Preparation and Performance Optimization of Original Aluminum Ash Coating Based on Plasma Spraying
Abstract
:1. Introduction
2. Experiments
2.1. Raw Materials
2.2. Preparation of Original Aluminum Ash Spray Powder
2.3. Preparation of Original Aluminum Ash Coating
2.4. Experiment Equipment and Testing Methods
3. Results and Discussion
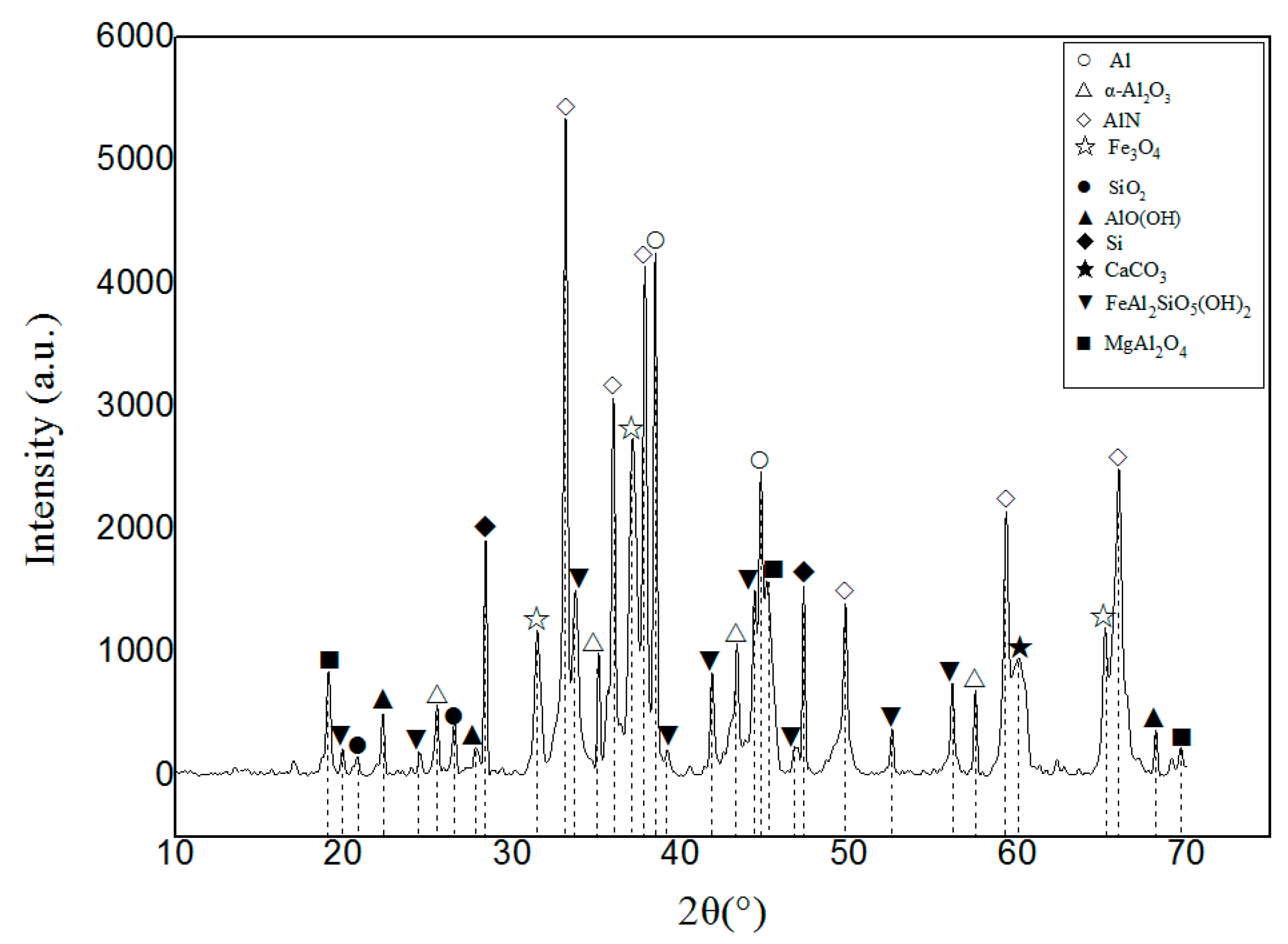
3.1. Characterization and Evaluation of Original Aluminum Ash Spray Powder
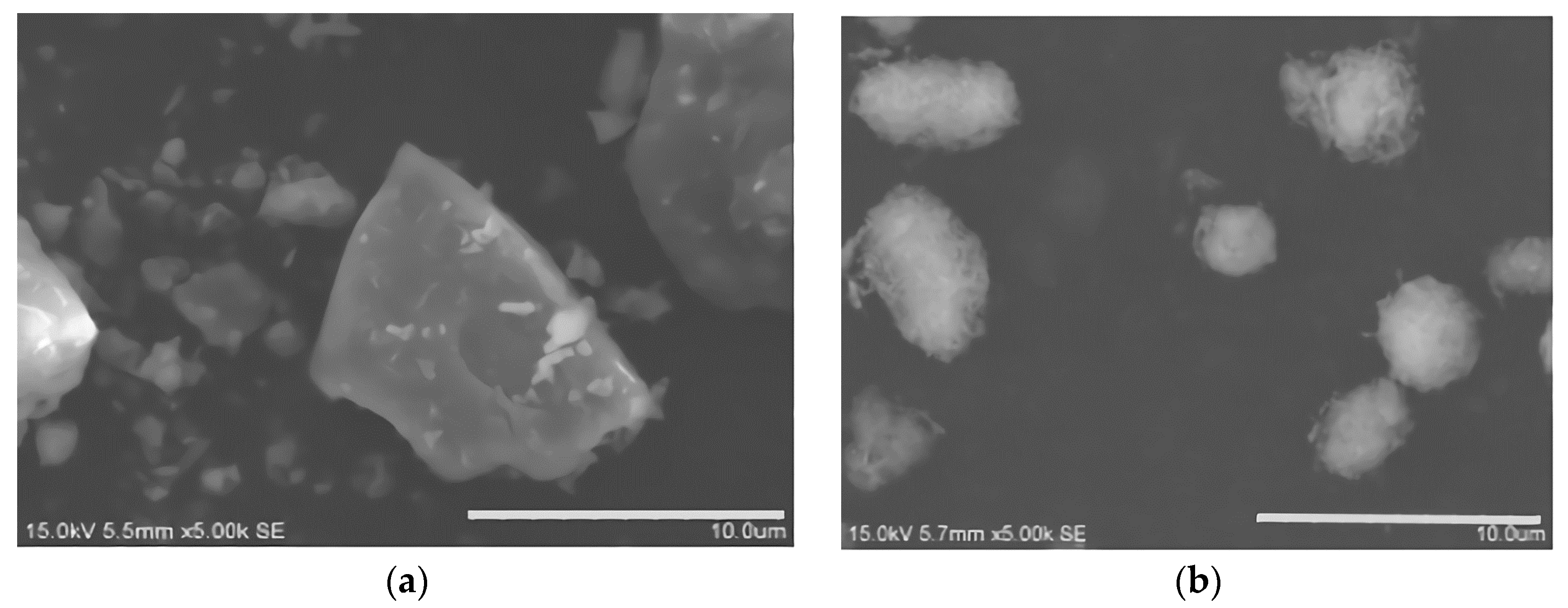
3.1.1. Observation and Analysis of Microstructure
3.1.2. Composition Analysis
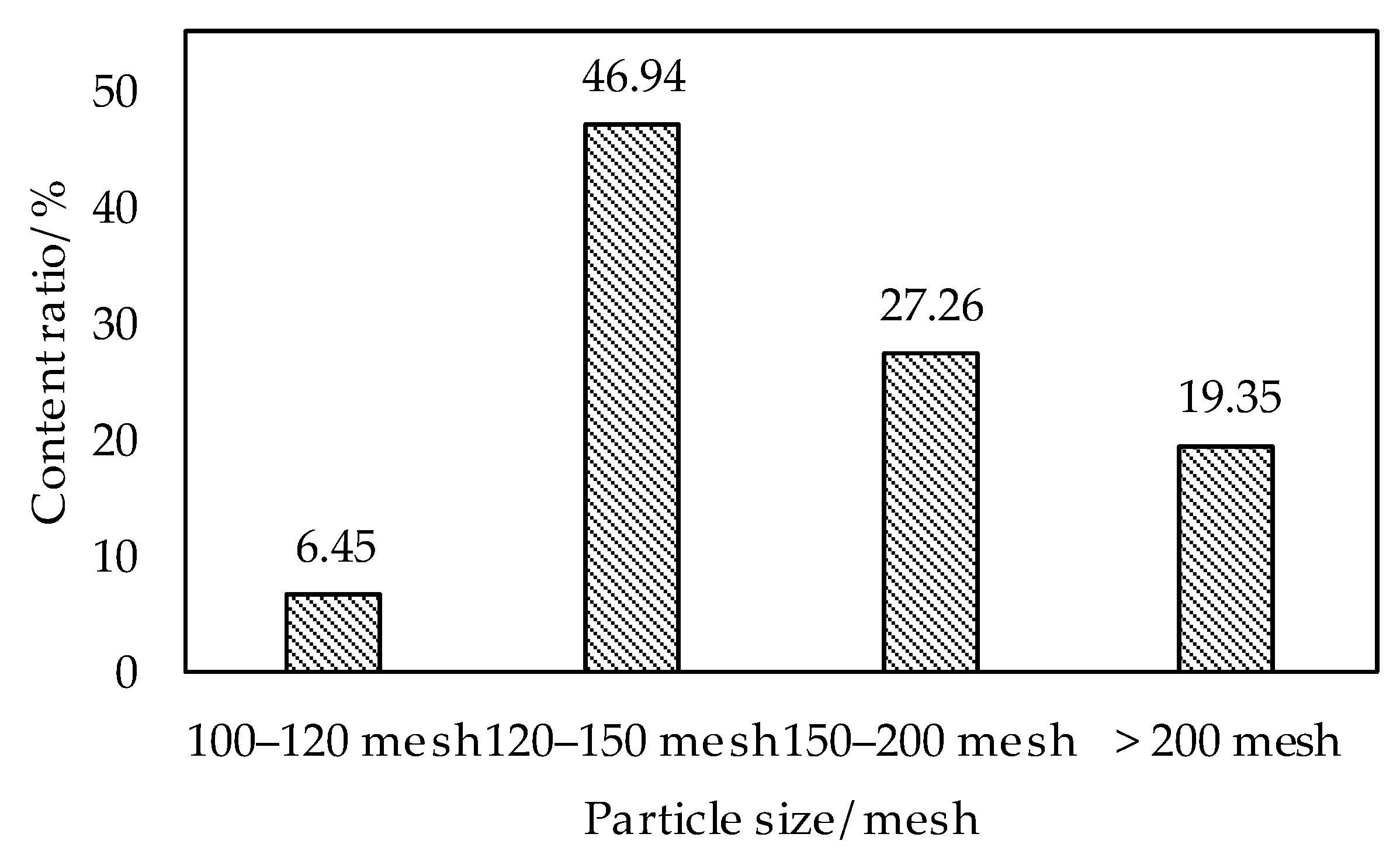
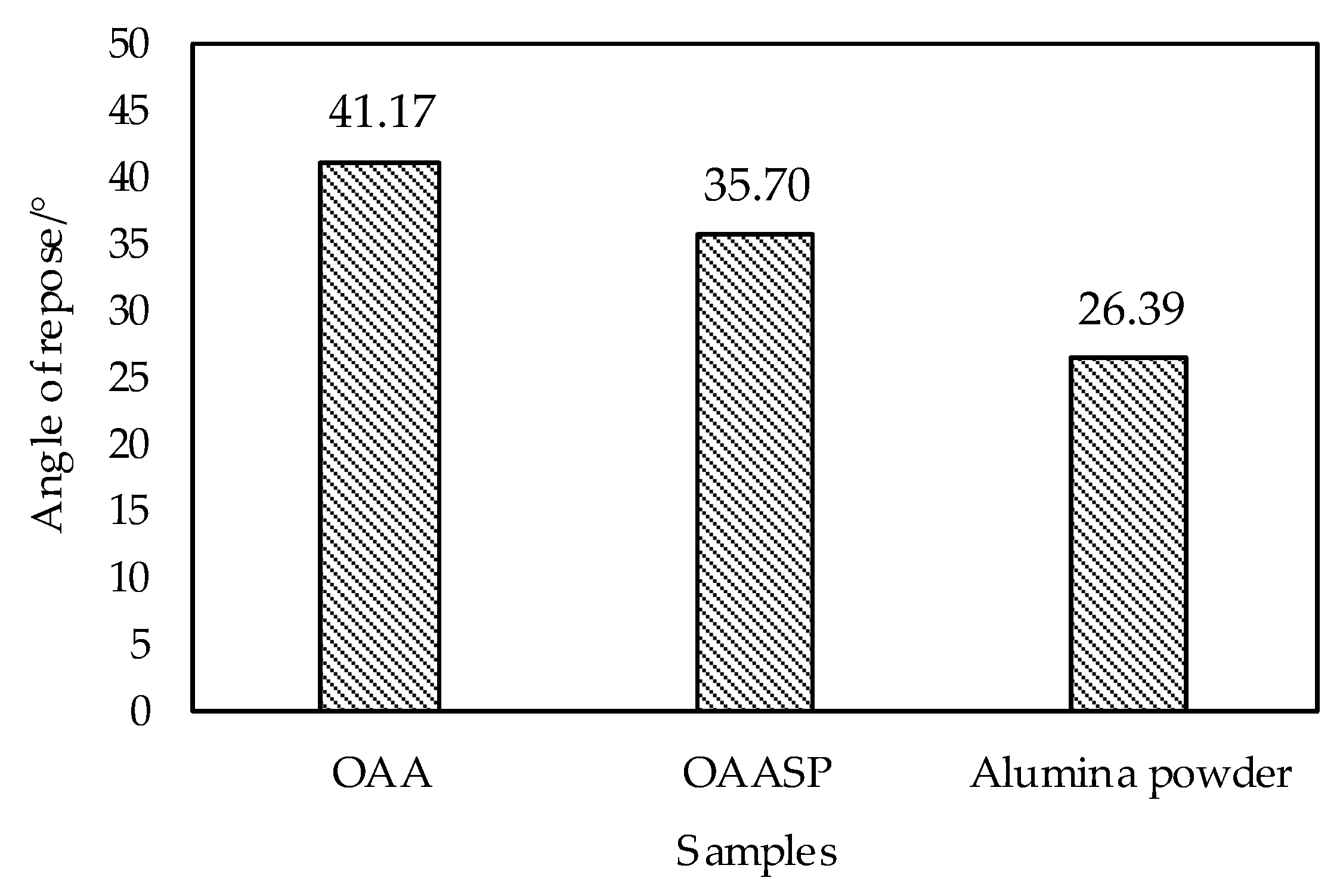
3.1.3. Evaluation of Flowability
3.2. Microstructure Analysis of Original Aluminum Ash Coating
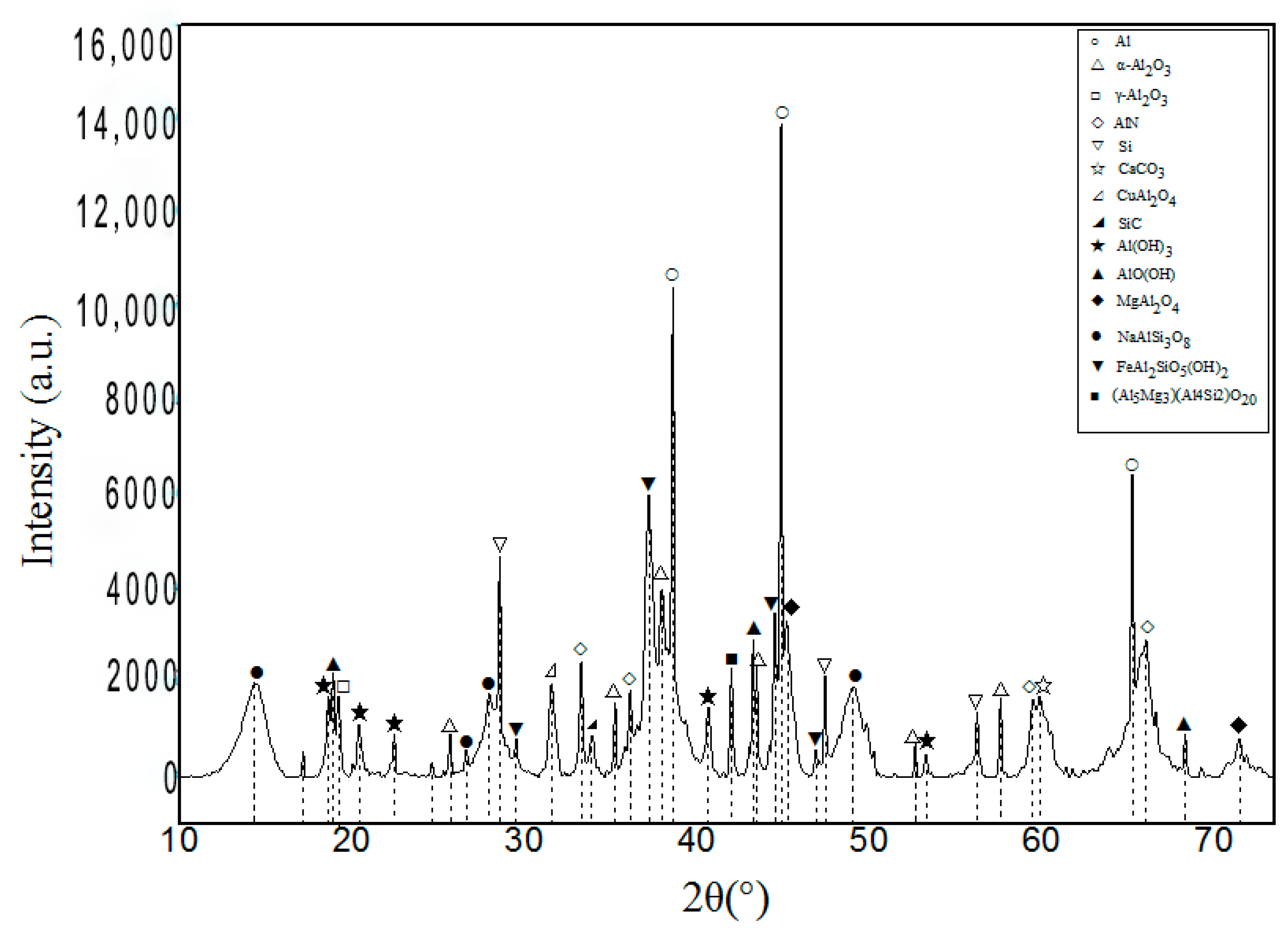
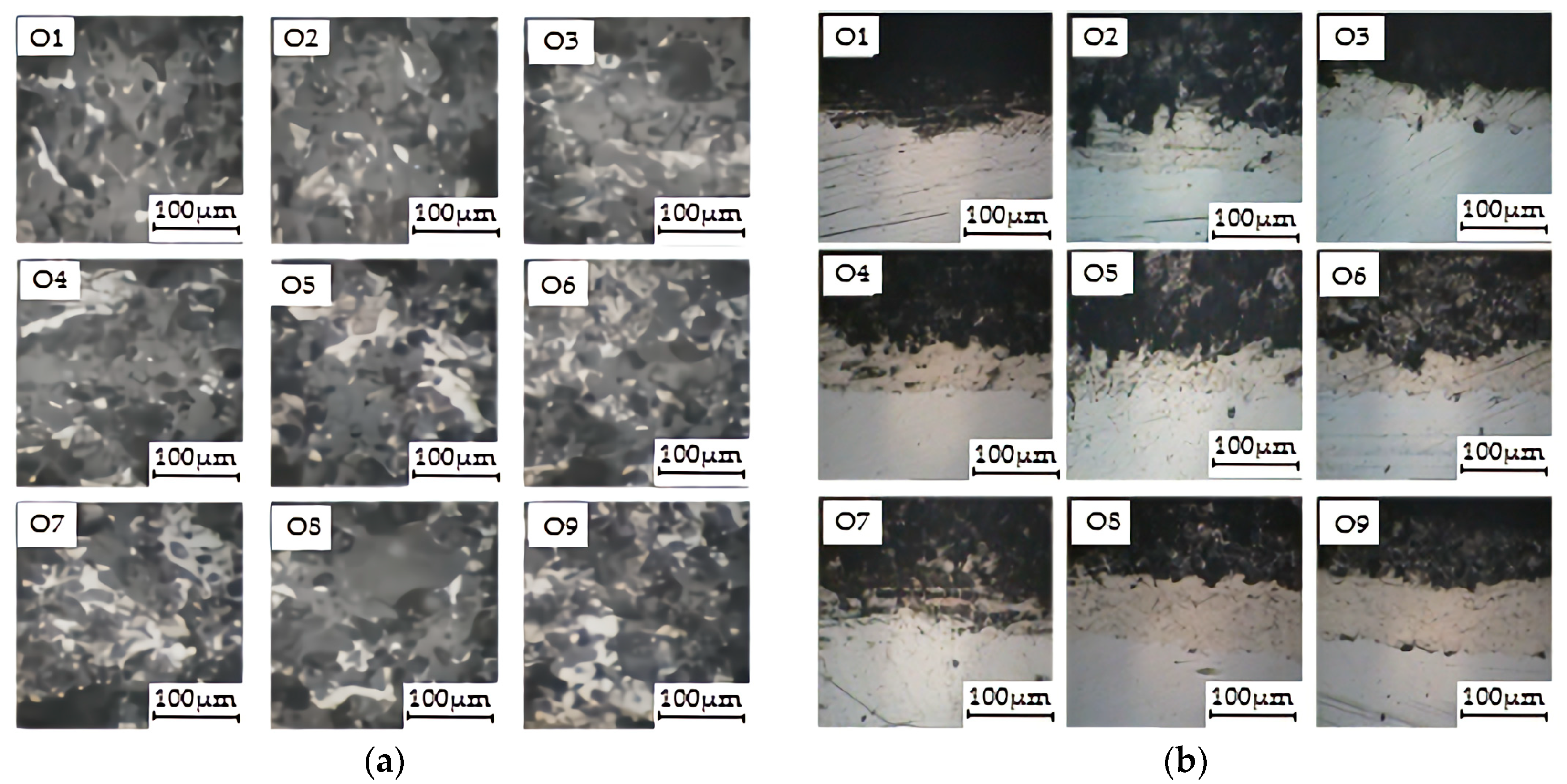
3.2.1. Microstructure of Coating
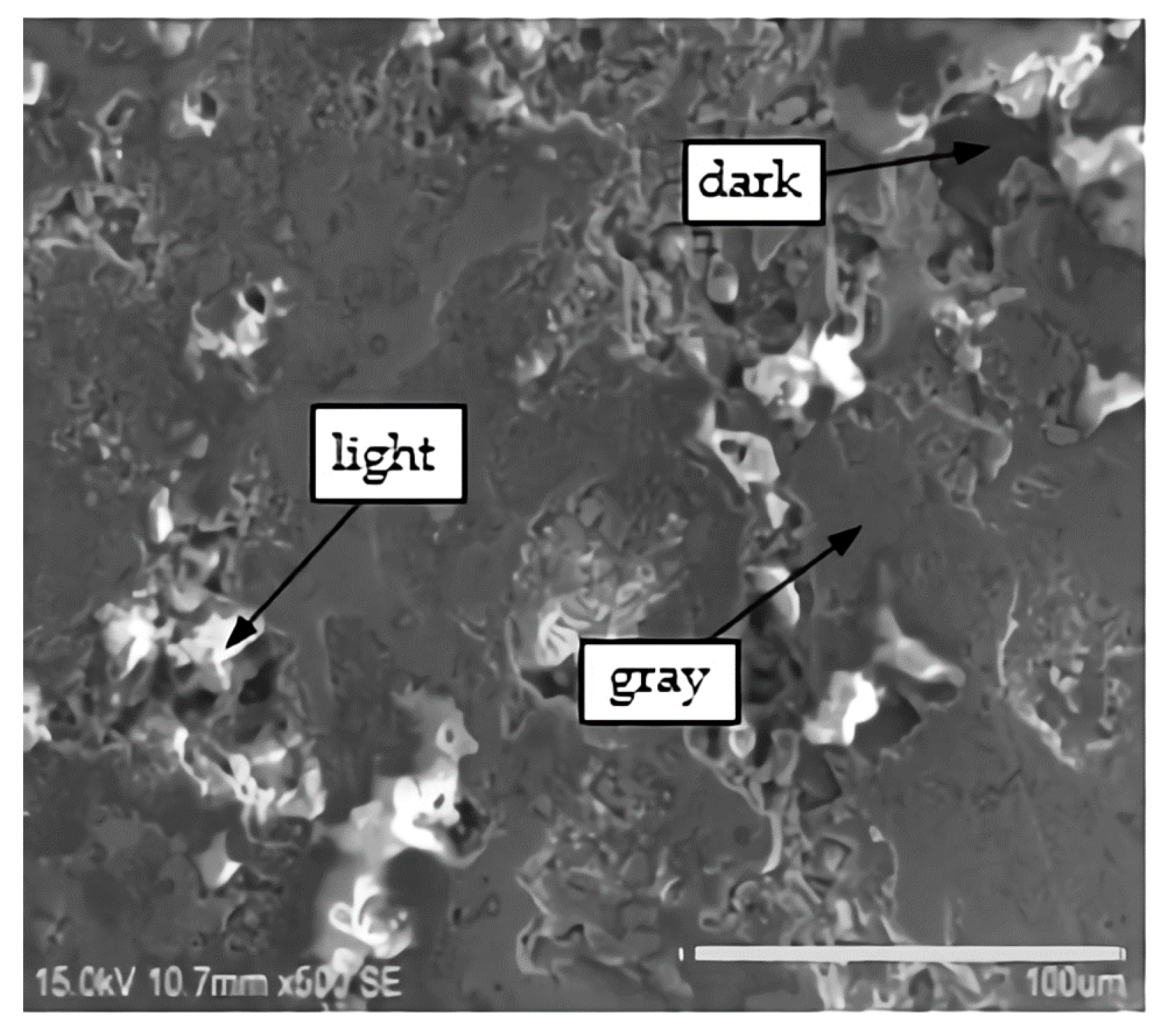
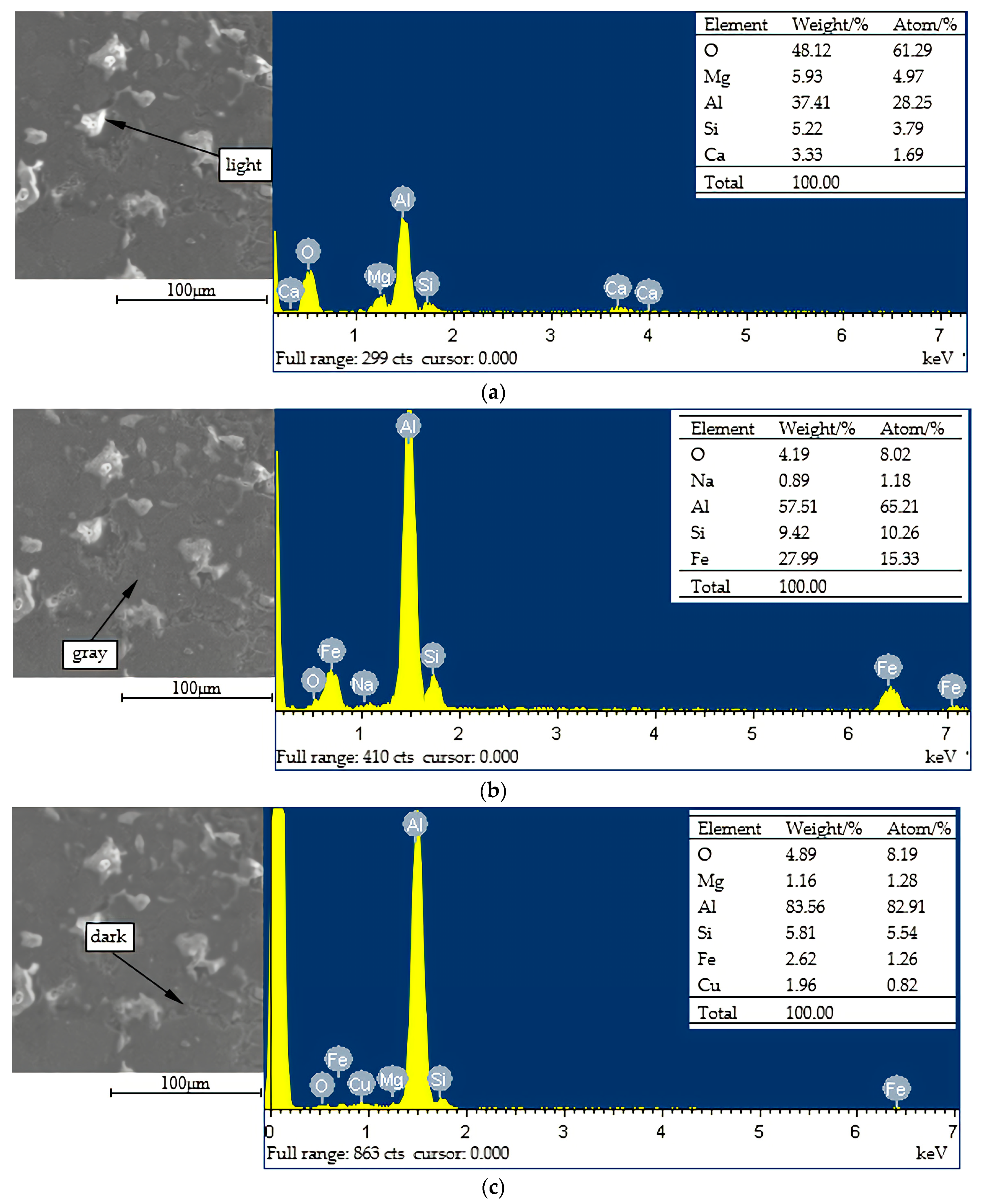
3.2.2. Energy Spectrum Analysis
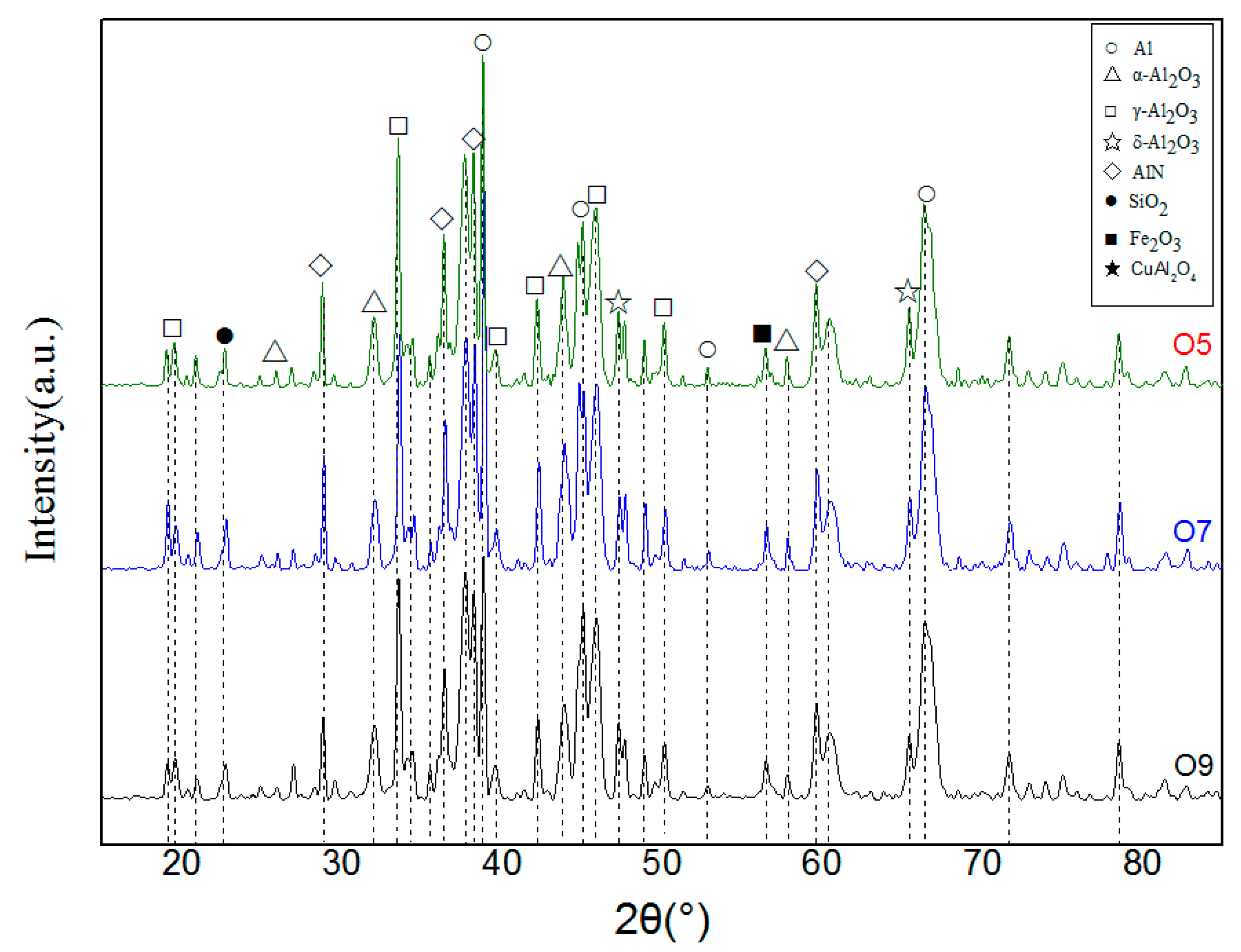
3.2.3. Phase Analysis
3.3. Coating Performance Optimization
3.3.1. Orthogonal Experiment
3.3.2. Optimization of Alumina Content in OAASP
4. Conclusions
- The plasma spray powder was prepared by OAA after hydrolyzing and ball milling; the roundness of the particles was improved; the AlN content was reduced and the angle of repose of OAASP was 32.19°. Compared with OAA, the angle of repose was reduced by 13.28%, and the flowability of powder was higher, which met the requirements of the plasma spraying.
