Formation Process of an LDHs Coating on Magnesium Alloy by a CO2 Pressurization Method
Abstract
:1. Introduction
2. Materials and Methods
3. Results
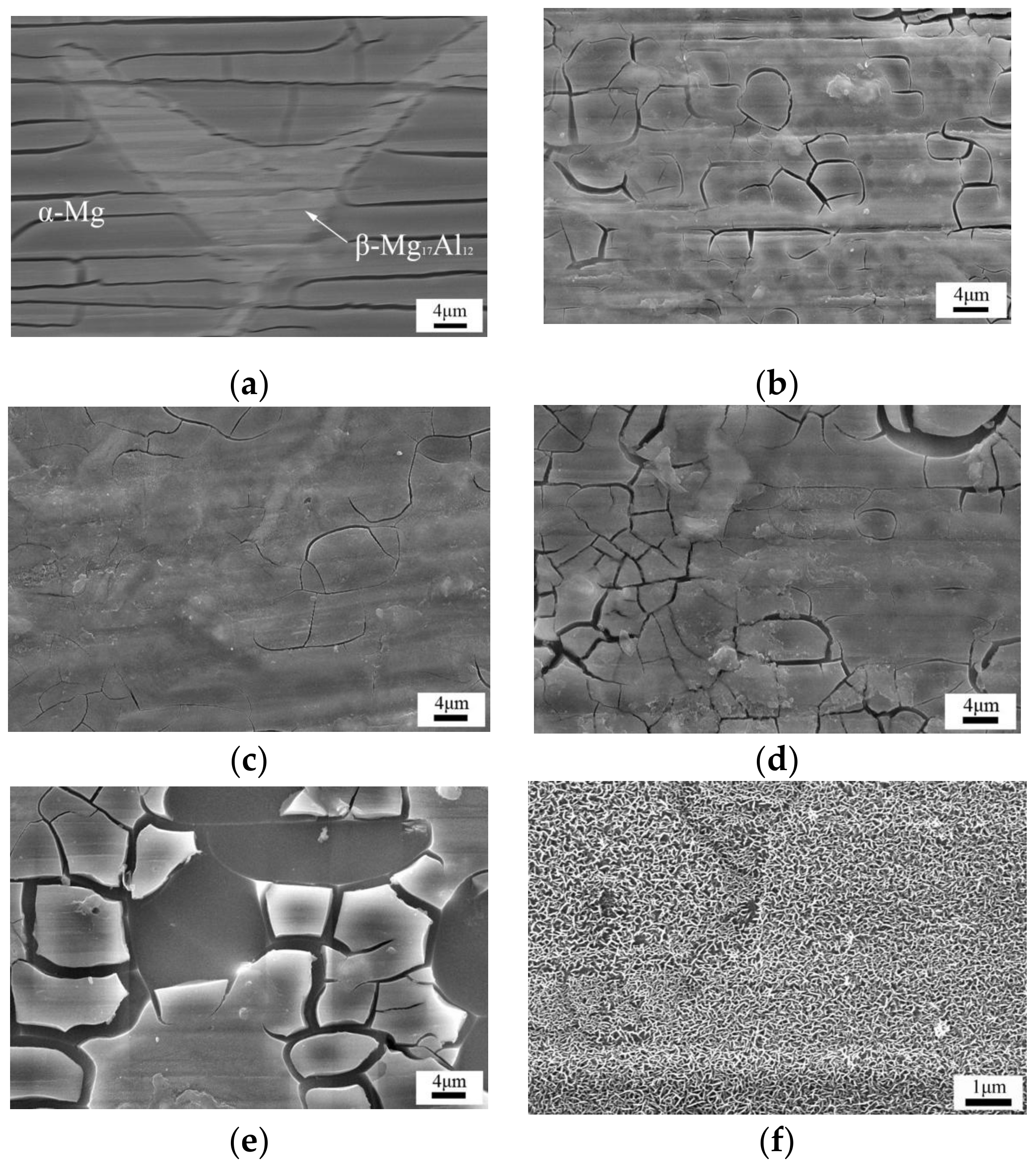
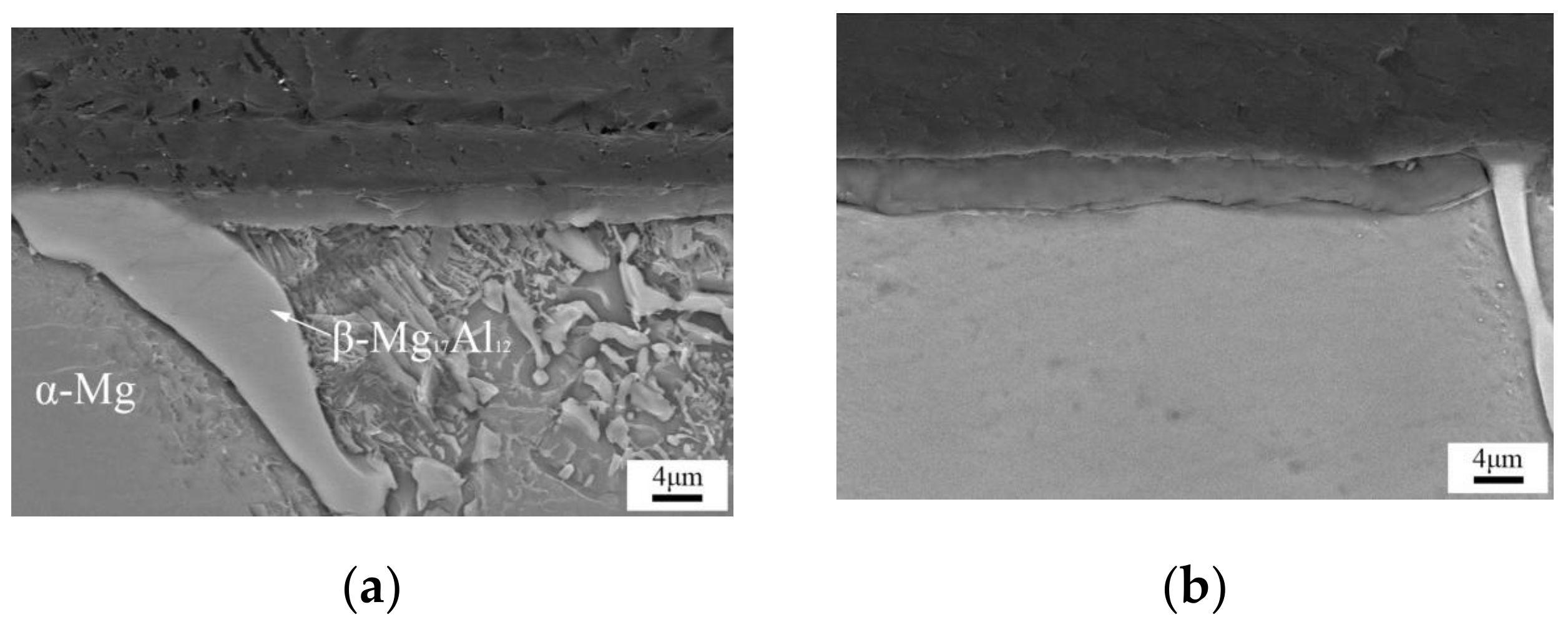
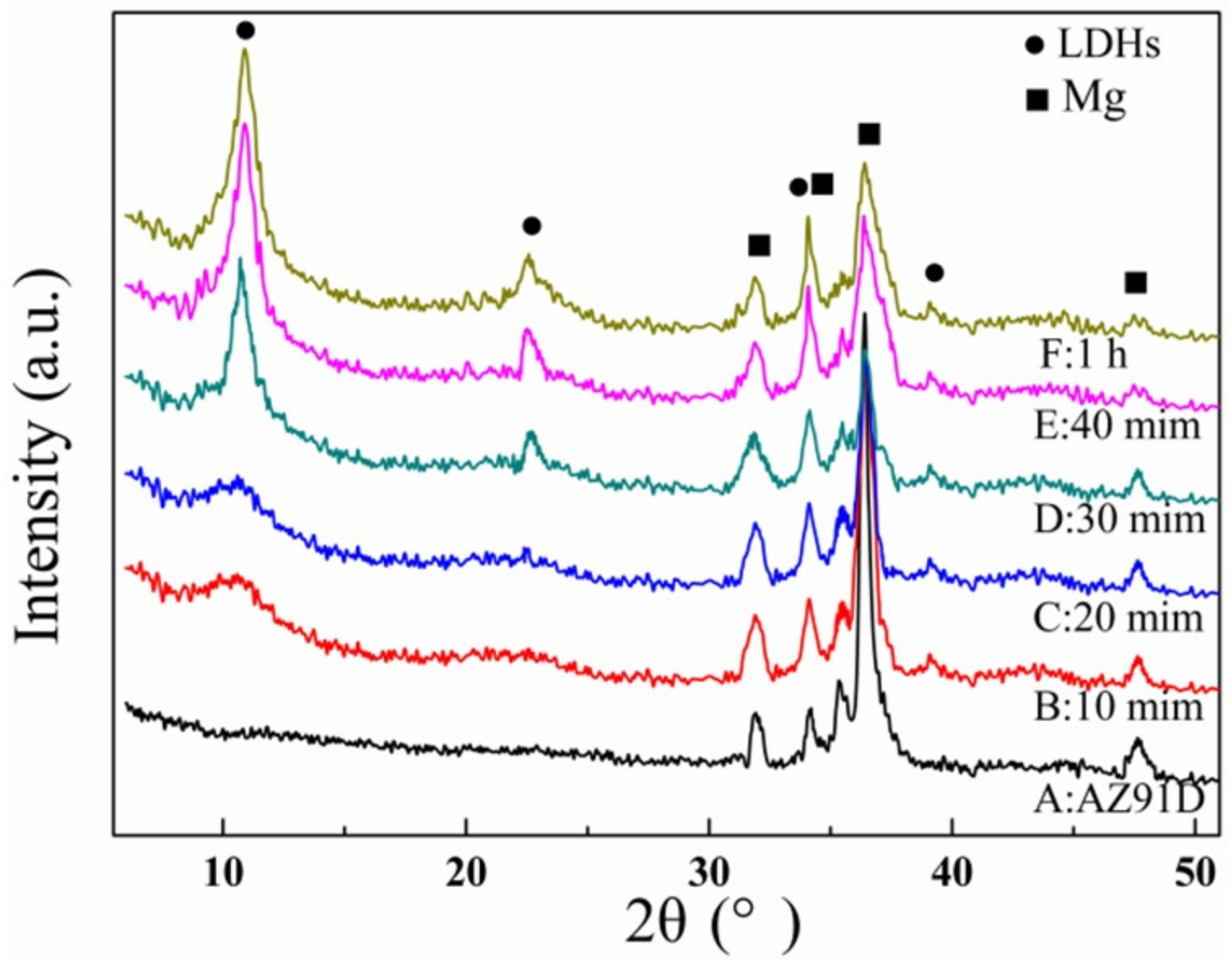
