Designing a Laboratory Bioassay for Evaluating the Efficacy of Antifouling Paints on Amphibalanus amphitrite Using a Flow-Through System
Abstract
:1. Introduction
2. Materials and Methods
2.1. AF Paints and Test Plates
2.2. Laboratory Bioassay
2.2.1. Test Organism
2.2.2. Evaluating the Inhibition Effects of Test Paints Using Cyprid Settlement as Index
2.2.3. Verification of the Validity of the Bioassay
2.3. Statistical Analysis
3. Results
3.1. Assessment of the Efficacy of Antifouling Paint on Cyprid Settlement Ratio
3.1.1. Parameters of Water Quality of the Test Water
3.1.2. The Activity of Cyprids Used in the Bioassay
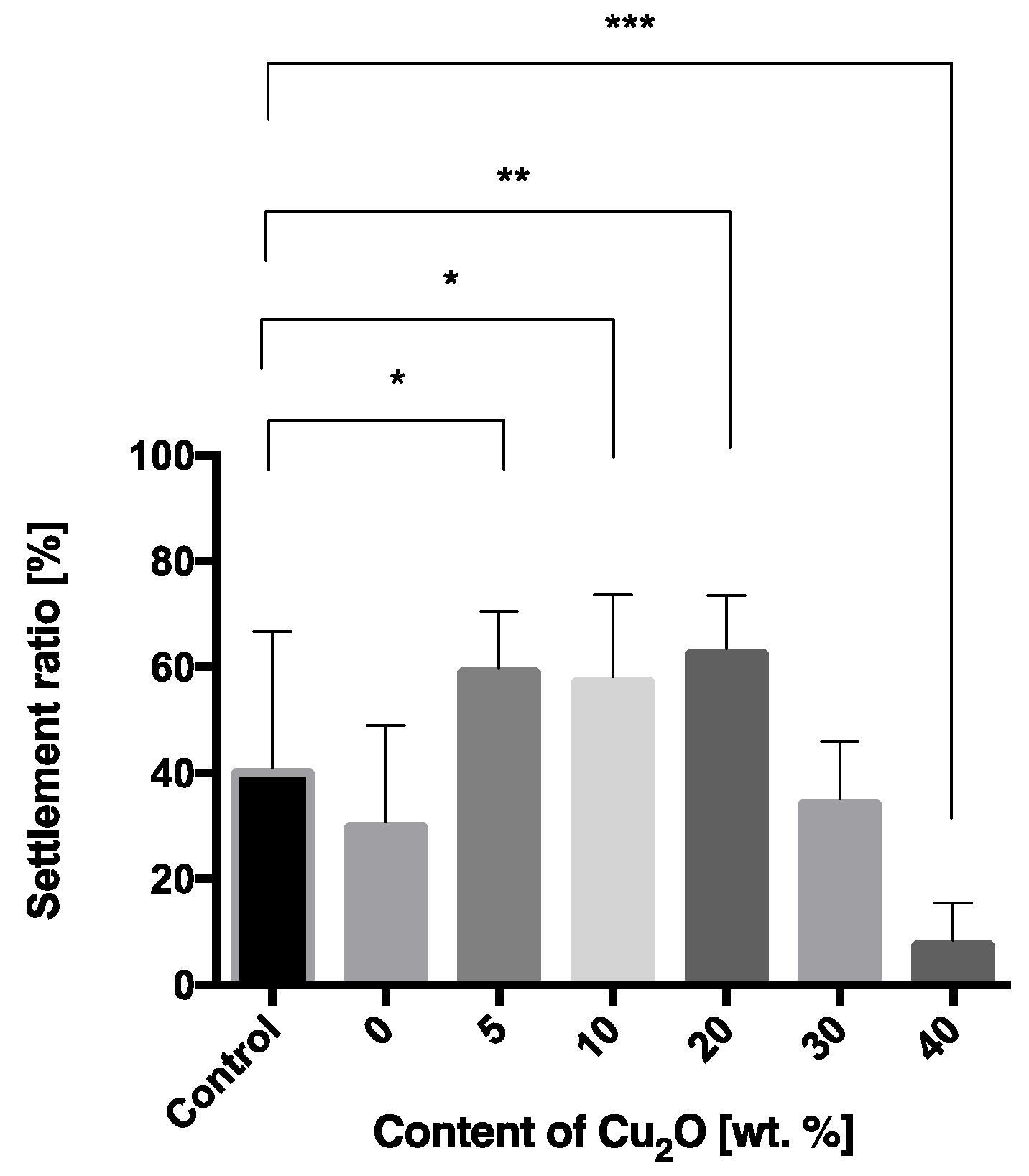
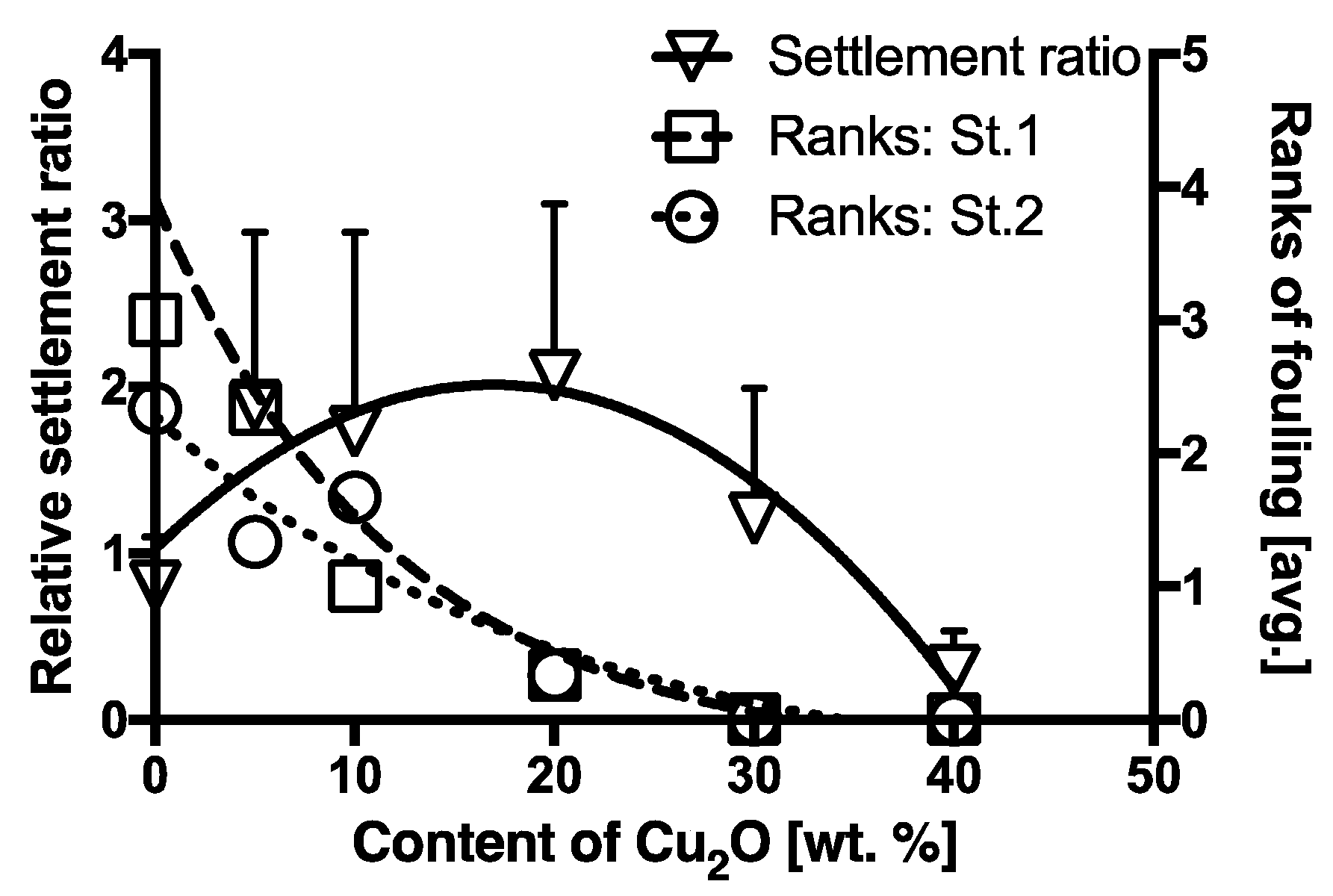
3.1.3. Relative Settlement Ratios (R) of Cyprids on Antifouling Paints
4. Discussion
4.1. The Concept of the Flow-Through Bioassay Designed for Barnacles in the Laboratory
4.2. The Repellent Effect of Cu2O on Barnacles
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Richmond, M.D.; Seed, R. A review of marine macrofouling communities with special reference to animal fouling. Biofouling 1991, 3, 151–168. [Google Scholar] [CrossRef]
- Briand, J.F. Marine antifouling laboratory bioassays: An overview of their diversity. Biofouling 2009, 25, 297–311. [Google Scholar] [CrossRef] [PubMed]
- Carlton, J.T. Pattern, process, and prediction in marine invasion ecology. Biol. Conserv. 1996, 78, 97–106. [Google Scholar] [CrossRef]
- Coutts, A.D.M.; Taylor, M.D. A preliminary investigation of biosecurity risks associated with biofouling on merchant vessels in New Zealand. N. Z. J. Mar. Freshw. Res. 2004, 38, 215–229. [Google Scholar] [CrossRef]
- Schultz, M.P. Effects of coating roughness and biofouling on ship resistance and powering. Biofouling 2007, 23, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Otani, M.; Oumi, T.; Uwai, S.; Hanyuda, T.; Prabowo, R.E.; Yamaguchi, T.; Kawai, H. Occurrence and diversity of barnacles on international ships visiting Osaka