- The sorts of the phases of the OAAC were greatly reduced, and the main phases were Al and different forms of Al2O3. Some regions with different brightness were formed on the surface of the coating. While the hardness of disparate regions differed, the hardness of the light region was the highest. Therefore, during the spraying process, the formation of dark areas should be averted in order to improve the hardness of the coating.
- The primary and secondary order of the influence of the spraying process parameters on the comprehensive performance of the OAAC was determined by the orthogonal experiment, i.e., the spray current > spray voltage > the main gas flow > the powder flow rate. The preferred spraying parameters were: spraying current 600 A, spraying voltage 60 V, main gas flow 33 slpm, and powder flow rate 22 g/min.
- The amount of alumina in the spray powder had a large effect on the properties of the coating. Therefore, in order to improve the quality of the aluminum ash coating, the spray powder can be purified or an appropriate amount of alumina can be added to the spray powder to increase the alumina content.
- The spray process parameters were optimized by orthogonal experiments, but simply four factors were considered in our research. In the future, factors like spray distance and spray angle can be taken into account to obtain an optimal solution.
5. Patents
Author Contributions
Funding
Conflicts of Interest
References
- Wu, C.Y.; Yu, H.F.; Zhang, H.F. Extraction of aluminum by pressure acid-leaching method from coal fly ash. Trans. Nonferrous Met. Soc. China 2012, 22, 2282–2288. [Google Scholar] [CrossRef]
- Li, X.L.; Ou, Y.J.; Li, C.L.; Zhu, J.K.; Zhi, P.G. Preparation of Alumina from Aluminum Ash by Sintering with Sodium Hydroxide; IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2018; Volume 233. [Google Scholar]
- Aubert, J.E.; Husson, B.; Vaquier, A. Metallic aluminum in MSWI fly ash: Quantification and influence on the properties of cement-based products. Waste Manag. 2004, 24, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, X.; Zhao, X.; Tang, Y.; Zuo, Y. Preparation of Ti–Zr-based conversion coating on 5052 aluminum alloy, and its corrosion resistance and antifouling performance. Coatings 2018, 8, 397. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, Y.Q.; Zhu, W.S.; Huang, J.B. Environmental impact assessment of China’s primary aluminum based on life cycle assessment. Trans. Nonferrous Met. Soc. China 2019, 29, 1784–1792. [Google Scholar] [CrossRef]
- Chen, Z.L.; Tang, M.H.; Lu, S.Y.; Alfons, B.; Ding, J.M.; Qiu, Q.L.; Yan, J.H. Mechanochemical degradation of PCDD/Fs in fly ash within different milling systems. Chemosphere 2019, 223, 188–195. [Google Scholar]
- Li, B.H.; Deng, Z.Y.; Wang, W.X.; Fang, H.S.; Zhou, H.B.; Deng, F.X.; Huang, L.; Li, H.Y. Degradation characteristics of dioxin in the fly ash by washing and ball-milling treatment. J. Hazard. Mater. 2017, 339, 191–199. [Google Scholar] [CrossRef]