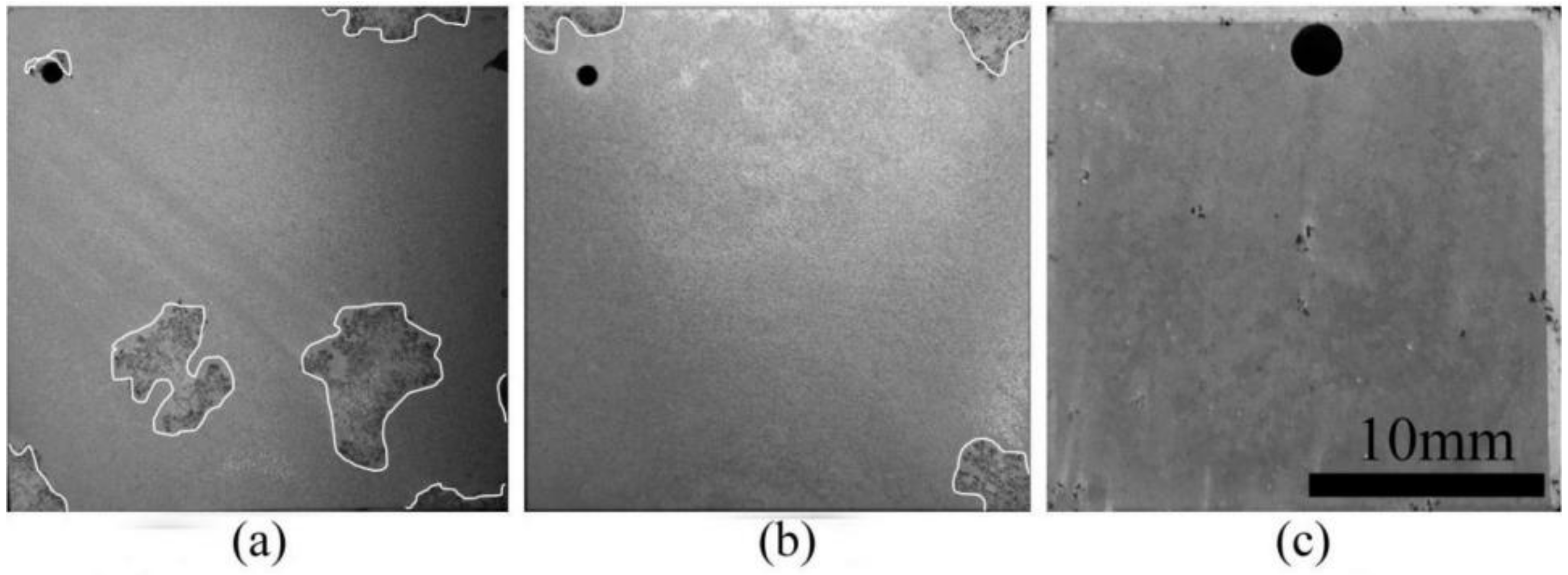
3.1. Micro-Structure
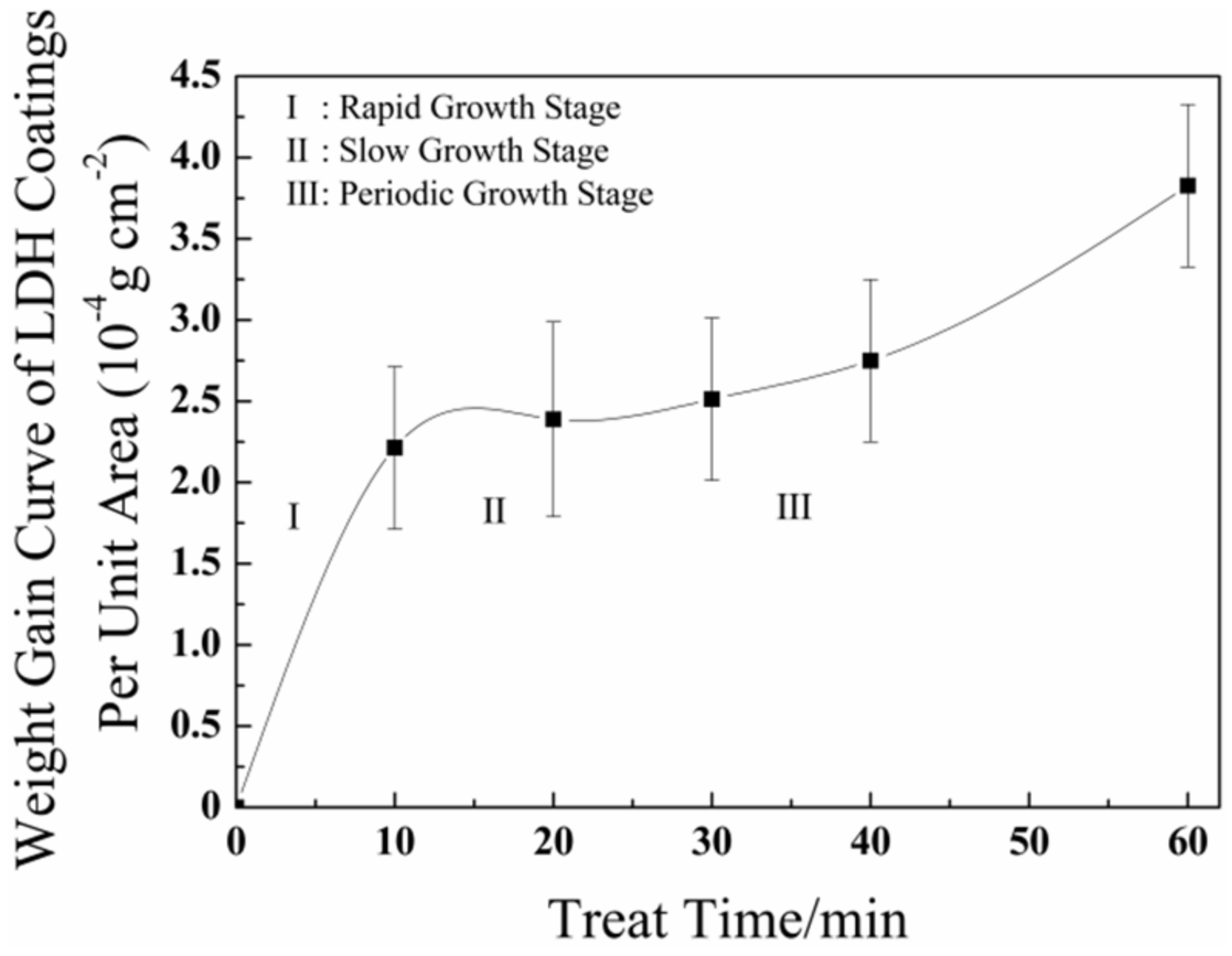
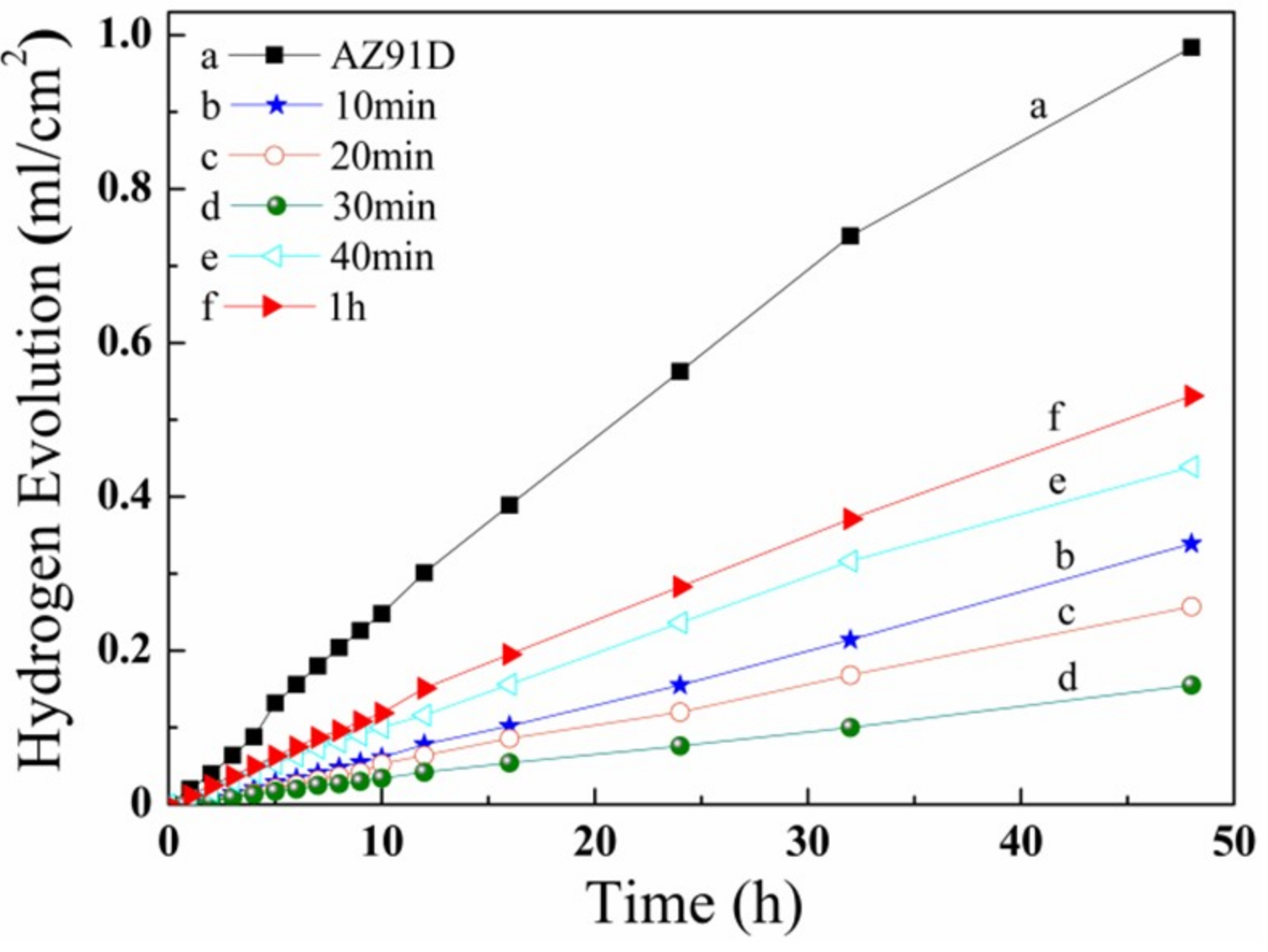
3.2. Weighted Gain Curve
3.3. Apparent Activation Energy
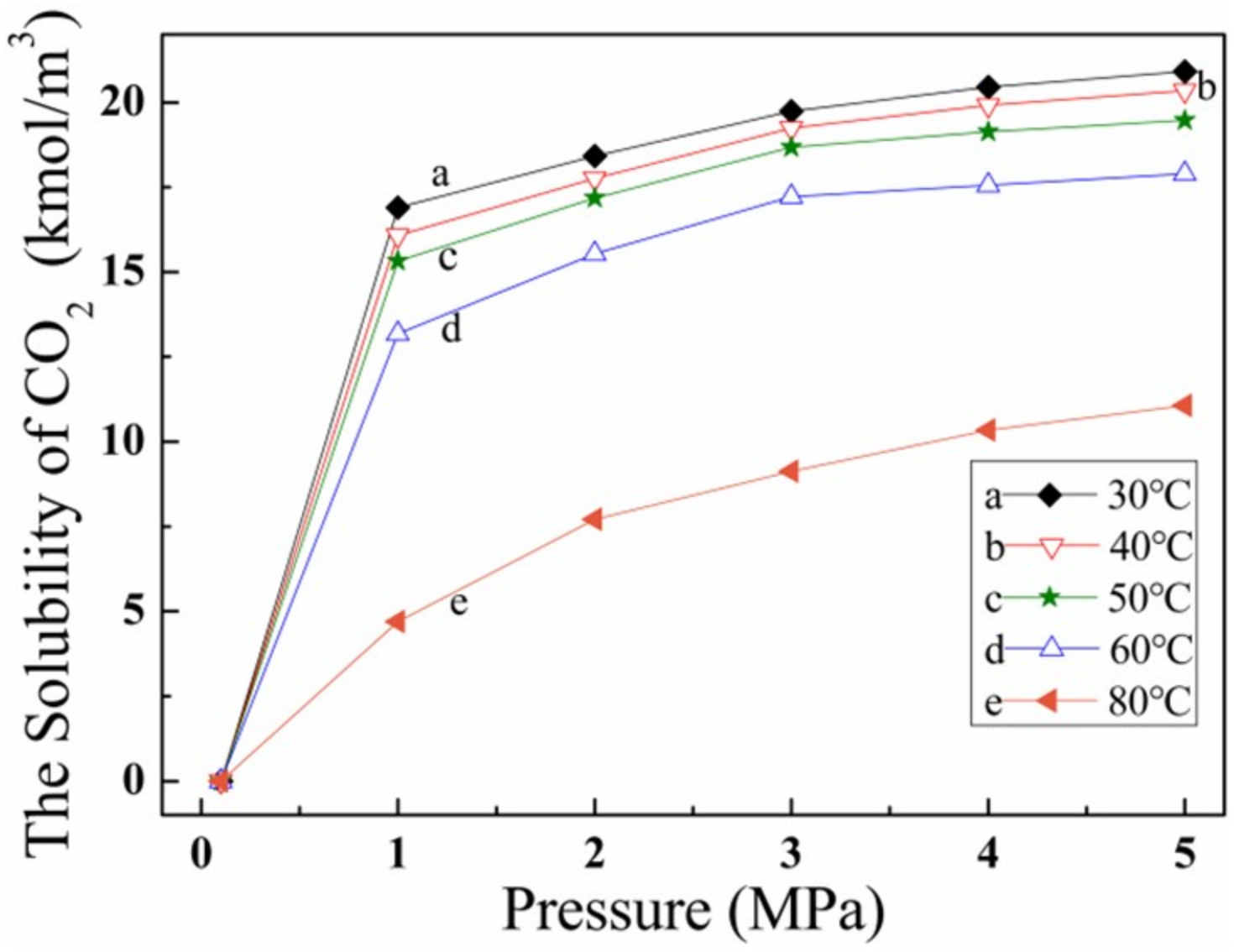