Bay, Japan, and the risk of their introduction. Biofouling 2007, 23, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Davidson, I.C.; Brown, C.W.; Sytsma, M.D.; Ruiz, G.M. The role of containerships as transfer mechanisms of marine biofouling species. Biofouling 2009, 25, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Piola, R.F.; Dafforn, K.A.; Johnston, E.L. The influence of antifouling practices on marine invasions. Biofouling 2009, 25, 633–644. [Google Scholar] [CrossRef]
- Coutts, A.D.M.; Valentine, J.P.; Edgar, G.J.; Davey, A.; Wilson, B.B. Removing vessels from the water for biofouling treatment has the potential to introduce mobile non-indigenous marine species. Mar. Pollut. Bull. 2010, 60, 1533–1540. [Google Scholar] [CrossRef]
- Coutts, A.D.M.; Piola, R.; Hewitt, C.L.; Connell, S.D.; Gardner, J.P.A. Effects of vessel voyage speed on survival of biofouling organisms: Implications for translocation of non-indigenous marine species. Biofouling 2010, 26, 1–13. [Google Scholar] [CrossRef]
- Hopkins, G.A.; Forrest, B.M. A preliminary assessment of biofouling and non-indigenous marine species associated with commercial slow-moving vessels arriving in New Zealand. Biofouling 2010, 26, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.L.; Hewitt, C.L. Assessing the port to port risk of vessel movements vectoring non-indigenous marine species within and across domestic Australian borders. Biofouling 2011, 27, 631–644. [Google Scholar] [CrossRef] [PubMed]
- Clare, A.S.; Matsumura, K. Nature and perception of barnacle settlement pheromones. Biofouling 2000, 15, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Rittschof, D.; Cohen, J.H. Crustacean peptide and peptide-like pheromones and kairomones. Peptides 2004, 25, 1503–1516. [Google Scholar] [CrossRef] [PubMed]