- Arumugam, N.; Amirhomayoun, S.; Takayuki, S. Hydrogen gas generation from metal aluminum-water interaction in municipal solid waste incineration (MSWI) bottom ash. Waste Manag. 2018, 73, 342–350. [Google Scholar]
- Arumugam, N.; Nithiya, A.; Takayuki, S. Aluminum and aluminum alloys in municipal solid waste incineration (MSWI) bottom ash: A potential source for the production of hydrogen gas. Int. J. Hydrogen Energy 2016, 41, 820–831. [Google Scholar]
- Vareda, J.P.; Valente, A.J.M.; Luisa, D. Assessment of heavy metal pollution from anthropogenic activities and remediation strategies: A review. J. Environ. Manag. 2019, 246, 101–118. [Google Scholar] [CrossRef]
- Abyzov, V.A. Refractory cellular concrete based on phosphate binder from waste of production and recycling of aluminum. Procedia Eng. 2017, 206, 783–789. [Google Scholar] [CrossRef]
- Abyzov, V.A. Lightweight refractory concrete based on aluminum-magnesium-phosphate binder. Procedia Eng. 2016, 150, 1440–1445. [Google Scholar] [CrossRef]
- Zhan, D.P.; Zhang, H.S.; Jiang, Z.H. Effects of AIMnCa and AIMnFe alloys on deoxidization of low carbon and low silicon aluminum killed steels. J. Iron Steel Res. Int. 2008, 15, 15–18. [Google Scholar] [CrossRef]
- Zhang, G.H.; Chou, K.C. Deoxidation of molten steel by aluminum. J. Iron Steel Res. Int. 2015, 22, 905–908. [Google Scholar] [CrossRef]