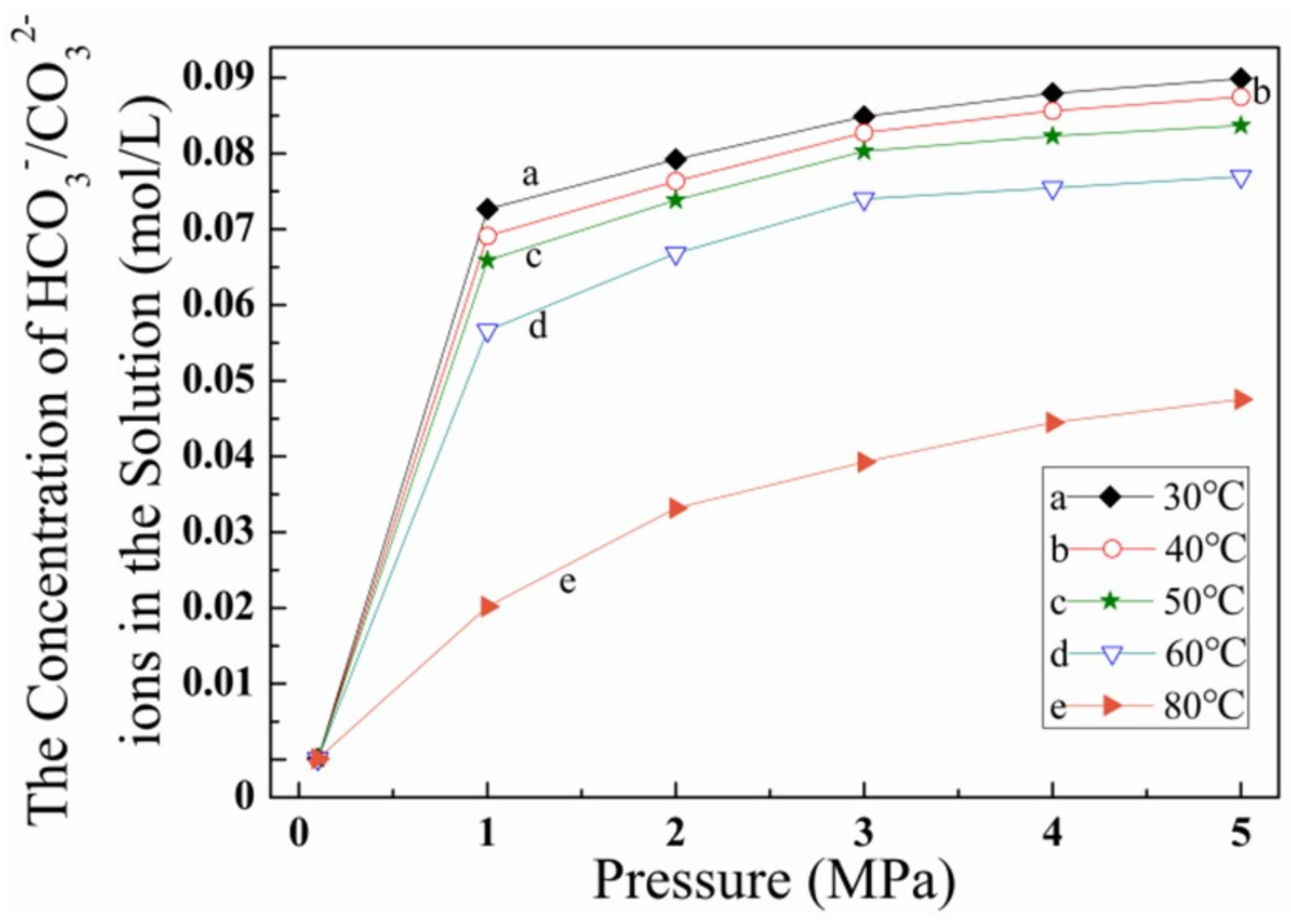
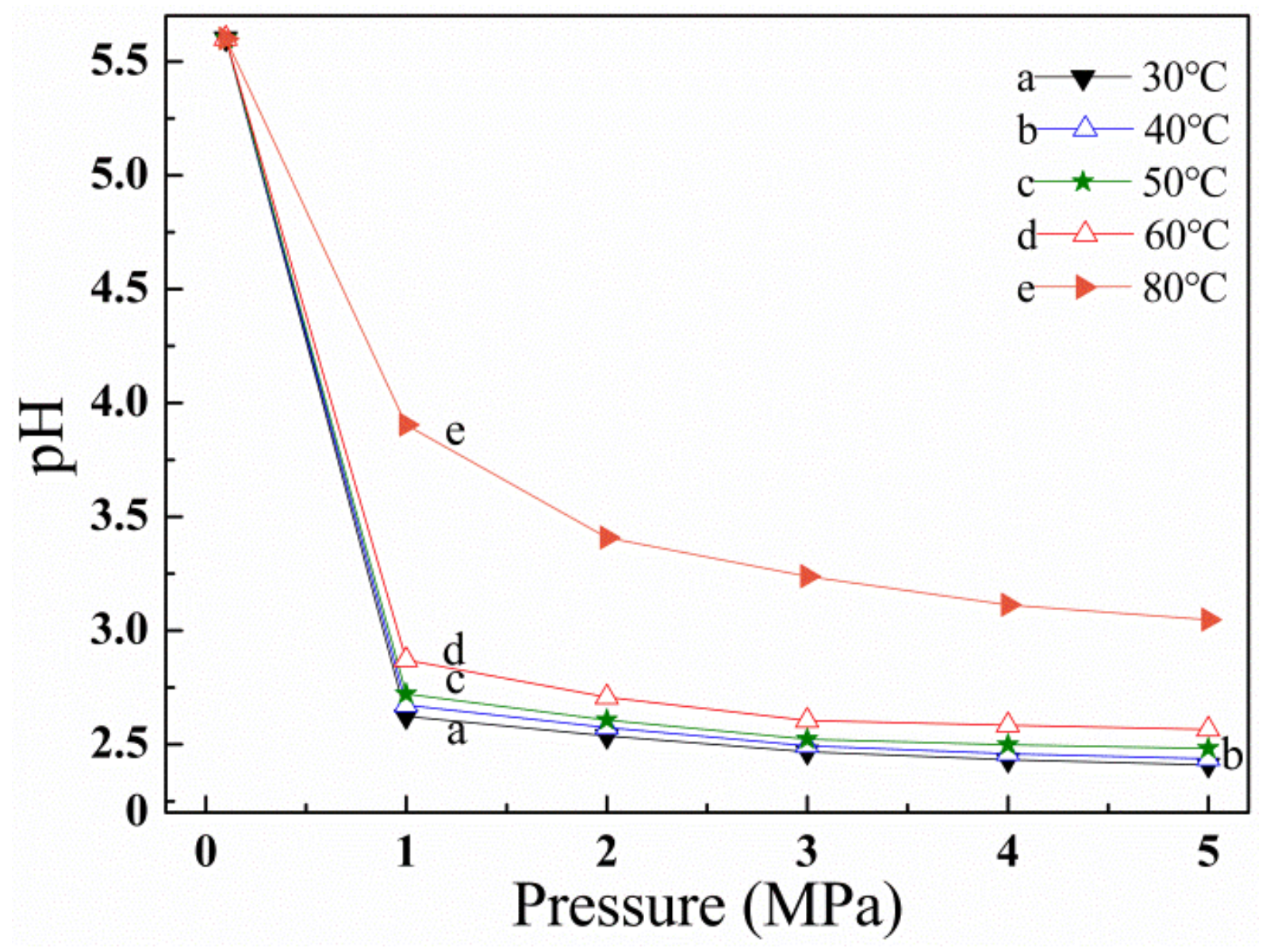
3.4. Effect of CO2 Solubility
3.5. Anti-Corrosion Properties
4. Discussion
4.1. Influence of the Micro-Structure on the Formation Process
4.2. Influence of Physical and Chemical Processes on the Formation Process
4.3. Influence of CO2 Pressurization on the Formation Process
4.4. Influence of the Formation Time on the Anti-Corrosion Properties
4.5. Comparison with the State-of-the-Art Technology
5. Conclusions
- •
- The initial formation position in the formation process of the LDHs coating on magnesium alloy is on the α-Mg phase, and subsequently on the β-Mg17Al12 phase. The most even and dense coating was found after 30 min, where the LDHs coating appeared to severe cracks and holes, and especially so after 1 h.