- Pansch, C.; Jonsson, P.R.; Berglin, M.; Pinori, E.; Wrange, A. A new flow-through bioassay for testing low-emission antifouling coatings. Biofouling 2017, 8, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Rittschof, D.; Bransconb, E.S.; Castlow, J.D. Settlement and behavior in relation to flow and surface in larval barnacle, Balanus amphitrite Darwin. J. Exp. Mar. Biol. Ecol. 1984, 82, 131–146. [Google Scholar] [CrossRef]
- Rittschof, D.; Clare, A.S.; Gerhart, D.J.; Mary Sister, A.; Bonaventura, J. Barnacle in vitro assays for biologically active substances: Toxicity and settlement assays using mass cultured Balanus amphitrite amphitrite darwin. Biofouling 1992, 6, 115–122. [Google Scholar] [CrossRef]
- Kitamura, H. Methods of larval culture and water-flow assay for larval settlement of the barnacle, Balanus amphitrite. Sess. Org. 1999, 15, 15–21. [Google Scholar] [CrossRef]
- Yoshimura, E.; Nogata, Y.; Sakaguchi, I. Simple methods for mass culture of barnacle larvae. Sess. Org. 2006, 23, 39–42. [Google Scholar] [CrossRef]
- Aldred, N.; Scardino, A.; Cavaco, A.; Nys, R.D.; Clare, A.S. Attachment strength is a key factor in the selection of surfaces by barnacle cyprids (Balanus Amphitrite) during settlement. Biofouling 2010, 26, 287–299. [Google Scholar] [CrossRef]
- Chen, H.N.; Tdang, L.M.; Chong, V.C.; Chan, B.K.K. Worldwide genetic differentiation in the common fouling barnacle, Amphibalanus amphitrite. Biofouling 2014, 30, 1067–1078. [Google Scholar] [CrossRef] [PubMed]
- Kon-ya, K.; Miki, W. All-seasonal assay for antifouling substances using reared barnacle larvae. J. Mar. Biotechnol. 1994, 1, 193–195. [Google Scholar]
- Kon-ya, K.; Miki, W. Effect of environmental factors on larval settlement of the barnacle Balanus amphitrite reared in the laboratory. Fish. Sci. 1994, 60, 563–565. [Google Scholar] [CrossRef]
- Usami, M. Prevention of barnacle settlement by electro-conductive coating system. Sess. Org. 1998, 15, 1–4. [Google Scholar] [CrossRef]
