Mechanical Durability of Engineered Superhydrophobic Surfaces for Anti-Corrosion
Abstract
:1. Introduction
2. Superhydrophobic Corrosion-Resistant Coatings
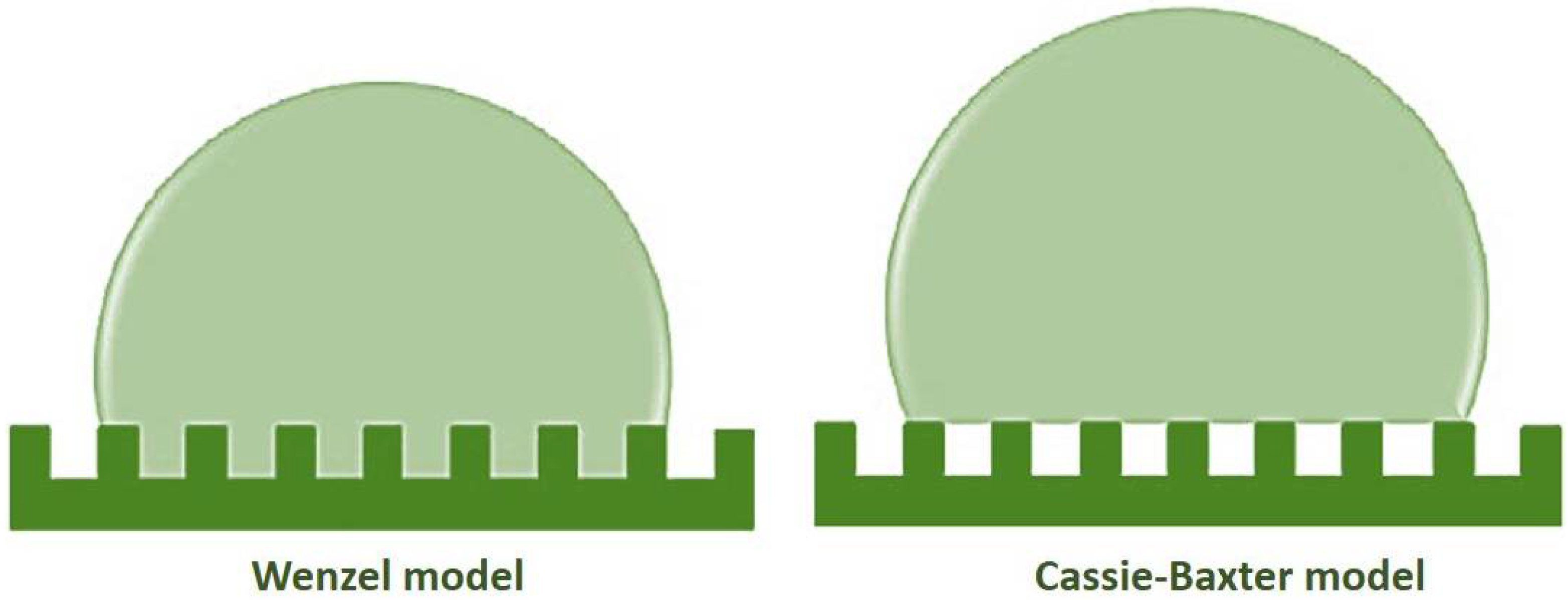
3. Theory of Superhydrophobic Behavior
4. Necessity for Mechanical Durability for Superhydrophobic Coatings
