Electrodeposition Behavior of Polycrystalline Ni–Mo–La Composite in Alkaline Solution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials and Preparation
2.2. Experimental Characterization
3. Composition and Structure of Coating
4. Analyses of Ni–Mo/La Composite Deposition Process
4.1. Analysis of Metal Ion Activity
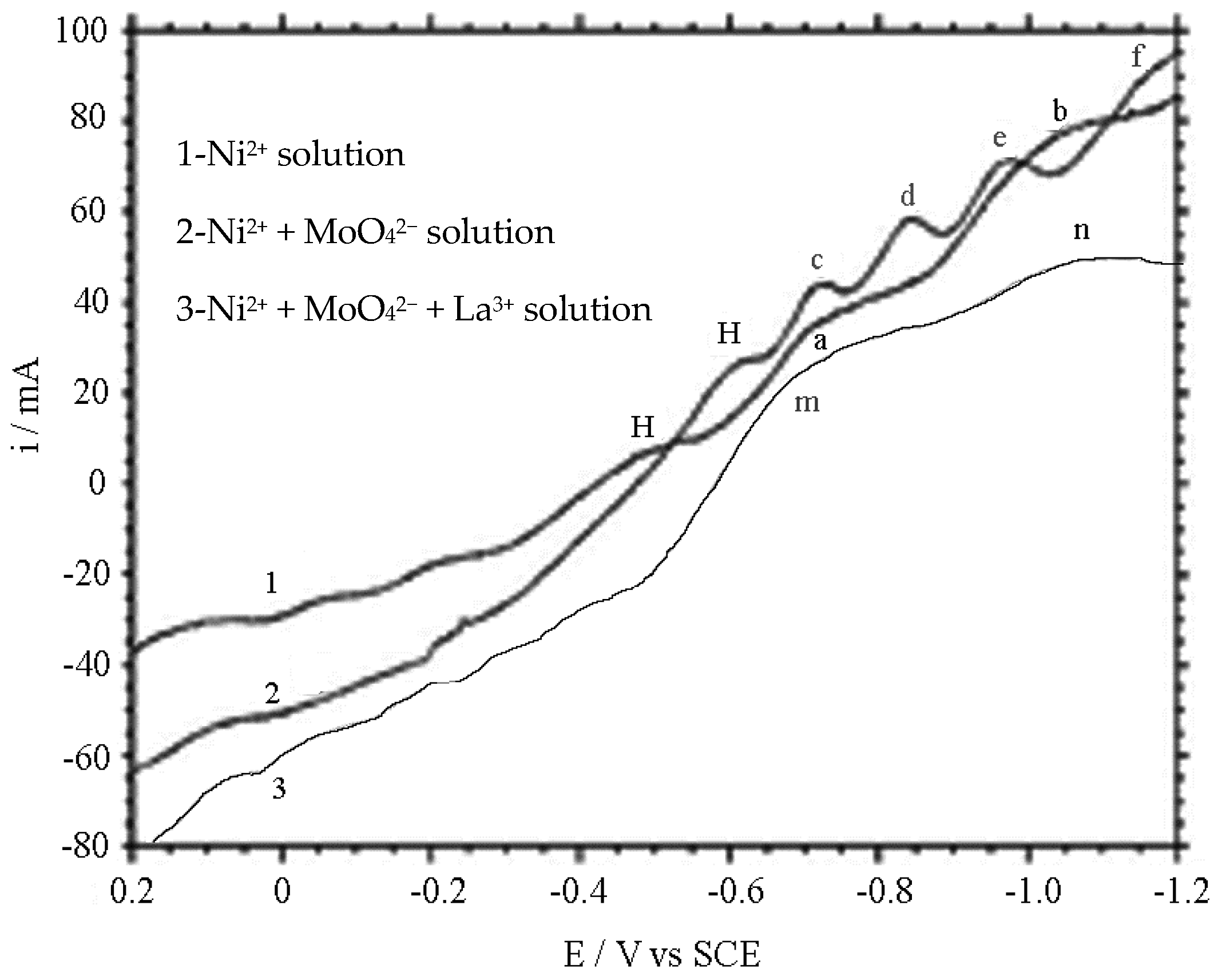
4.2. Trimetal Reduction Experiment
4.3. Co-Deposition Kinetic Analysis
5. Conclusions
- The La3+ ion solution was added into the weak alkaline solution of Ni and Mo ions main salt to obtain the Ni–Mo–La composite. The addition of lanthanum element not only changed the structural composition and the spatial configuration of the composite, also showed some impact on the surface morphology and the deposition mechanism of the coating.