- Kitano, Y.; Yokoyama, A.; Nogata, Y.; Shishima, K.; Yoshimura, E.; Chiba, K.; Tada, M.; Sakaguchi, I. Synthesis and anti-barnacle activities of novel 3-Isocyanotheonellin analogues. Biofouling 2003, 19, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Pettitt, M.E.; Henry, S.L.; Callow, M.E.; Callow, J.A.; Clare, A.S. Activity of commercial enzymes on settlement and adhesion of cypris larvae of the barnacle Balanus amphitrite, spores of the green alga Ulva linza, and the diatom Navicula perminuta. Biofouling 2004, 20, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, R.; Kobayashi, S.; Kameyama, Y.; Mizutani, M.; Komotori, J.; Katsuyama, I. Barnacle settlement behavior on controlled micro-textured surfaces. Zair. Kankyo 2009, 58, 302–307. (In Japanese) [Google Scholar] [CrossRef]
- Murosaki, T.; Noguchi, T.; Kakugo, A.; Putra, A.; Kurokawa, T.; Furukawa, H.; Osada, Y.; Gong, J.P.; Nogata, Y.; Matsumura, K.; et al. Antifouling activity of synthetic polymer gels against cyprids of the barnacle (Balanus amphitrite) in vitro. Biofouling 2009, 25, 313–320. [Google Scholar] [CrossRef]
- Petrone, L.; Fino, A.D.; Aldred, N.; Sukkaew, P.; Ederth, T.; Clare, A.S.; Liedberg, B. Effects of surface charge and Gibbs surface energy on the settlement behavior of barnacle cyprids (Balanus amphitrite). Biofouling 2011, 27, 1043–1055. [Google Scholar] [CrossRef]
- Guo, S.; Lee, H.P.; Teo, S.L.M.; Khoo, B.C. Inhibition of barnacle cyprid settlement using low frequency and intensity ultrasound. Biofouling 2012, 28, 131–141. [Google Scholar] [CrossRef]
- Maleschlijski, S.; Bauer, S.; Fino, A.D.; Sendra, G.H.; Clare, A.S.; Rosenhahn, A. Barnacle cyprid motility and distribution in the water column as an indicator of the settlement-inhibiting potential of nontoxic antifouling chemistries. Biofouling 2014, 30, 1055–1065. [Google Scholar] [CrossRef] [PubMed]
- Katsuyama, I.; Kado, R.; Kominami, H.; Kitamura, H. A screening method for the test substances on attachment using larval barnacle, Balanus amphitrite, in the laboratory. Mar. Fouling 1992, 9, 13–14. [Google Scholar] [CrossRef]