- Peng, H.L.; Zhong, S.X.; Xiang, J.X.; Lin, Q.T.; Yao, C.; Dong, J.H.; Yin, G.C.; Yao, K.; Zeng, S.Y.; Zhong, J. Characterization and secondary sludge dewatering performance of a novel combined aluminum-ferrous-starch flocculant (CAFS). Chem. Eng. Sci. 2017, 173, 335–345. [Google Scholar] [CrossRef]
- Lin, Q.T.; Peng, H.L.; Zhong, S.X.; Xiang, J.X. Synthesis, characterization, and secondary sludge dewatering performance of a novel combined silicon–aluminum–iron–starch flocculant. J. Hazard. Mater. 2015, 285, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Kachalova, G.S. Modern Coagulants and Flocculants in the Cleaning of Washing Waters of Water Treatment Plants; IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2018; Volume 451. [Google Scholar]
- Tripathy, A.K.; Mahalik, S.; Sarangi, C.K.; Tripathy, B.C.; Sanjay, K.; Bhattacharya, I.N. A pyro-hydrometallurgical process for the recovery of alumina from waste aluminium dross. Miner. Eng. 2019, 137, 181–186. [Google Scholar] [CrossRef]
- Ramaswamy, P.; Ranjit, S.; Bhattacharjee, S.; Gomes, S.A. Synthesis of high temperature (1150 °C) resistant materials after extraction of oxides of Al and Mg from aluminum dross. Mater. Proc. 2019. [Google Scholar] [CrossRef]
- Leiva, C.; Galiano, Y.L.; Arenas, C.; Fariñas, B.A.; Pereira, F. A porous geopolymer based on aluminum-waste with acoustic properties. Waste Manag. 2019, 95, 504–512. [Google Scholar] [CrossRef]