- In the process of electro-deposition of Ni–Mo–La coating in alkaline solution, the OH− ions were reduced. The addition of lanthanum and molybdenum ions negatively shifted the reduction potential of nickel ions and broadened the peak positions, which delayed the reduction of Ni ions speed, thus reducing the current efficiency.
- The polarization patterns and cyclic voltammetry patterns of the reduction deposition show that the electron transfer process of multivalent molybdenum is a step-by-step valance-lowing one.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, X.; Cheng, Z.; Jin, Y.; Wu, Y.; Dai, X.; Liu, G. Magneto-electronic properties and tetragonal deformation of rare-earth-element-based quaternary Heusler half-metals: A first-principles prediction. J. Alloys Compd. 2018, 734, 329–341. [Google Scholar] [CrossRef]
- Martins, A.; Silva, J.M.; Ribeiro, M.F. Influence of rare earth elements on the acid and metal sites of Pt/HBEA catalyst for short chain n-alkane hydroisomerization. Appl. Catal. A Gen. 2013, 466, 293–299. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, C.; Hua, Y.; Wang, M.; Su, Z. Electrochemical preparation of Ni–La alloys from the EMIC-EG eutectic-based ionic liquid. Ionics 2017, 23, 1703–1710. [Google Scholar] [CrossRef]
- Gao, C.; Li, N. Hydrogen evolution reaction activity of electrodeposited amorphous/nanocrystalline Ni–Mo–La alloy electrode. Chin. J. Nonferrous Met. 2011, 21, 2819–2824. [Google Scholar]