- Kojima, R.; Kobayashi, S.; Satuito, C.G.P.; Katsuyama, I.; Ando, H.; Seki, Y.; Senda, T. A method for evaluating the efficacy of antifouling paints using Mytilus galloprovincialis in the laboratory in a flow-through system. PLoS ONE 2016, 11, e0168172. [Google Scholar] [CrossRef] [PubMed]
- IMO. Resolution MSC.215 (82): Performance Standards for Protection Coatings for Dedicated Seawater Ballast Tanks in All Types of Ships and Double-Side Skin Spaces of Bulk Carriers. 82/24/Add.1, ANNEX 1; IMO: London, UK, 2006. [Google Scholar]
- Kado, R.; Hirano, R. Rearing methods of planktonic larvae of marine sessile animals. Mar. Fouling 1979, 1, 11–19. [Google Scholar] [CrossRef]
- Kitamurta, H.; Nakashima, Y. Influence of storage temperatures and period on settlement rate and substrate discrimination in cyprids of the barnacle Balanus amphitrite. Fish. Sci. 1996, 62, 998–999. [Google Scholar] [CrossRef]
- Sakaguchi, I. Bivavle. In Attaching Organisms and Aquaculture; Kajihara, T., Ed.; Kouseishya Kouseikaku Press: Tokyo, Japan, 2007; Chapter 9; pp. 100–107. (In Japanese) [Google Scholar]
- Tomoda, K.; Ninomiya, K.; Azuma, K.; Suzuki, T.; Satuito, C.G.; Kitamura, H. Larval settlement of the barnacle Amphibalanus amphitrite on coal-flyash concrete plates in the laboratory. Sess. Org. 2008, 25, 25–29. [Google Scholar] [CrossRef]
- Nogata, Y.; Tokikuni, N.; Yoshimura, E.; Sato, K.; Endo, N.; Matsumura, K.; Sugita, H. Salinity limitation on larval settlement of four barnacle species. Sess. Org. 2011, 28, 47–54. [Google Scholar] [CrossRef]
- Kawahara, H.; Tamura, R.; Ajioka, S.; Shizuri, Y. Convenient assay for settlement inducing substances of barnacles. Mar. Biotechnol. 1999, 1, 98–101. [Google Scholar] [CrossRef]
- Dahms, H.U.; Jin, T.; Qian, P.T. Adrenoceptor compounds prevent the settlement of marine invertebrate larvae: Balanus amphitrite (Cirripedia), Bugula neritina (Bryozoa), and Hydroides elegans (Plychaeta). Biofouling 2004, 20, 313–321. [Google Scholar] [CrossRef]
