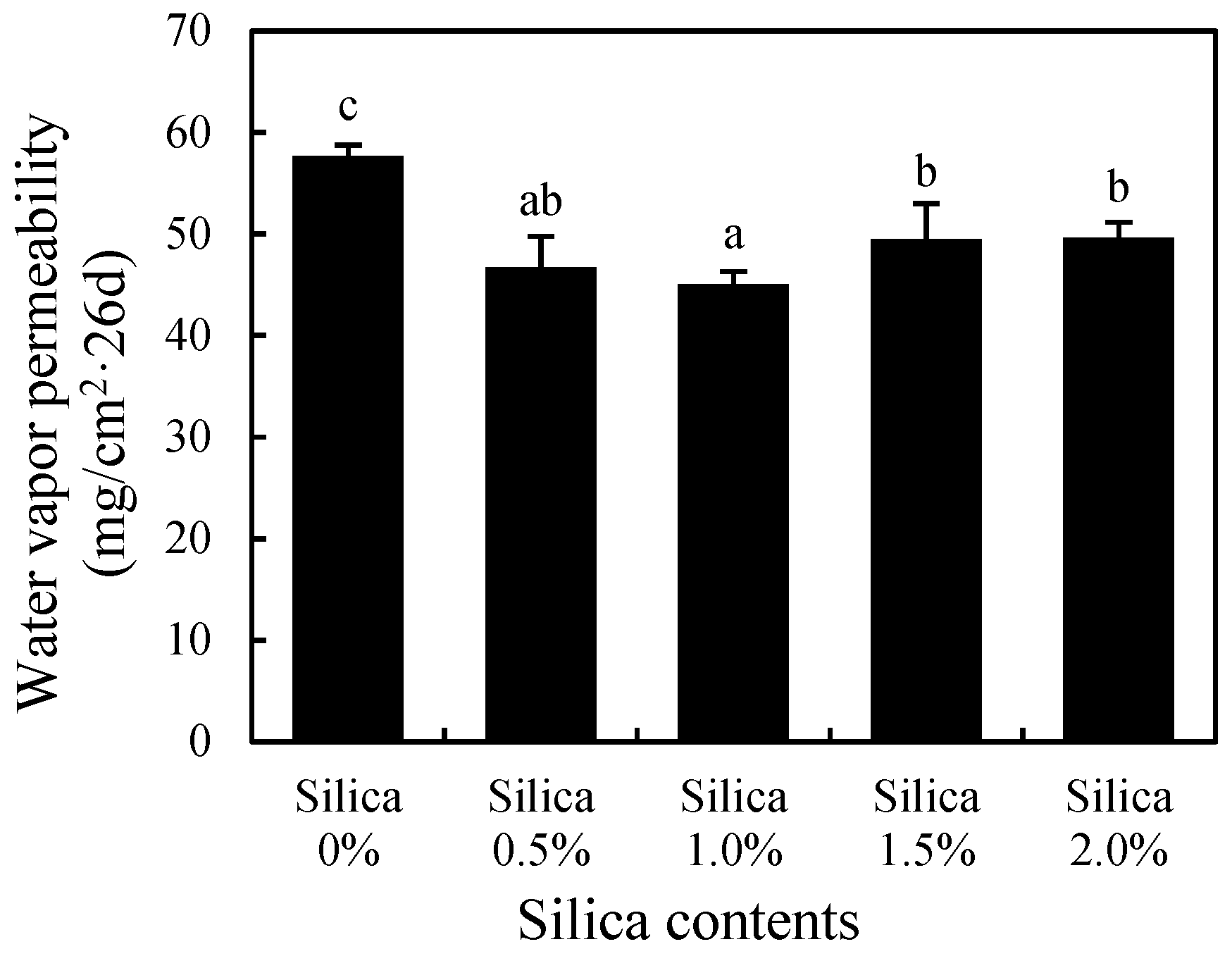
| Silica Content (wt.%) | Preliminary Solubility (%) | Release Duration (days) |
|---|---|---|
| 0 | 38.3 a | 8 C |
| 0.5 | 4.3 b | 33 A |
| 1.0 | 2.2 b | 27 B |
| 1.5 | 5.4 b | 32 A |
| 2.0 | 9.1 b | 28 B |
| Silica Content (wt.%) | Tg (°C) | Hardness (Share A) | Tensile Stress at Break (MPa) | Elongation at Break (%) | Young’s Modulus (MPa) |
|---|---|---|---|---|---|
| 0 | 6.38 | 34 b | 3.47 b | 1033.54 a | 29.42 b |
| 0.5 | 9.54 | 38 a | 3.78 ab | 1181.62 a | 32.40 ab |
| 1.0 | 9.97 | 38 a | 4.66 a | 1013.23 a | 36.00 a |
| 1.5 | 8.50 | 38 a | 3.00 bc | 887.66 a | 24.04 c |
| 2.0 | 6.23 | 38 a | 2.48 c | 962.99 a | 23.69 c |
| Release Parameters | Water Uptake (%) | Water Vapor Permeability (mg/cm2·26d) | Tg (°C) | Hardness (Share A) | Stress (MPa) | Modulus (MPa) |
|---|---|---|---|---|---|---|
| Preliminary solubility | 0.150 | 0.935 ** | −0.668 ** | −0.959 ** | −0.159 | −0.104 |
| Release duration | −0.296 | −0.863 ** | 0.473 | 0.965 ** | 0.088 | −0.066 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, Y.; Zhou, J.; Du, C. Development of a Polyacrylate/Silica Nanoparticle Hybrid Emulsion for Delaying Nutrient Release in Coated Controlled-Release Urea. Coatings 2019, 9, 88. https://doi.org/10.3390/coatings9020088
Shen Y, Zhou J, Du C. Development of a Polyacrylate/Silica Nanoparticle Hybrid Emulsion for Delaying Nutrient Release in Coated Controlled-Release Urea. Coatings. 2019; 9(2):88. https://doi.org/10.3390/coatings9020088
Chicago/Turabian StyleShen, Yazhen, Jianmin Zhou, and Changwen Du. 2019. "Development of a Polyacrylate/Silica Nanoparticle Hybrid Emulsion for Delaying Nutrient Release in Coated Controlled-Release Urea" Coatings 9, no. 2: 88. https://doi.org/10.3390/coatings9020088