- Ning, L.; Chenghui, G.; Suzhen, Y. Effects of lanthanum on microstructure and properties of amorphous-nanocrystalline Ni–Mo alloy coating. J. Chin. Soc. Rare Earths 2009, 27, 251. (In Chinese) [Google Scholar]
- Frommen, C.; Sørby, M.H.; Heere, M.; Humphries, T.D.; Olsen, J.E.; Hauback, B.C. Rare earth borohydrides-crystal structures and thermal properties. Energies 2017, 10, 2115. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Biswas, K. Effect on de-hydrogenation efficiency on doping of rare earth elements (Pr, Nd, Gd, Dy) in MgH2—A density functional theory study. Int. J. Hydrogen Energy 2017, 42, 1012–1017. [Google Scholar] [CrossRef]
- Lee, D.; Chen, Y. Hydrogenation of p-chloronitrobenzene on La-doped NiMoB nanocluster catalysts. Chin. J. Catal. 2013, 34, 2018–2028. [Google Scholar] [CrossRef]
- Paschoalino, W.J.; Thompson, S.J.; Russell, A.E.; Ticianelli, E.A. The borohydride oxidation reaction on La-Ni-based hydrogen-storage alloys. Chemphyschem 2014, 15, 2170–2176. [Google Scholar] [CrossRef] [PubMed]
- Kemula, W.; Kublik, Z. Application de la goutte pendante de mercure à la détermination de minimes quantités de différents ions: Communiqué préliminaire. Anal. Chim. Acta 1958, 18, 104–111. [Google Scholar] [CrossRef]
- Zeng, Y.; Yu, S.; Li, Z.; Chen, K.; Zhou, S. Kinetic model of ethanol oxidation on Ni–Mo alloy electrode. Acta Phys. Chim. Sin. 2000, 16, 1013–1021. [Google Scholar] [CrossRef]