- Zhou, X.; Xu, Y.; Jin, C.; Qian, P.Y. Reversible anti-settlement activity against Amphibalanus (=Balanus) amphitrite, Bulgula neritina, and Hydroides elegans by a nontoxic pharamaceutical compounds, mizolastine. Biofouling 2009, 25, 739–747. [Google Scholar] [CrossRef]
- ISO. ISO 8486-1:1996 Bonded Abrasives—Determination and Designation of Grain Size Distribution—Part 1: Macrogrits F4 to F220; ISO: Geneva, Switzerland, 1996. [Google Scholar]
- Japanese Standard Association. Bonded Abrasives—Determination and Designation of Grain Size Distribution—Part 1: Macrogrits F4 to F220; JIS R 6001-1; Japanese Standard Association: Tokyo, Japan, 2017. [Google Scholar]
- Qiu, J.W.; Thiyagarajan, V.; Cheung, S.; Qian, P.Y. Toxic effects of copper on larval development of the barnacle Balanus amphitrite. Mar. Pollut. Bull. 2005, 51, 688–693. [Google Scholar] [CrossRef] [PubMed]
- Khandepaker, L.; Anil, A.C. Underwater adhesion: The barnacle way. Int. J. Adhes. Adhes. 2007, 27, 165–172. [Google Scholar] [CrossRef]
- Aldred, N.; Clare, A.S. The adhesion strategies of cyprids and development of barnacle-resistant marine coatings. Biofouling 2008, 24, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Weiss, C.M. The comparative tolerance of some fouling organisms to copper and mercury. Biol. Bull. 1947, 93, 56–63. [Google Scholar] [CrossRef]
- Rao, Y.P.; Devi, V.U.; Rao, D.G.V.P. Copper toxicity in tropical barnacles, Balanus amphitrite and Balanus tintinnabulum tintinnabulum. Water Air Soil Pollut. 1985, 27, 109–115. [Google Scholar] [CrossRef]
- Rainbow, P.S.; Blackmore, G.; Wang, W.X. Effects of previous field-exposure history on the uptake of trace metals from water and food by the barnacle Balanus amphitrite. Mar. Ecol. Prog. Ser. 2003, 259, 201–213. [Google Scholar] [CrossRef]
- Rainbow, P.S. Trace metal bioaccumulation: Models, metabolic availability and toxicity. Environ. Int. 2007, 33, 576–582. [Google Scholar] [CrossRef]