- •
- The formation process of the LDHs coating can be divided into three stages: a rapid growth stage, a slow growth stage, and a periodic growth stage. During the rapid growth stage (0–10 min), the weight of the LDHs coatings increasing rapidly, with a minimum value of activation energy (21.78 kJ mol−1). During the slow growth stage (10–20 min), the weight of the LDHs coatings grew stably, with a growing value of activation energy (31.86 kJ mol−1). Finally, during the periodic growth period, the formation and dissolution of the LDHs coatings tended to be balanced, with a maximum value of activation energy (34.92 kJ mol−1).
- •
- CO2 pressurization promotes the shifts of the ionization reaction and the electrochemical reaction to the right, thereby accelerating the formation reaction, which increases the formation reaction rate and formation stability.
- •
- The anti-corrosion of the LDHs coating is gradually improved with the increase in formation time. The icorr (corrosion current) of the LDHs coating was minimal at 30 min, and had a higher efficiency percentage. The LDHs coating has a compact micro-structure, and better anti-corrosion at a pressure of 3 MPa, a temperature of 50 °C, and a time of 30 min. Comparing with the state-of-the-art technology, the CO2_3 MPa_0.5 h treatment is observably better than CO2_24 h and CO2_2 h/pH 11.5_2 h treatments.
Author Contributions
Funding
Conflicts of Interest
References
- Chen, X.H.; Geng, Y.X.; Pan, F.S. Research Progress in Magnesium Alloys as Functional Materials. Rare Met. Mater. Eng. 2016, 45, 2269–2274. [Google Scholar] [CrossRef]
- Song, G.; Xu, Z. The surface, microstructure and corrosion of magnesium alloy AZ31 sheet. Electrochim. Acta 2010, 55, 4148–4161. [Google Scholar] [CrossRef]
- Shi, F.; Wang, C.Q.; Zhang, Z.M. Microstructures, corrosion and mechanical properties of as-cast MgZnY(Gd) alloys. Trans. Nonferrous Met. Soc. China 2015, 25, 2172–2180. [Google Scholar] [CrossRef]
- Atrens, A.; Song, G.L.; Liu, M. Review of recent devel-opments in the field of magnesium corrosion. Adv. Eng. Mater. 2015, 17, 400–453. [Google Scholar] [CrossRef]
- Cao, F.; Song, G.L.; Atrens, A. Corrosion and passivation of magnesium alloys. Corros. Sci. 2016, 111, 835–845. [Google Scholar] [CrossRef]
- Brown, R. International Mg Association 55th Annual World Conference. Light Met. Age. 1998, 56, 86–93. [Google Scholar]
- Eliezer, D.; Aghion, E.; Fores, F.H. Magnesium science, technology and applications. Adv. Perform. Mater. 1998, 5, 201–212. [Google Scholar] [CrossRef]
- Toshihide, T.; Yuji, N.; Nobuo, U.; Masahiro, K. Improvement of corrosion resistance of magnesium metal by rare earth elements. Electrochim. Acta 2007, 1, 117–121. [Google Scholar] [CrossRef]
- Song, Y.L.; Liu, Y.H.; Wang, S.H. Effect of cerium addition on microstructure and corrosion resistance of die cast AZ91 magnesium alloy. Mater. Corros. 2007, 3, 189–192. [Google Scholar] [CrossRef]
- Tong, G.D.; Liu, H.F.; Liu, Y.H. Effec of rare earth additions on microstructure and mechanical properties of AZ91 magnesium alloys. Trans. Nonferrous Met. Soc. China 2010, 20, 336–340. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, M.; Teng, X.; Zuo, M. Effect of Gd addition on microstructure and corrosion behaviors of Mg-Zn-Y alloy. J. Magn. Alloy 2016, 4, 319–325. [Google Scholar] [CrossRef]
- Zhou, H.; Xu, W.Z.; Jian, W.W. A new metastable precipitate phase in Mg-Gd-Y-Zr alloy. Philos. Mag. 2014, 21, 2403–2409. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, Q.D.; Guo, W.; Ye, B.; Jian, W.W.; Xu, W.Z.; Ma, X.L.; Moering, J. Finite element simulation and experimental investigation on homogeneity of Mg-9.8Gd-2.7Y-0.4Zr magnesium alloy processed by repeated-up setting. J. Mater. Process. Technol. 2015, 225, 310–317. [Google Scholar] [CrossRef]
- Zhou, H.; Cheng, G.M.; Ma, X.L.; Xu, W.Z.; Mathaudhu, S.N.; Wang, Q.D.; Zhu, Y.T. Effect of Ag on interfacial segregation in Mg-Gd-Y-(Ag)-Zr alloy. Acta Mater. 2015, 95, 20–29. [Google Scholar] [CrossRef]
- Liang, C.H.; Wang, S.S.; Huang, N.B. Effects of Lanthanum and Cerium Mixed Rare Earth Metal on Abrasion and Corrosion Resistance of AM60 Magnesium Alloy. Rare Metal Mater. Eng. 2015, 3, 521–526. [Google Scholar] [CrossRef]
- Zhou, H.; Ning, H.Y.; Ma, X.L. Microstructural evolution and mechanical properties of Mg-9.8Gd-2.7Y-0.4Zr alloy produced by repetitive upsetting. J. Mater. Sci. Technol. 2018, 34, 1067–1075. [Google Scholar] [CrossRef]
- Emley, E.F. Principles of Magnesium Technology; Pegamon Press: New York, NY, USA, 1966. [Google Scholar]
- Zhang, Y.; Yan, C.; Wang, F. Study on the environmentally friendly anodizing of AZ91D magnesium alloy. Surf. Coat. Technol. 2002, 1, 36–43. [Google Scholar] [CrossRef]
- Song, G.L. Recent progress in corrosion and protection of magnesium alloys. Adv. Eng. Mater. 2005, 7, 563–586. [Google Scholar] [CrossRef]
- Pommiers, S.; Frayret, J.; Castetbon, A. Alternative conversion coatings to chromate for the protection of magnesium alloys. Corros. Sci. 2014, 84, 135–146. [Google Scholar] [CrossRef]