- Wu, D.; Liang, G.; Li, L.; Wu, H. Microstructural investigation of electrochemical hydrogen storage in amorphous Mg–Ni–La alloy. Mater. Sci. Eng. B 2010, 175, 248–252. [Google Scholar] [CrossRef]
- Paul, M.S. Electrodeposition of thin-film rare-earth-metal oxocuprates. J. Mater. Chem. 1996, 6, 183–186. [Google Scholar] [CrossRef]
- Herbst, J.F.; Hector, L.G., Jr. La(TM)5 hydrides (TM = Fe, Co, Ni): Theoretical perspectives. J. Alloys Compd. 2007, 446–447, 188–194. [Google Scholar] [CrossRef]
- Huang, Z.; Li, J.; Rao, Q.; Zhou, Y. Effects of La content on the glass transition and crystallization process of Al–Ni–La amorphous alloys. Intermetallics 2007, 15, 1139–1146. [Google Scholar] [CrossRef]
- Scherrer, P. Bestimmung der Größe und der inneren Struktur von Kolloidteilchen mittels Röntgenstrahlen. In Kolloidchemie Ein Lehrbuch; Zsigmondy, R., Ed.; Springer-Verlag: Berlin, Germany, 1918; pp. 98–100. [Google Scholar]
- Spiller, E. X-ray optics. In Encyclopedia of Optical Engineering; Driggers, R.G., Hoffman, C., Driggers, R., Eds.; CRC Press: Boca Raton, FL, USA, 2003; pp. 3042–3048. [Google Scholar]
- Kuang, W.; Fan, Y.; Chen, Y. Structure and reactivity of ultrafine Ce–Mo oxide particles. Catal. Today 2001, 68, 75–82. [Google Scholar] [CrossRef]
- Jiang, C.; Yang, Z.; Xiang, L.; Shang, J.; Cheng, M.; Min, D. Dose-dependent effects of lanthanum chloride on wear particle-induced aseptic inflammation in a murine air-pouch model. J. Rear Earths 2013, 4, 420–427. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, X.; Yang, Y.; Yang, Y.; Peng, H.; Luo, H. Preparation of La–Ni–Mo–B amorphous catalyst and its catalytic properties for hydrodeoxygenation of phenol. Acta Phys. Chim. Sin. 2012, 28, 1243–1251. [Google Scholar]