- Rainbow, P.S. Phylogeny of trace metal accumulation in crustaceans. In Metal Metabolism in Aquatic Environment; Langston, W.J., Bebianno, M.J., Eds.; Chapman & Hall: London, UK, 1998; pp. 285–319. [Google Scholar]
- Walker, G. Copper granules in the barnacle Balanus balanoides. Mar. Biol. 1997, 39, 343–349. [Google Scholar] [CrossRef]
- Rainbow, P.S. Heavy metals in barnacles. In Barnacle Biology; Southward, A.J., Ed.; A. A. Balkema: Rotterdam, The Netherlands, 1987; pp. 405–417. [Google Scholar]
- Yang, J.L.; Satuito, C.G.; Bao, W.Y.; Kitamura, H. Induction of metamorphosis of pediveliger larvae of the mussel Mytilus galloprovincialis Lamarck, 1819 using neuroactive compounds, KCl, NH4Cl and organic solvents. Biofouling 2008, 24, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Dubilier, N. H2S-A settlement cure or a toxic substance for Cplitella sp. I Larvae? Biol. Bull. 1998, 174, 30–38. [Google Scholar] [CrossRef]
- Ketchum, B.H.; Ferry, J.D.; Redfield, A.C. Evaluation of antifouling paints by leaching rate determinations. Ind. Eng. Chem. 1945, 37, 456–460. [Google Scholar] [CrossRef]
- Berntsson, K.M.; Jonson, P.R. Temporal and spatial patterns in recruitment and succession of a temperate marine fouling assemblage: A comparison of static panels and boat hulls during the boating season. Biofouling 2003, 19, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Gatley-Montross, C.M.; Finlay, J.A.; Aldred, N.; Cassady, H.; Destino, J.F.; Orihuela, B.; Hickner, M.A.; Clare, A.S.; Rittschof, D.; Holm, E.R.; et al. Multivariate analysis of attachment of biofouling organisms in response to material surface characteristics. Biointerphases 2017, 12, 051003. [Google Scholar] [CrossRef] [PubMed]

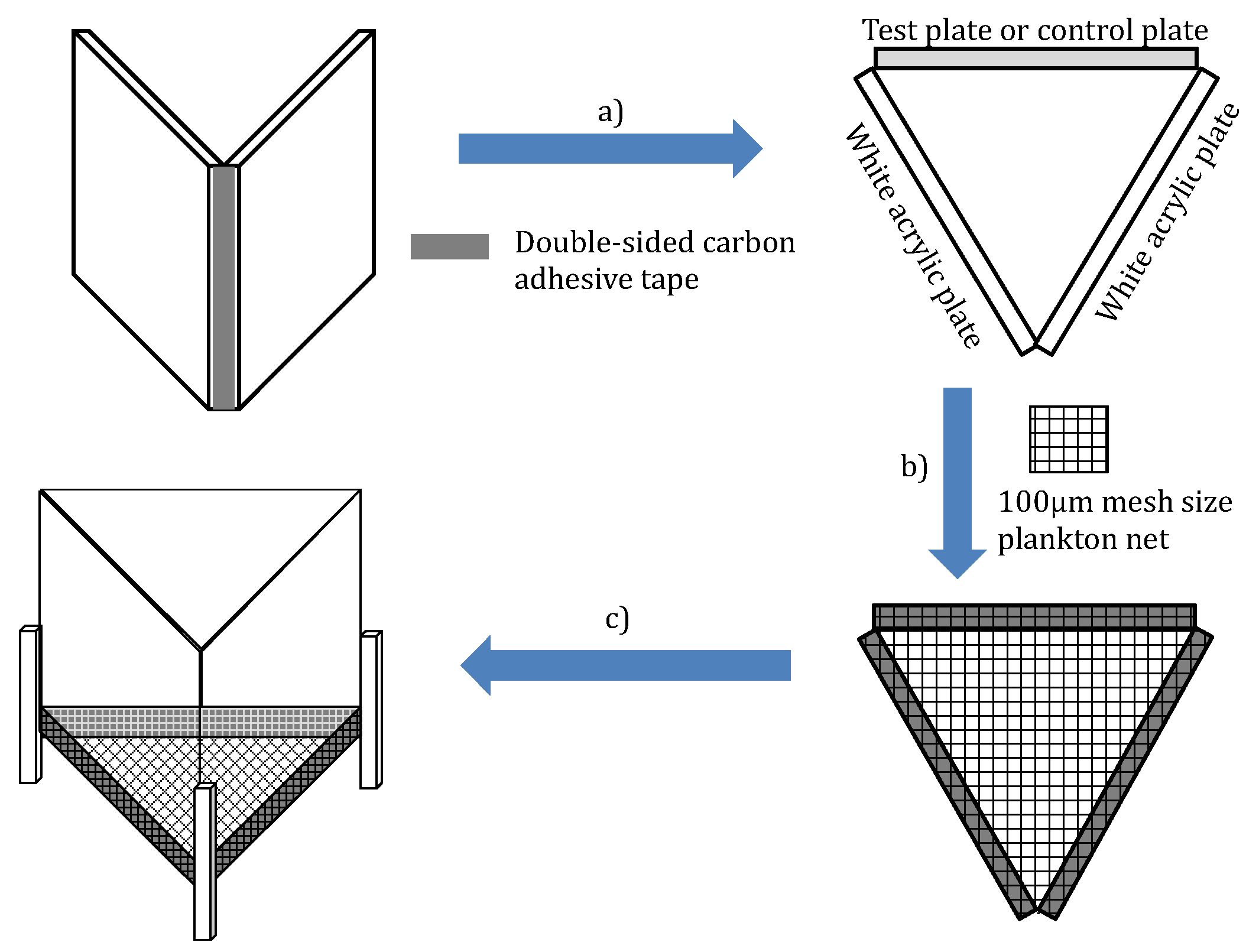
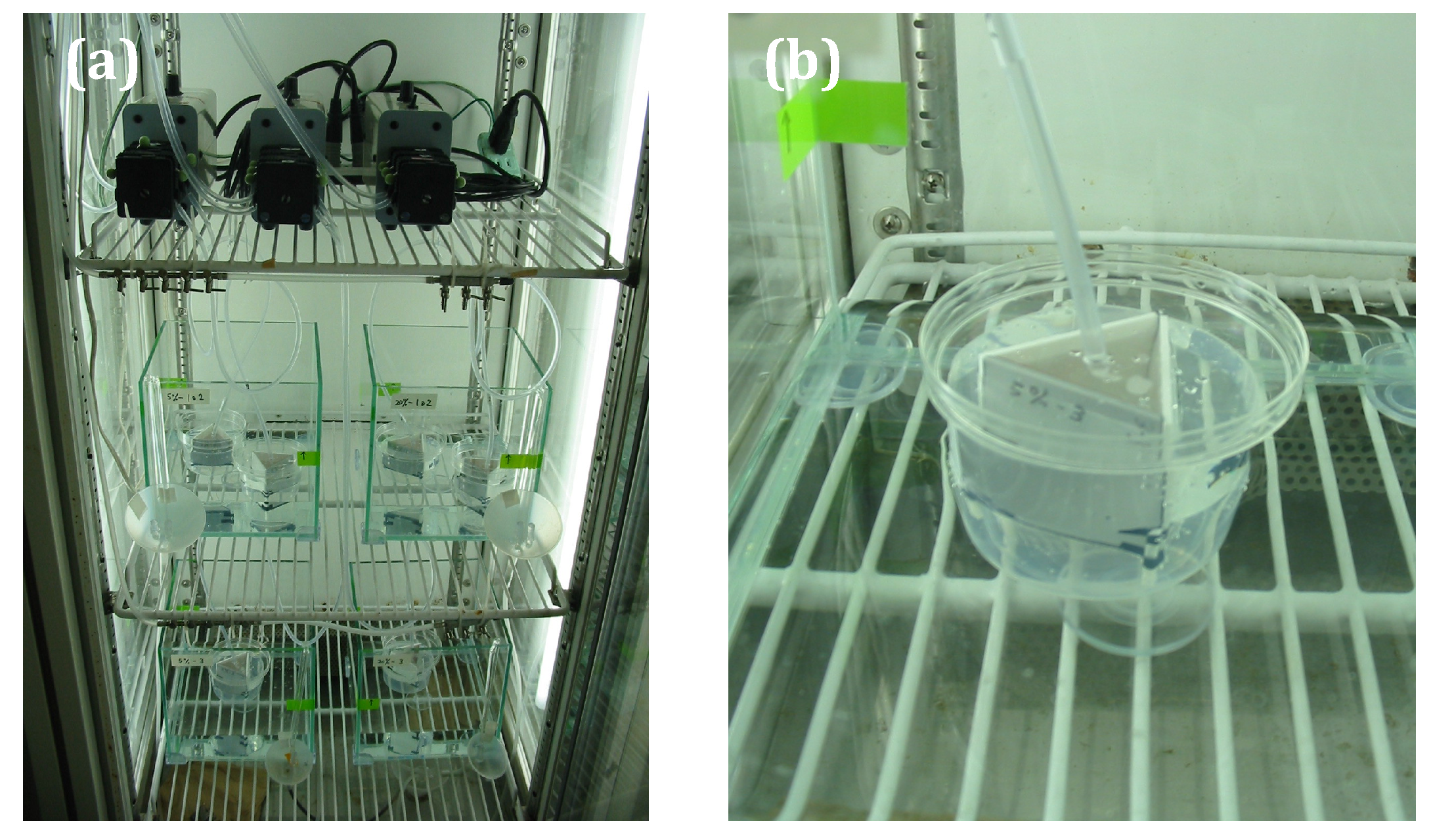
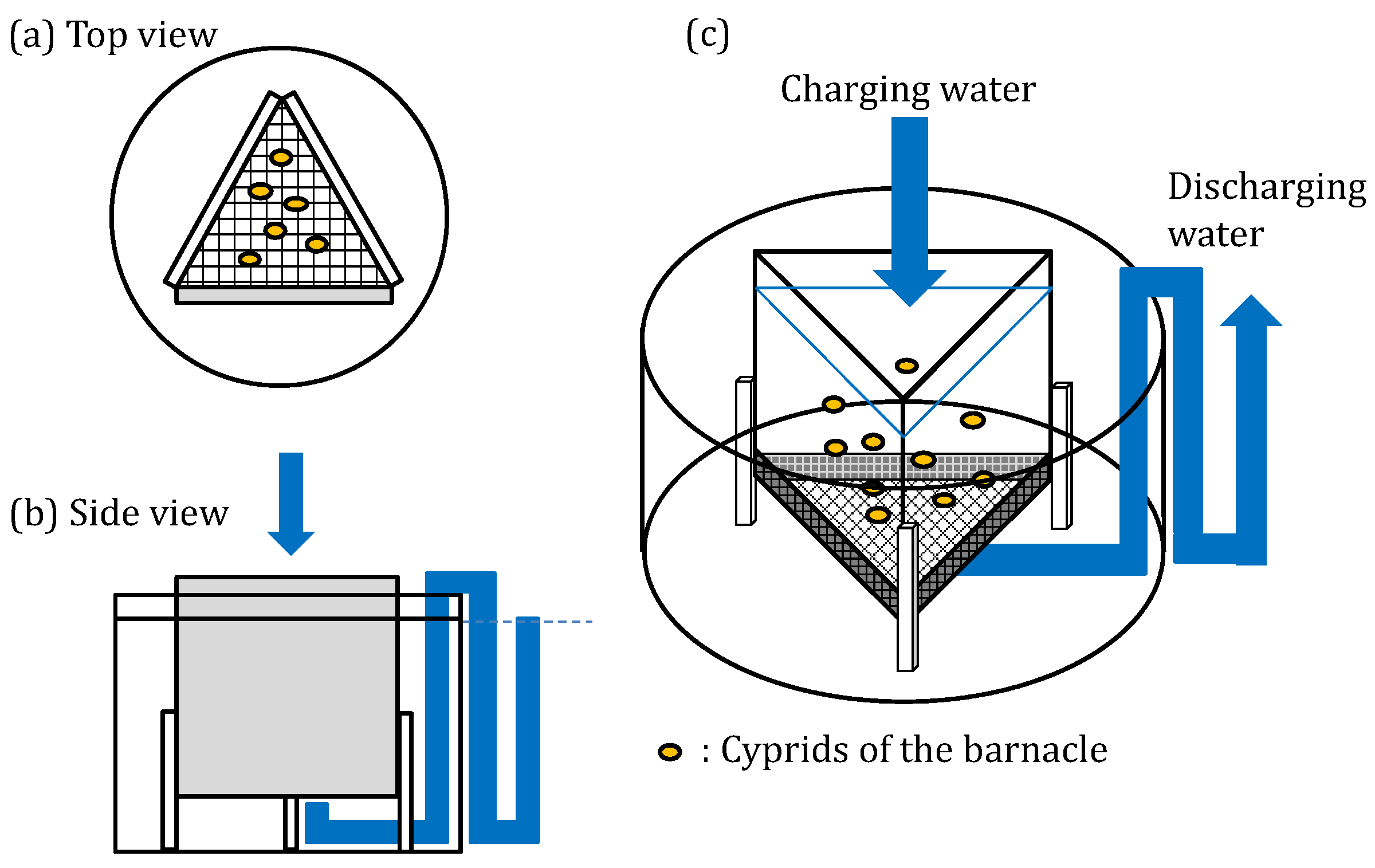
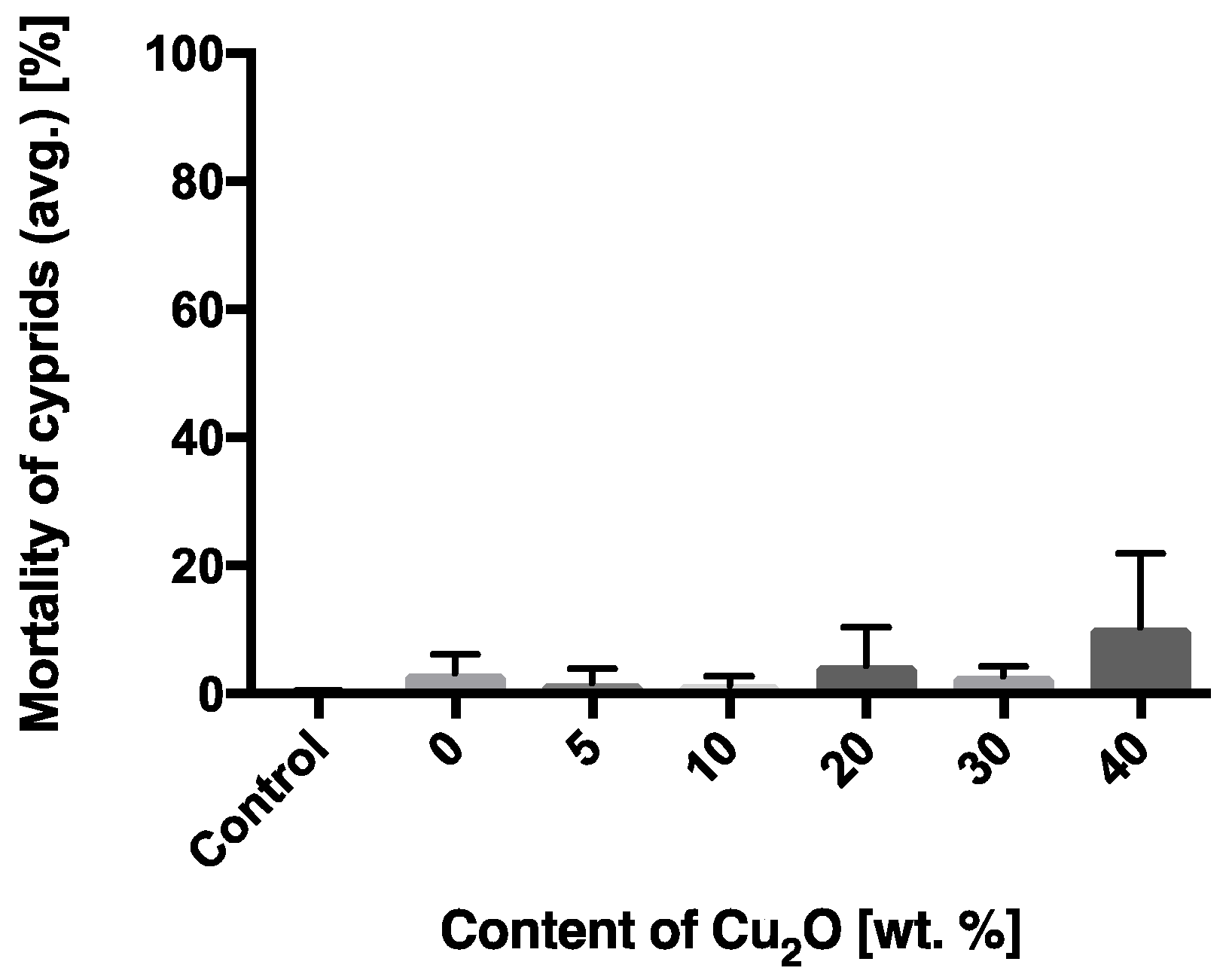

| Composition/Paint Name | A-0 | A-1 | A-2 | A-3 | A-4 | A-5 |
|---|---|---|---|---|---|---|
| Cuprous oxide | 0 | 5 | 10 | 20 | 30 | 40 |
| Xylene | 23 | 23.6 | 24 | 25 | 26 | 27 |