- Jiang, X.; Guo, R.G.; Jiang, S.Q. Microstructure and corrosion resistance of Ce–V conversion coatingon AZ31 magnesium alloy. Appl. Surf. Sci. 2015, 341, 166–174. [Google Scholar] [CrossRef]
- Zeng, R.C.; Hu, Y.; Zhang, F. Corrosion resistance of cerium-doped zinc calcium phosphate chemical conversion coatings on AZ31 magnesium alloy. Trans. Nonferrous Met. Soc. 2016, 26, 472–483. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.B.; Yang, H.Y.; Abbott, T.B. Corrosion protection of magnesium and itsalloys by metal phosphate conversion coatings. Surf. Eng. 2014, 30, 871–879. [Google Scholar] [CrossRef]
- Phuong, N.V.; Gupta, M.; Moon, S. Enhanced corrosion performance of magnesium phosphate conversion coating on AZ31 magnesium alloy. Trans. Nonferrous Met. Soc. 2017, 27, 1087–1095. [Google Scholar] [CrossRef]
- Jian, S.Y.; Chu, Y.R.; Lin, C.S. Permanganate conversion coating on AZ31 magnesium alloys with enhanced corrosion resistance. Corros. Sci. 2015, 93, 301–309. [Google Scholar] [CrossRef]
- Lee, Y.L.; Chu, Y.R.; Li, W.C. Effect of permanganate concentration on the formation and properties of phosphate/permanganate conversion coating on AZ31 magnesium alloy. Corros. Sci. 2013, 70, 74–81. [Google Scholar] [CrossRef]
- Mosiałek, M.; Mordarski, G.; Nowak, P. Phosphate–permanganate conversion coatings on the AZ81 magnesium alloy; SEM, EIS and XPS studies. Surf. Coat. Technol. 2011, 206, 51–62. [Google Scholar] [CrossRef]
- Yang, L.H.; Zhang, M.L.; Li, J.Q. Stannate conversion coatings on Mg–8Li alloy. J. Alloys Compd. 2009, 471, 97–200. [Google Scholar] [CrossRef]
- Lee, Y.L.; Chu, Y.R.; Chen, F.J. Mechanism of the formation of stannate and cerium conversion coatings on AZ91D magnesium alloys. Appl. Surf. Sci. 2013, 276, 578–585. [Google Scholar] [CrossRef]
- Ma, Y.B.; Li, N.; Li, D.Y.; Huang, X.M. Characteristics and corrosion studies of vanadate conversion coating formed on Mg–14 wt % Li–1 wt % Al–0.1 wt % Ce alloy. Appl. Surf. Sci. 2012, 261, 59–67. [Google Scholar] [CrossRef]
- Niu, L.Y.; Chang, S.H.; Tong, X. Analysis of characteristics of vanadate conversion coating on the surface of magnesium alloy. J. Alloy. Compd. 2014, 617, 214–218. [Google Scholar] [CrossRef]
- Loperena, A.P.; Lehr, I.L.; Saidman, S.B. Formation of a cerium conversion coating on magnesium alloy using ascorbic acid as additive. Characterisation and anticorrosive properties of the formed film. J. Magn. Alloy 2016, 4, 278–285. [Google Scholar] [CrossRef]
- Lei, L.; Shi, J.; Wang, X. Microstructure and electrochemical behavior of cerium conversion coating modified with silane agent on magnesium substrates. Appl. Surf. Sci. 2016, 376, 161–171. [Google Scholar] [CrossRef]
- Kong, G.; Liu, L.Y.; Lu, J.T. Study on lanthanum salt conversion coating modified with citric acid on hot dip galvanized steel. J. Rare Earths 2010, 28, 461–465. [Google Scholar] [CrossRef]
- Xu, S.; Liao, M.C.; Zeng, H.Y. Surface modification and dissolution behavior of Mg–Al hydrotalcite particles. J. Taiwan. Inst. Chem. Eng. 2015, 56, 174–180. [Google Scholar] [CrossRef]
- Wang, L.D.; Zhang, K.Y.; He, H.R. Enhanced corrosion resistance of MgAl hydrotalcite conversion coating on aluminum by chemical conversion treatment. Surf. Coat. Technol. 2013, 235, 484–488. [Google Scholar] [CrossRef]
- Lin, J.K.; Hsia, C.L.; Uan, J.Y. Characterization of Mg, Al-hydrotalcite conversion film on Mg alloy and Cl− and CO32− anion-exchangeability of the film in a corrosive environment. Scr. Mater. 2007, 56, 927–930. [Google Scholar] [CrossRef]
- Lin, J.K.; Uan, J.Y. Formation of Mg, Al-hydrotalcite conversion coating on Mg alloy in aqueous HCO3−/CO32− and corresponding protection against corrosion by the coating. Corros. Sci. 2009, 51, 1181–1188. [Google Scholar] [CrossRef]
- Uan, J.Y.; Lin, J.K.; Sun, Y.S. Surface coatings for improving the corrosion resistance and cell adhesion of AZ91D magnesium alloy through environmentally clean methods. Thin Solid Films 2010, 518, 7563–7567. [Google Scholar] [CrossRef]
- Uan, J.Y.; Lin, J.K.; Tung, Y.S. Direct growth of oriented Mg–Al layered double hydroxide film on Mg alloy in aqueous HCO3−/CO32− solution. J. Mater. Chem. 2010, 20, 761–766. [Google Scholar] [CrossRef]
- Lin, J.K.; Jeng, K.L.; Uan, J.Y. Crystallization of a chemical conversion layer that forms on AZ91D magnesium alloy in carbonic acid. Corros. Sci. 2011, 53, 3832–3839. [Google Scholar] [CrossRef]
- Syu, J.H.; Uan, J.Y.; Lin, M.C. Optically transparent Li–Al–CO3 layered double hydroxide thin films on an AZ31 Mg alloy formed by electrochemical deposition and their corrosion resistance in a dilute chloride environment. Corros. Sci. 2013, 68, 238–248. [Google Scholar] [CrossRef]
- Yu, B.L.; Lin, J.K.; Uan, J.Y. Applications of carbonic acid solution for developing conversion coating on Mg alloy. Trans. Nonferrous Met. Soc. China 2010, 20, 1331–1339. [Google Scholar] [CrossRef]
- Chen, J.; Song, Y.W.; Shan, D.Y. In situ growth of Mg–Al hydrotalcite conversion film on AZ31 magnesium alloy. Corros. Sci. 2011, 53, 3281–3288. [Google Scholar] [CrossRef]