- You, Y.; Yan, M. Behaviors and interactions of La atom with other foreign substitutional atoms (Al, Si, Ti, V, Cr, Mn, Co, Ni, Cu, Nb or Mo) in iron based solid solution from first principles. Comput. Mater. Sci. 2013, 73, 120–127. [Google Scholar] [CrossRef]
- Terashima, M.; Takada, N.; Saga, H.; Kohn, K. Metallic conductivity of the oxides of La–Ni–O system. Phase Transit. 1993, 41, 123–128. [Google Scholar] [CrossRef]
- Lačnjevac, U.; Jović, B.M.; Baščarević, Z.; Maksimović, V.M.; Jović, V.D. Morphology and phase composition of as-deposited and recrystallized Ni–Mo–O powders. Electrochim. Acta 2009, 54, 3115–3123. [Google Scholar] [CrossRef]
- Zeng, L.; Xiao, L.; Xiao, C.; Gong, B. Study on leaching of molybdenum and nickel from Ni–Mo ore using sodium chlorate. Can. Met. Q. 2013, 52, 335–341. [Google Scholar] [CrossRef]
- De la Torre, A.I.; Banda, J.A.; García, U.P.; Alvarado, D.I.; García, M.A.; Martínez, B.P. Crystallographic properties of the unsupported Ni–Mo carbides phases. Adv. Mater. Phys. Chem. 2013, 3, 206–208. [Google Scholar] [CrossRef]
- Li, Y.; Tao, Y.; Ke, D.; Yang, S.; Han, S. Facile synthesis of Mo–Ni particles and their effect on the electrochemical kinetic properties of La–Mg–Ni-based alloy electrodes. J. Alloys Compd. 2014, 615, 91–95. [Google Scholar] [CrossRef]