| Methylisobutylketone | 5 | 5 | 5 | 5 | 5 | 5 |
| Base polymer | 9 | 8.7 | 8.5 | 8 | 7.5 | 7 |
| Rosin | 9 | 8.7 | 8.5 | 8 | 7.5 | 7 |
| Barium sulphate | 50 | 45 | 40 | 30 | 20 | 10 |
| Anhydrous ferric oxide | 1 | 1 | 1 | 1 | 1 | 1 |
| Oxidized polyethylene wax | 1 | 1 | 1 | 1 | 1 | 1 |
| Amide wax | 2 | 2 | 2 | 2 | 2 | 2 |
| Condition | Remarks |
|---|---|
| Seawater | 0.22 μm filtered seawater (Nylon filter membrane, Merck) with the salinity adjusted to 28 using purified water |
| Density of larvae | 2 to 3 larvae per 1 mL |
| Diet and density | The diatom Chaetoceros gracilis (200,000 to 400,000 cells/mL), other diatoms can also be used, such as Skeletonema costatum (1,000,000 to 2,000,000 cells/mL) |
| Antibiotics | Streptomycin sulfate (30 μg/mL), Penicillin G sodium salt (20 μg/mL) |
| Water temperature | 25 ± 1 °C |
| Light | The light intensity is approximately 3000 lux., and the photoperiod was 12 h. light, 12 h. dark period |
| Aeration | Approximately 20 mL/min. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kojima, R.; Kobayashi, S.; Matsumura, K.; Satuito, C.G.P.; Seki, Y.; Ando, H.; Katsuyama, I. Designing a Laboratory Bioassay for Evaluating the Efficacy of Antifouling Paints on Amphibalanus amphitrite Using a Flow-Through System. Coatings 2019, 9, 112. https://doi.org/10.3390/coatings9020112
Kojima R, Kobayashi S, Matsumura K, Satuito CGP, Seki Y, Ando H, Katsuyama I. Designing a Laboratory Bioassay for Evaluating the Efficacy of Antifouling Paints on Amphibalanus amphitrite Using a Flow-Through System. Coatings. 2019; 9(2):112. https://doi.org/10.3390/coatings9020112
Chicago/Turabian StyleKojima, Ryuji, Seiji Kobayashi, Kiyotaka Matsumura, Cyril Glenn Perez Satuito, Yasuyuki Seki, Hirotomo Ando, and Ichiro Katsuyama. 2019. "Designing a Laboratory Bioassay for Evaluating the Efficacy of Antifouling Paints on Amphibalanus amphitrite Using a Flow-Through System" Coatings 9, no. 2: 112. https://doi.org/10.3390/coatings9020112