- Dirisu, J.O.; Fayomi, O.S.I.; Oyedepo, S.O.; Jolayemi, K.J.; Moboluwarin, D.M. Critical evaluation of aluminium dross composites and other potential building ceiling materials. Procedia Manuf. 2019, 35, 1205–1210. [Google Scholar] [CrossRef]
- Echarri-Iribarren, V.; Echarri-Iribarren, F.; Rizo-Maestre, C. Ceramic panels versus aluminium in buildings: Energy consumption and environmental impact assessment with a new methodology. Procedia Manuf. 2019, 233, 959–974. [Google Scholar] [CrossRef]
- Sobolev, A.; Kossenko, A.; Zinigrad, M.; Borodianskiy, K. An investigation of oxide coating synthesized on an aluminum alloy by plasma electrolytic oxidation in molten salt. Appl. Sci. 2017, 7, 889. [Google Scholar] [CrossRef]
- Takajo, S.; Hollis, K.J.; Cummins, D.R.; Tegtmeier, E.L.; Dombrowski, D.E.; Vogel, S.C. Texture evolution in U-10Mo nuclear fuel foils during plasma spray coating with Zr. Quantum Beam Sci. 2018, 2, 12. [Google Scholar] [CrossRef]
- Sanjai, S.G.; Anathakrishna, B.; Sumanth, M.S.; Srideep, S.; Ramaswamy, P. Process development to synthesize plasma sprayable powders from nano alumina ceramic powders. Mater. Today Proc. 2019. [Google Scholar] [CrossRef]
- Rahmati, M.; Raeissi, K.; Toroghinejad, M.R.; Hakimizad, A.; Santamaria, M. Effect of pulse current mode on microstructure, composition and corrosion performance of the coatings produced by plasma electrolytic oxidation on AZ31 Mg alloy. Coatings 2019, 9, 688. [Google Scholar] [CrossRef]
- He, L.; Zhao, Y.; Xing, L.; Liu, P.; Zhang, Y.; Wang, Z. Low infrared emissivity coating based on graphene surface-modified flaky Aluminum. Materials 2018, 11, 1502. [Google Scholar] [CrossRef]