- Wang, W.; Yang, Y.; Luo, H.; Liu, W. Effect of additive (Co, La) for Ni–Mo–B amorphous catalyst and its hydrodeoxygenation properties. Catal. Commun. 2010, 11, 803–807. [Google Scholar] [CrossRef]
- Gangopadhyay, A.K.; Kelton, K.F. Effect of rare-earth atomic radius on the devitrification of Al88Re8Ni4 amorphous alloys. Philos. Mag. 2000, 80, 1193–1206. [Google Scholar] [CrossRef]
- Suran, F.; Lemin, L. Determination of the stability constants of rare earth-tetracycline-citric (malic, tartaric, salicylic) acids mixed ligand complexes. In New Frontiers in Rare Earth Science and Applications; Xu, G., Xiao, J., Eds.; Academic Press: Cambridge, MA, USA, 1985; pp. 127–130. [Google Scholar]
- Gómez, E.; Pellicer, E.; Vallés, E. Influence of the bath composition and the pH on the induced cobalt-molybdenum electrodeposition. Electroanal. Chem. 2003, 556, 137–145. [Google Scholar] [CrossRef]
- Chen, Q.; Chen, M.; Mao, S.; Zhou, Z. Progress in coordinated structural chemistry to citrate process. Sci. Sin. Chim. 2011, 41, 645–653. [Google Scholar] [CrossRef]
- Yang, J.B.; Tai, C.Y.; Marasinghe, G.K.; Waddill, G.D.; Pringle, O.A.; James, W.J.; Kong, Y. Structural, electronic and magnetic properties of LaNi5−xTx (T = Fe, Mn) compounds. Phys. Rev. B 2001, 63, 4407–4440. [Google Scholar] [CrossRef]
- Yang, J.B.; Tai, C.Y.; Marasinghe, G.K.; Waddill, G.D.; Pringle, O.A.; James, W.J. Electronic structures and magnetism of LaNi5−xFex compounds. J. Appl. Phys. 2001, 89, 7311–7313. [Google Scholar] [CrossRef]