- Chen, J.; Song, Y.W.; Shan, D.Y. Study of the corrosion mechanism of the in situ grown Mg–Al–CO32− hydrotalcite film on AZ31 alloy. Corros. Sci. 2012, 65, 268–277. [Google Scholar] [CrossRef]
- Chen, J.; Song, Y.W.; Shan, D.Y. Modifications of the hydrotalcite film on AZ31 Mg alloy by phytic acid: The effects on morphology, composition and corrosion resistance. Corros. Sci. 2013, 74, 130–138. [Google Scholar] [CrossRef]
- Chen, J.; Xu, Q.; Song, Y.W. Characterization of the in situ growth manasseite/carbonates composite conversion film on Mg2Zn alloy. Mater. Lett. 2015, 150, 65–68. [Google Scholar] [CrossRef]
- Chen, J.; Song, Y.W.; Shan, D.Y. Influence of alloying elements and microstructure on the formation of hydrotalcite film on Mg alloys. Corros. Sci. 2015, 93, 90–99. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, Z.G.; Zeng, R.C. Corrosion resistance of Mg-Al-LDH coating on magnesium alloy AZ31. Surf. Coat. Technol. 2014, 258, 1152–1158. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, C.L.; Song, L. Corrosion of in-situ grown MgAl-LDH coating on aluminum alloy. Trans. Nonferrous Met. Soc. China 2015, 25, 3498–3504. [Google Scholar] [CrossRef]
- Starljngke, K.E.; Han, M.S. Thermo data refined for LPG–14. Mixtures. Hydrocarb. Process. 1972, 51, 129–132. [Google Scholar]
- Liauo, C.S.; Huang, J.C. Relationship between apparent and true activation energies. Scripta Mater. 1998, 39, 873–879. [Google Scholar] [CrossRef]
- Zhang, T.; Shao, Y.W.; Meng, G.Z. Corrosion of hot extrusion AZ91 magnesium alloy; I-relation between the microstructure and corrosion behavior. Corros. Sci. 2011, 53, 1960–1968. [Google Scholar] [CrossRef]
- Song, G.L.; Atrens, A.; Wu, X.L.; Zhang, B. Corrosion behavior of AZ21, AZ501 and AZ91 in sodium chloride. Corros. Sci. 1998, 40, 1769–1791. [Google Scholar] [CrossRef]
- Cao, Y.; Li, G.; Li, X. Graphene/layered double hydroxide nanocomposite: Properties, synthesis and applications. Chem. Eng. J. 2016, 292, 207–223. [Google Scholar] [CrossRef]
- Alibakhshi, E.; Ghasemi, E.; Mahdavian, M.; Ramezanzadeh, B. A comparative study on corrosion inhibitive efect of nitrate and phosphate intercalated Zn-Al-layered double hydroxides (LDHs) nanocontainers incorporated into a hybrid silane layer and their efect on cathodic delamination of epoxy topcoat. Corros. Sci. 2017, 115, 159–174. [Google Scholar] [CrossRef]
- Song, G.L. Corrosion behavior of magnesium alloys and protection techniques. Surf. Eng. Light Alloy. 2010, 3–39. [Google Scholar] [CrossRef]
- Zhang, T.; Li, Y.; Wang, F.H. Roles of beta phase in the corrosion process of AZ91D magnesium alloy. Corros. Sci. 2006, 48, 1249–1264. [Google Scholar] [CrossRef]
- Fajardoa, S.; Frankel, G.S. A kinetic model explaining the enhanced rates of hydrogen evolution on anodically polarized magnesium in aqueous environments. Electrochem. Commun. 2017, 84, 36–39. [Google Scholar] [CrossRef] [Green Version]
- Thomas, S.; Medhekar, N.V.; Franke, G.S.; Birbilis, N. Corrosion mechanism and hydrogen evolution on Mg. Curr. Opin. Solid State Mater. Sci. 2015, 19, 85–94. [Google Scholar] [CrossRef]
- Song, D.; Ma, A.B.; Jiang, J.H. Corrosion behaviour of bulk ultra-fine grained AZ91D magnesium alloy fabricated by equal-channel angular pressing. Corros. Sci. 2011, 53, 362–373. [Google Scholar] [CrossRef]




| Condition | 10 min, 50 °C | 20 min, 50 °C | 30 min, 50 °C | |||
|---|---|---|---|---|---|---|
| Δm (mg) | 1.39 | 1.81 | 1.41 | 2.06 | 1.41 | 2.17 |
| Ea (KJ mol−1) | 21.78 | 31.86 | 34.92 | |||
| i | ||
|---|---|---|
| 1 | 0.443 | 0.115 |
| 2 | 1.284 | −0.921 |
| 3 | 0.356 | 1.708 |
| 4 | 0.545 | −0.171 |
| 5 | 0.528 | 0.349 |
| 6 | 0.484 | 0.754 |
| 7 | 0.071 | −0.044 |
| 8 | 0.504 | 1.322 |
| 9 | 0.031 | 0.179 |
| 10 | 0.073 | 0.463 |
| 11 | 0.006 | −0.022 |
| Sample | Ecorr (V vs. SCE) | Icorr (μA cm−2) | Efficiency % |
|---|---|---|---|
| AZ91D | −1.41 (0.059) | 83.62 (1.67) | - |
| 10 min | −1.39 (0.047) | 30.11 (1.69) | 63.99% |
| 20 min | −1.37 (0.031) | 10.53 (1.63) | 87.41% |
| 30 min | −1.36 (0.034) | 8.92 (1.63) | 89.33% |
| 40 min | 1.38 (0.042) | 20.19 (1.73) | 75.86% |
| 1 h | −1.39 (0.47) | 28.73 (1.62) | 65.64% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Wang, J.; Zhang, C.; Liu, B.; Jiang, P.; Zhao, Y.; Buhe, B.; Zhang, T.; Meng, G.; Wang, F. Formation Process of an LDHs Coating on Magnesium Alloy by a CO2 Pressurization Method. Coatings 2019, 9, 47. https://doi.org/10.3390/coatings9010047
Zhang X, Wang J, Zhang C, Liu B, Jiang P, Zhao Y, Buhe B, Zhang T, Meng G, Wang F. Formation Process of an LDHs Coating on Magnesium Alloy by a CO2 Pressurization Method. Coatings. 2019; 9(1):47. https://doi.org/10.3390/coatings9010047
Chicago/Turabian StyleZhang, Xiaochen, Jiaoxiang Wang, Chunyan Zhang, Bin Liu, Peng Jiang, Yang Zhao, Bateer Buhe, Tao Zhang, Guozhe Meng, and Fuhui Wang. 2019. "Formation Process of an LDHs Coating on Magnesium Alloy by a CO2 Pressurization Method" Coatings 9, no. 1: 47. https://doi.org/10.3390/coatings9010047