- Algahtani, A.; Mahmoud, E.R.; Khan, S.Z.; Tirth, V. Experimental studies on corrosion behavior of ceramic surface coating using different deposition techniques on 6082-T6 aluminum alloy. Processes 2018, 6, 240. [Google Scholar] [CrossRef]
- Gatzen, C.; Mack, D.E.; Guillon, O.; Vaßen, R. YAlO3-A novel environmental barrier coating for Al2O3/Al2O3-ceramic matrix composites. Coatings 2019, 9, 609. [Google Scholar] [CrossRef]
- Chen, X.; Li, C.D.; Xu, S.J.; Hu, Y.; Ji, G.C.; Wang, H.T. Microstructure and microhardness of Ni/Al-TiB2 composite coatings prepared by cold spraying combined with postannealing treatment. Coatings 2019, 9, 565. [Google Scholar] [CrossRef]
- Cheng, K.C.; Chen, J.H.; Stadler, S.; Chen, S.H. Properties of atomized AlCoCrFeNi high-entropy alloy powders and their phase-adjustable coatings prepared via plasma spray process. Appl. Surf. Sci. 2019, 478, 478–486. [Google Scholar] [CrossRef]
- Yusoff, N.H.N.; Ghazali, M.J.; Isa, M.C.; Daud, A.R.; Muchtar, A. Effects of powder size and metallic bonding layer on corrosion behaviour of plasma-sprayed Al2O3-13% TiO2 coated mild steel in fresh tropical seawater. Ceram. Int. 2013, 39, 2527–2533. [Google Scholar] [CrossRef]
- ISO7784.2-97. Paints and Varnishes-Determination of Resistance to Abrasion. Part 2: Rotating Abrasive Rubber Wheel Method; International Organization for Standardization: Geneva, Switzerland, 1997.