- Dias, C.; Pessine, E.J. The electrochemical behaviour of La3+ over Ni and Mo, in molten salts chloride. In Hydrogen Materials Science and Chemistry of Metal Hydrides; Hampton, M.D., Schur, D.V., Zaginaichenko, S.Y., Trefilov, V.I., Eds.; Springer: Dordrecht, The Netherlands, 2002; pp. 349–356. [Google Scholar]
- Şahin, Ö.; Ekinci, A.; Balbay, A.; Saka, C. Effects of plasma treatment, La content and temperature on the La–Ni–Mo–B catalysts for hydrogen production from NaBH4 hydrolysis. Surf. Eng. 2017, 33, 499–505. [Google Scholar] [CrossRef]
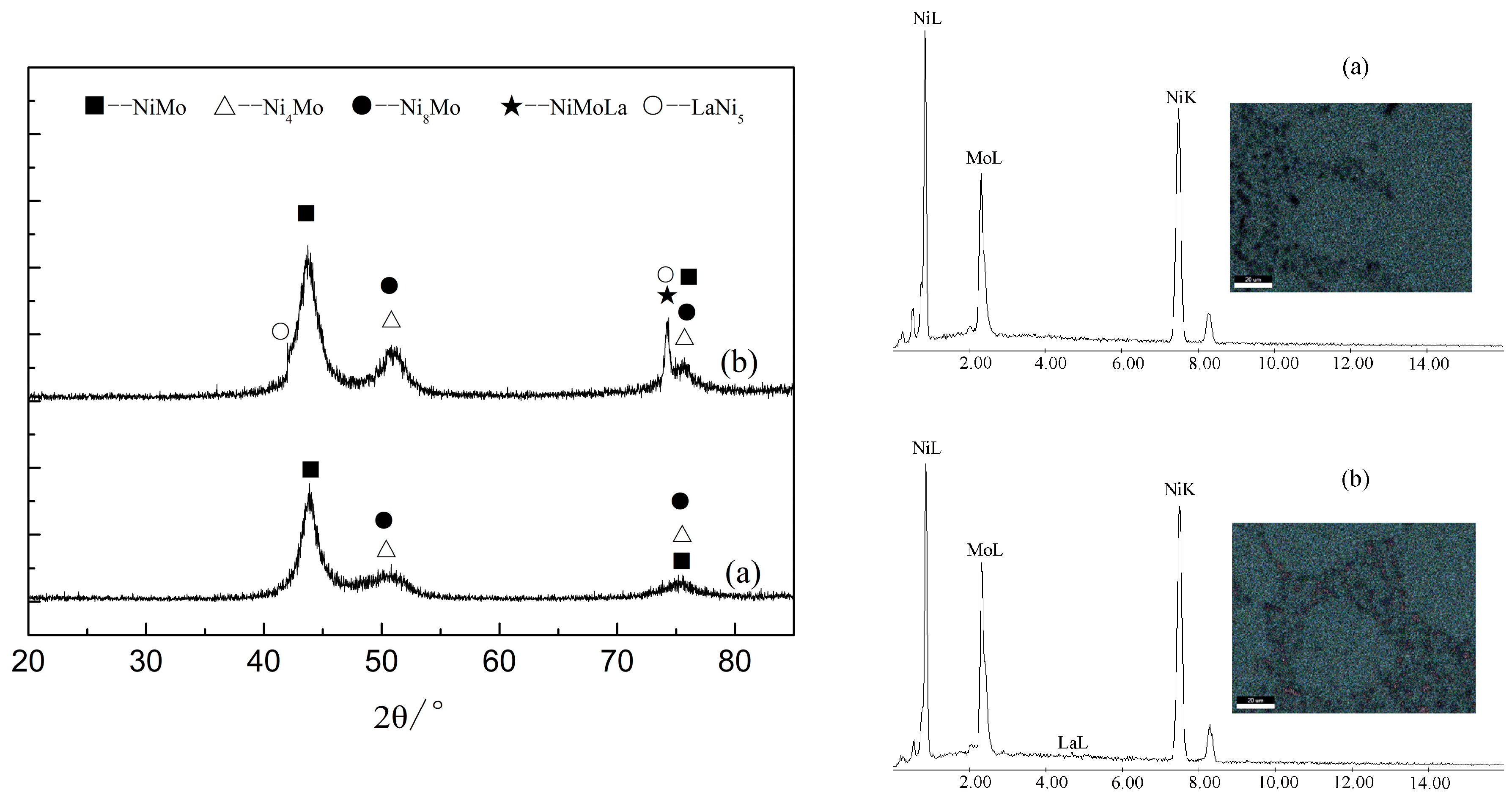
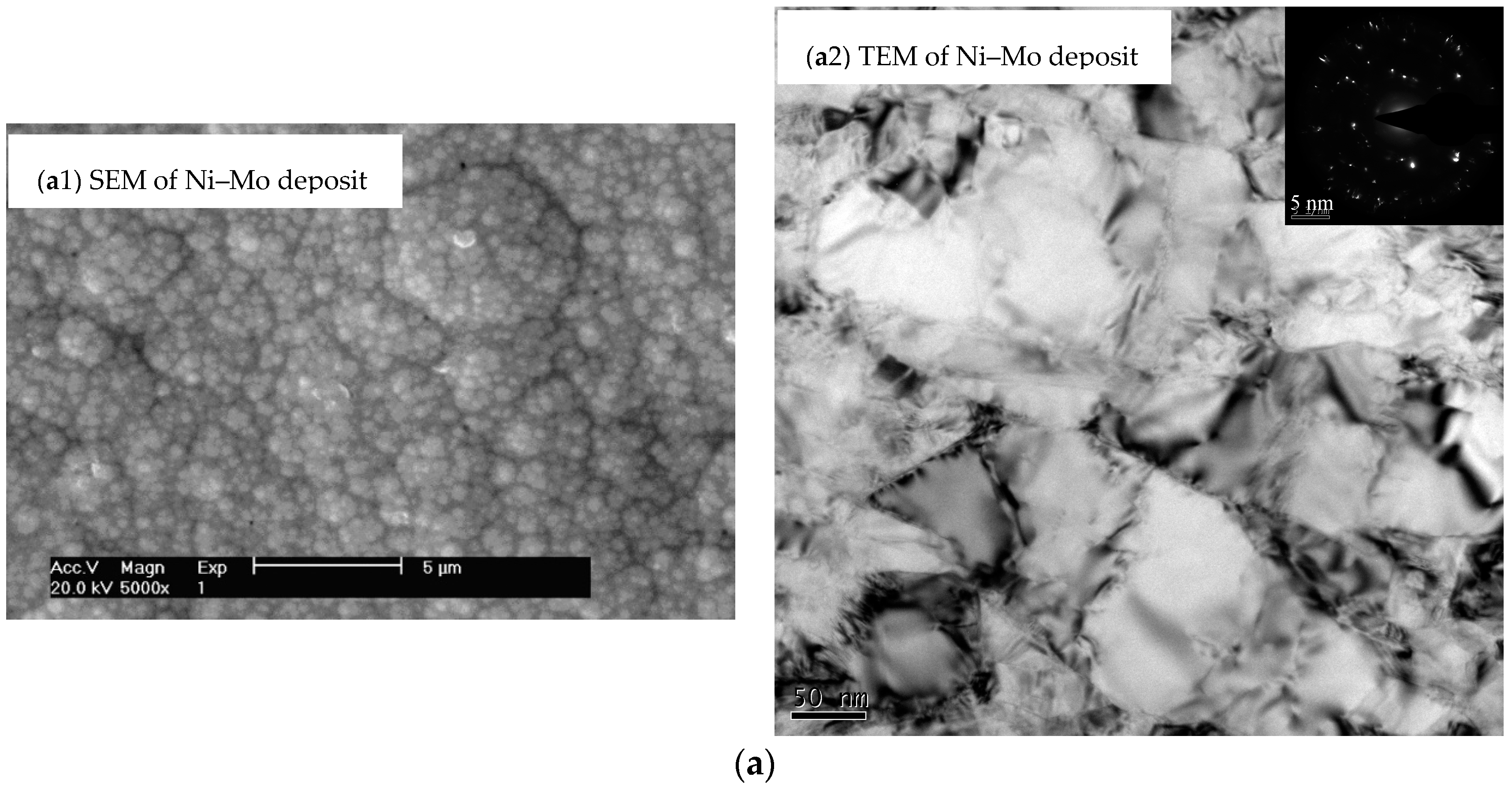
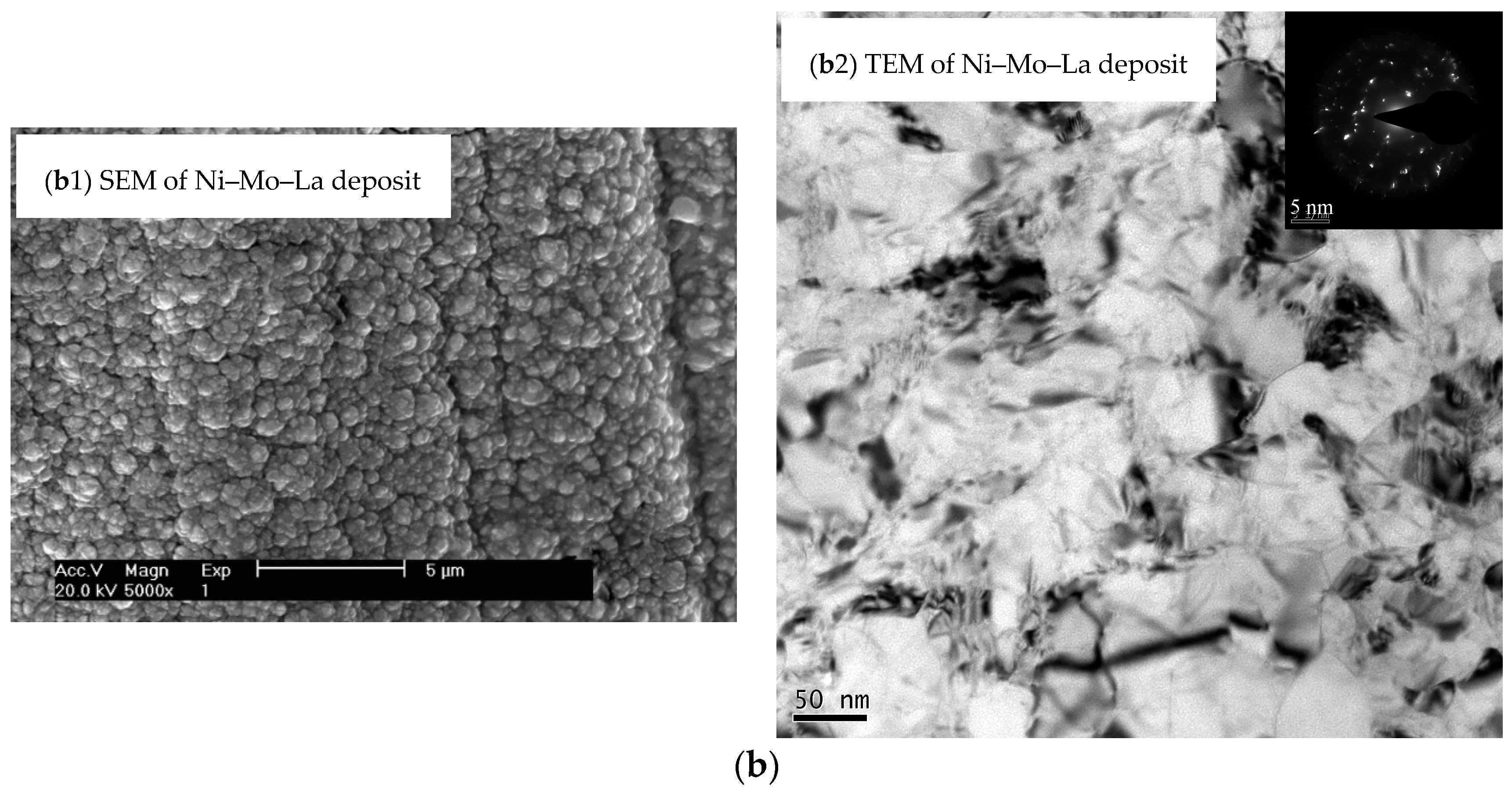




| Element Content | ρ (La) (g/L) | W (Ni) (at.%) | W (Mo) (at.%) | W (La) (at.%) |
|---|---|---|---|---|
| Ni–Mo | 0 | 82.94 | 17.06 | 0 |
| Ni–Mo–La | 1.6 | 74.05 | 25.03 | 0.92 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, N.; Chen, W.; Lu, L.; Gao, C. Electrodeposition Behavior of Polycrystalline Ni–Mo–La Composite in Alkaline Solution. Coatings 2018, 8, 299. https://doi.org/10.3390/coatings8090299
Li N, Chen W, Lu L, Gao C. Electrodeposition Behavior of Polycrystalline Ni–Mo–La Composite in Alkaline Solution. Coatings. 2018; 8(9):299. https://doi.org/10.3390/coatings8090299
Chicago/Turabian StyleLi, Ning, Weizeng Chen, Lirong Lu, and Chenghui Gao. 2018. "Electrodeposition Behavior of Polycrystalline Ni–Mo–La Composite in Alkaline Solution" Coatings 8, no. 9: 299. https://doi.org/10.3390/coatings8090299