- GB/T 8642-2002. Thermal Spraying-Determination of Tensile Adhesive Strength; China Machinery Industry Federation: Beijing, China, 2002. (In Chinese)
- GB4342-84. Metal microscopic Vickers Hardness Test Method; China Ministry of Metallurgical Industry: Beijing, China, 1984. (In Chinese)
- John, G.O.; Li, W.G.; Zhao, Y.T.; Li, C.L. Porosity and its significance in plasma-sprayed coatings. Coatings 2019, 9, 460. [Google Scholar]
- Geldart, D.; Abdullah, E.C.; Hassanpour, A.; Nwoke, L.C.; Wouters, I. Characterization of powder flowability using measurement of angle of repose. China Particuol. 2006, 4, 104–107. [Google Scholar] [CrossRef]
- Tan, A.W.Y.; Sun, W.; Bhowmik, A.; Lek, J.Y.; Marinescu, I.; Li, F.; Khun, N.W.; Dong, Z.L.; Liu, E. Effect of coating thickness on microstructure, mechanical properties and fracture behaviour of cold sprayed Ti6Al4V coatings on Ti6Al4V substrates. Surf. Coat. Technol. 2018, 349, 303–317. [Google Scholar] [CrossRef]

| Element | Al | Fe | Ca | Cu | Si | Ti | Zn | Cl | Mn | Others |
| Wt.% | 61.802 | 13.301 | 4.372 | 2.167 | 10.143 | 0.986 | 0.743 | 1.137 | 1.661 | 3.688 |
| Molecular Formula | Al | Al2O3 | AlN | AlO(OH) | SiO2 | Others |
| Semi-Quantitative (%) | 21 ± 3 | 5 ± 2 | 44 ± 3 | 7 ± 2 | 1 | 22 ± 3 |
| Powder | Spray Voltage/V | Spray Current/A | Powder Flow Rate/g·min−1 | Main Gas Flow/slpm |
|---|---|---|---|---|
| Al/Ni | 55 | 550 | 24 | 33 |
| Levels | Factors | |||
|---|---|---|---|---|
| A Spray Voltage/V | B Spray Current/A | C Powder Flow Rate/g·min−1 | D Main Gas Flow/slpm | |
| 1 | 50 | 500 | 22 | 30 |
| 2 | 55 | 550 | 24 | 33 |
| 3 | 60 | 600 | 26 | 36 |
| Element | Al | Fe | Ca | Cu | Si | Cl | Ti | Zn | Others |
| Wt.% | 62.51 | 13.32 | 4.57 | 2.36 | 10.17 | 1.45 | 1.12 | 0.85 | 3.65 |
| No. | A | B | C | D | Microhardness/HV | Porosity/% | Abrasion Rate 10−3 g/min | Adhesive Strength/MPa | Comprehensive Score | |
|---|---|---|---|---|---|---|---|---|---|---|
| O1 | 1 | 1 | 1 | 1 | 104.95 | 0.14 | 29.76 | 11 | −59.82 | |
| O2 | 1 | 2 | 2 | 2 | 372.99 | 0.16 | 10.98 | 13 | −29.28 | |
| O3 | 1 | 3 | 3 | 3 | 391.61 | 0.16 | 16.85 | 4 | −28.08 | |
| O4 | 2 | 1 | 2 | 3 | 390.97 | 0.15 | 17.89 | 6 | −46.64 | |
| O5 | 2 | 2 | 3 | 1 | 655.23 | 0.21 | 9.76 | 10 | −39.39 | |
| O6 | 2 | 3 | 1 | 2 | 605.75 | 0.16 | 8.78 | 16 | −10.94 | |
| O7 | 3 | 1 | 3 | 2 | 611.02 | 0.19 | 20.27 | 16 | −35.12 | |
| O8 | 3 | 2 | 1 | 3 | 689.81 | 0.14 | 23.08 | 17 | −15.32 | |
| O9 | 3 | 3 | 2 | 1 | 624.44 | 0.15 | 12.40 | 12 | −18.58 | |
| k1 | −117.18 | −141.59 | −86.09 | −117.80 | – | |||||
| k2 | −96.97 | −84.00 | −94.50 | −75.35 | ||||||
| k3 | −69.03 | −57.60 | −102.59 | −90.04 | ||||||
| R | 48.16 | 83.99 | 16.50 | 42.44 | ||||||
| Factors primary to secondary | B > A > D > C | |||||||||
| Preferred scheme | B3A3D2C1 | |||||||||
| Spray Voltage/V | Spray Current/A | Powder Flow Rate/g·min−1 | Main Gas Flow/slpm | Micro Hardness/HV | Porosity/% | Abrasion Rate 10−3 g/min | Adhesive Strength/MPa |
|---|---|---|---|---|---|---|---|
| 60 | 600 | 22 | 33 | 606.54 | 0.16 | 12.86 | 16 |
| Samples | OAASP/% | Al2O3/% |
|---|---|---|
| H0 | 100 | 0 |
| H10 | 90 | 10 |
| H20 | 80 | 20 |
| H30 | 70 | 30 |
| H40 | 60 | 40 |
| H50 | 50 | 50 |
| Samples | Microhardness/HV | Porosity/% | Abrasion Rate 10−3 g/min | Adhesive Strength/MPa |
|---|---|---|---|---|
| H0 | 606.54 | 0.163 | 12.86 | 16.25 |
| H10 | 625.32 | 0.158 | 12.33 | 16.52 |
| H20 | 648.64 | 0.153 | 11.84 | 16.73 |
| H30 | 667.74 | 0.147 | 11.39 | 16.82 |
| H40 | 681.97 | 0.139 | 10.87 | 16.90 |
| H50 | 713.36 | 0.131 | 10.31 | 17.12 |
| H100 | 950–1000 | 0.1–0.12 | 7–7.5 | 20–25 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ni, H.; Zhang, J.; Lv, S.; Wang, X.; Zhu, Y.; Gu, T. Preparation and Performance Optimization of Original Aluminum Ash Coating Based on Plasma Spraying. Coatings 2019, 9, 770. https://doi.org/10.3390/coatings9110770
Ni H, Zhang J, Lv S, Wang X, Zhu Y, Gu T. Preparation and Performance Optimization of Original Aluminum Ash Coating Based on Plasma Spraying. Coatings. 2019; 9(11):770. https://doi.org/10.3390/coatings9110770
Chicago/Turabian StyleNi, Hongjun, Jiaqiao Zhang, Shuaishuai Lv, Xingxing Wang, Yu Zhu, and Tao Gu. 2019. "Preparation and Performance Optimization of Original Aluminum Ash Coating Based on Plasma Spraying" Coatings 9, no. 11: 770. https://doi.org/10.3390/coatings9110770