5. Various Superhydrophobic Coatings for Corrosion Prevention of Metal Substrates
5.1. Silane-Modified Superhydrophobic Coatings
5.2. Polymer-Based Superhydrophobic Coatings
5.3. Soft Lithography Method
5.4. Titanium Oxide-Based Superhydrophobic Coatings
5.5. Nanoparticle-Based Superhydrophobic Coatings
6. Corrosion Measurement Techniques
6.1. Potentiodynamic Polarization Method
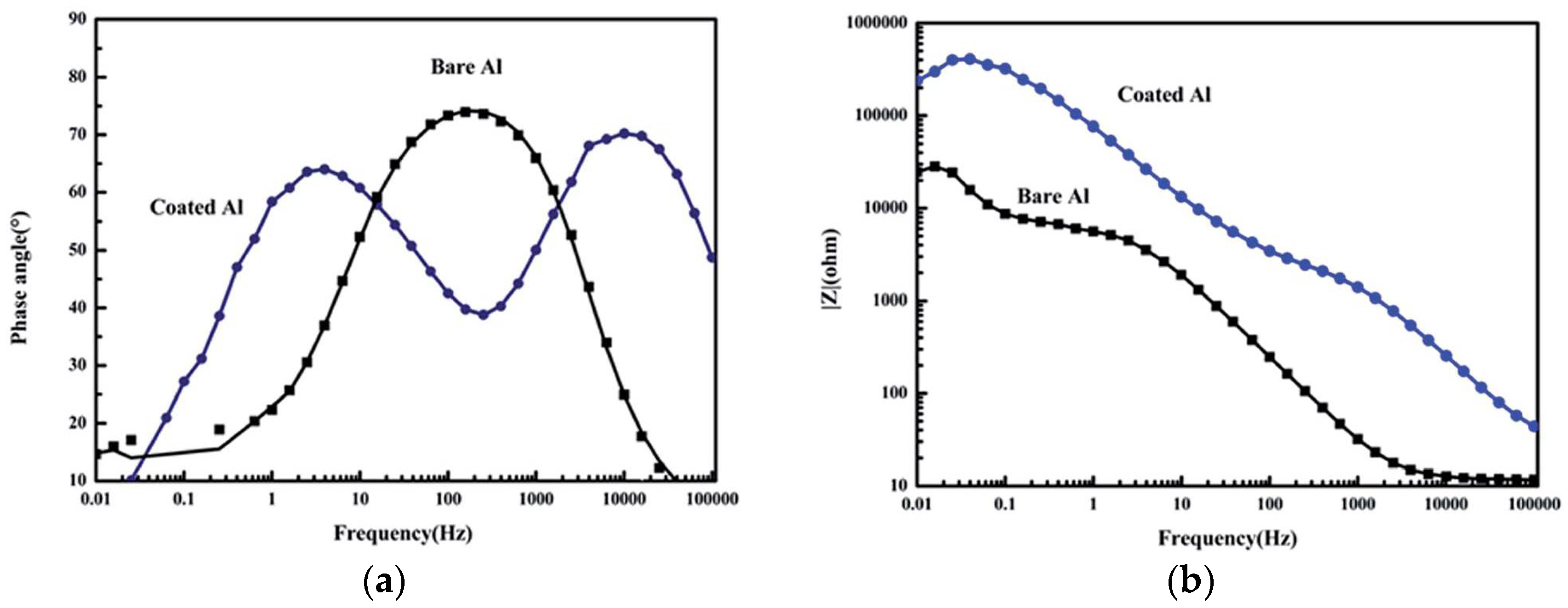
6.2. Electrochemical Impedance Spectroscopy
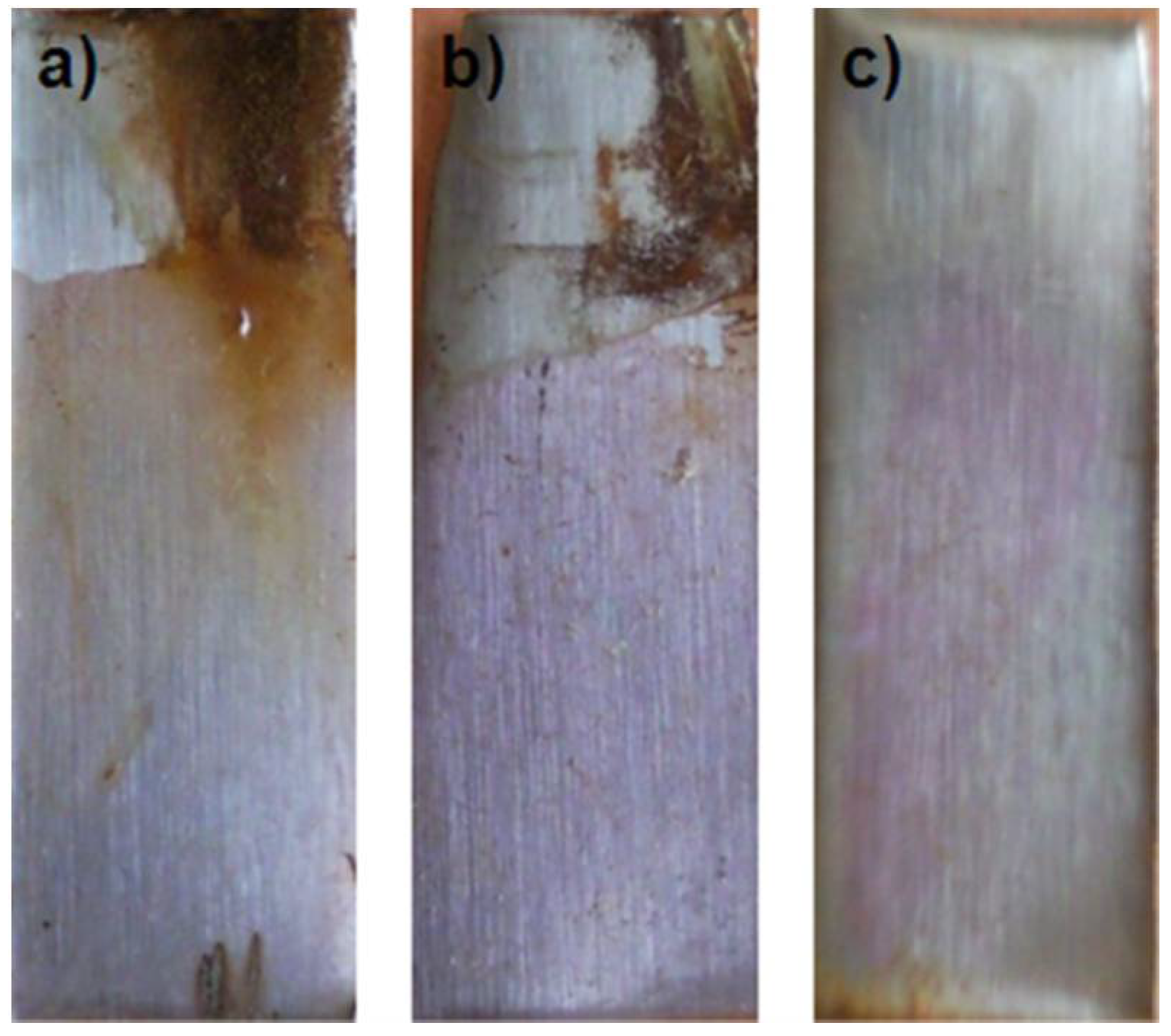
6.3. Salt Fog Test
7. Procedures for Determining Mechanical Stability
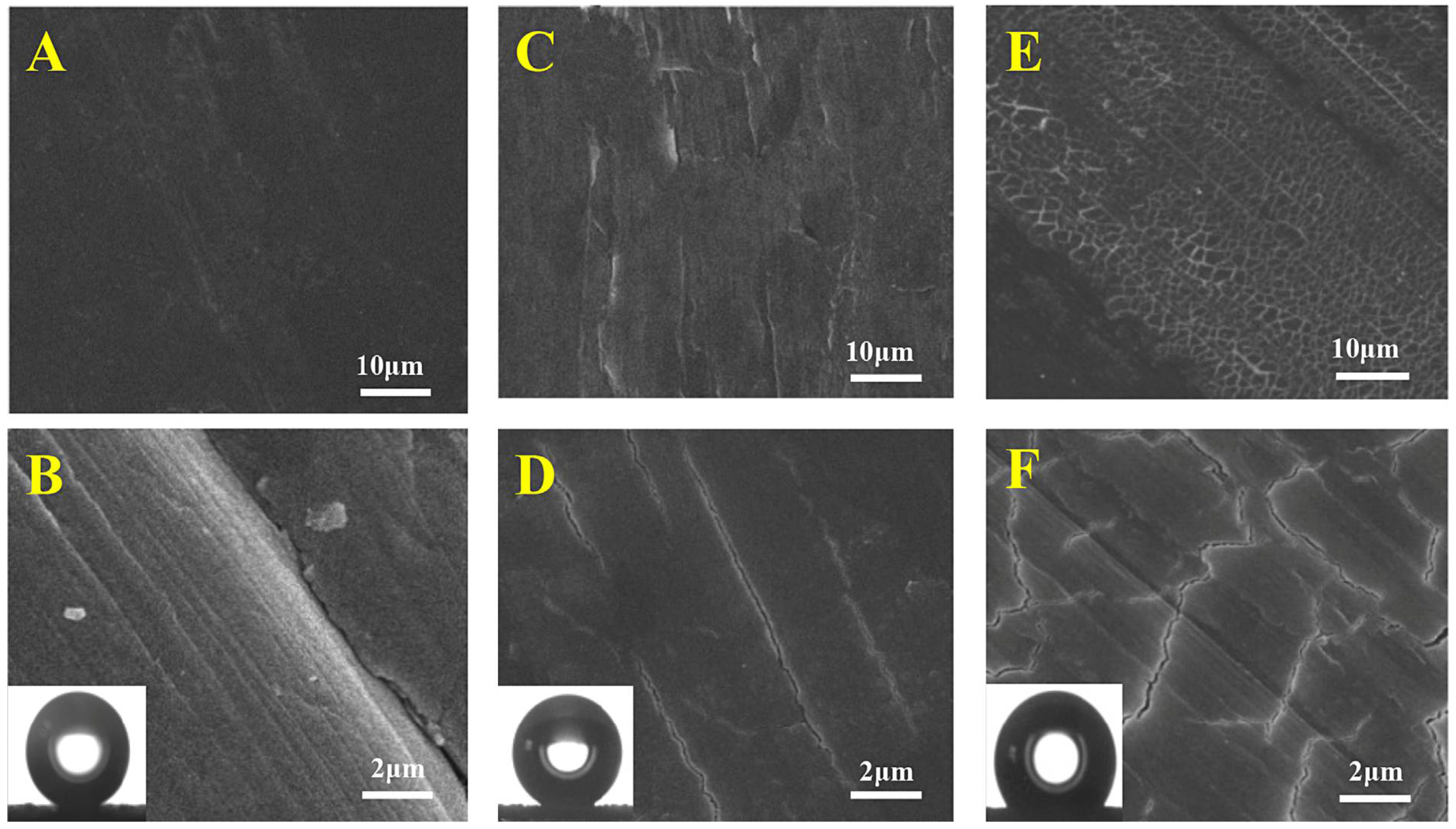
7.1. Abrasion Tests
7.2. AFM Measurements
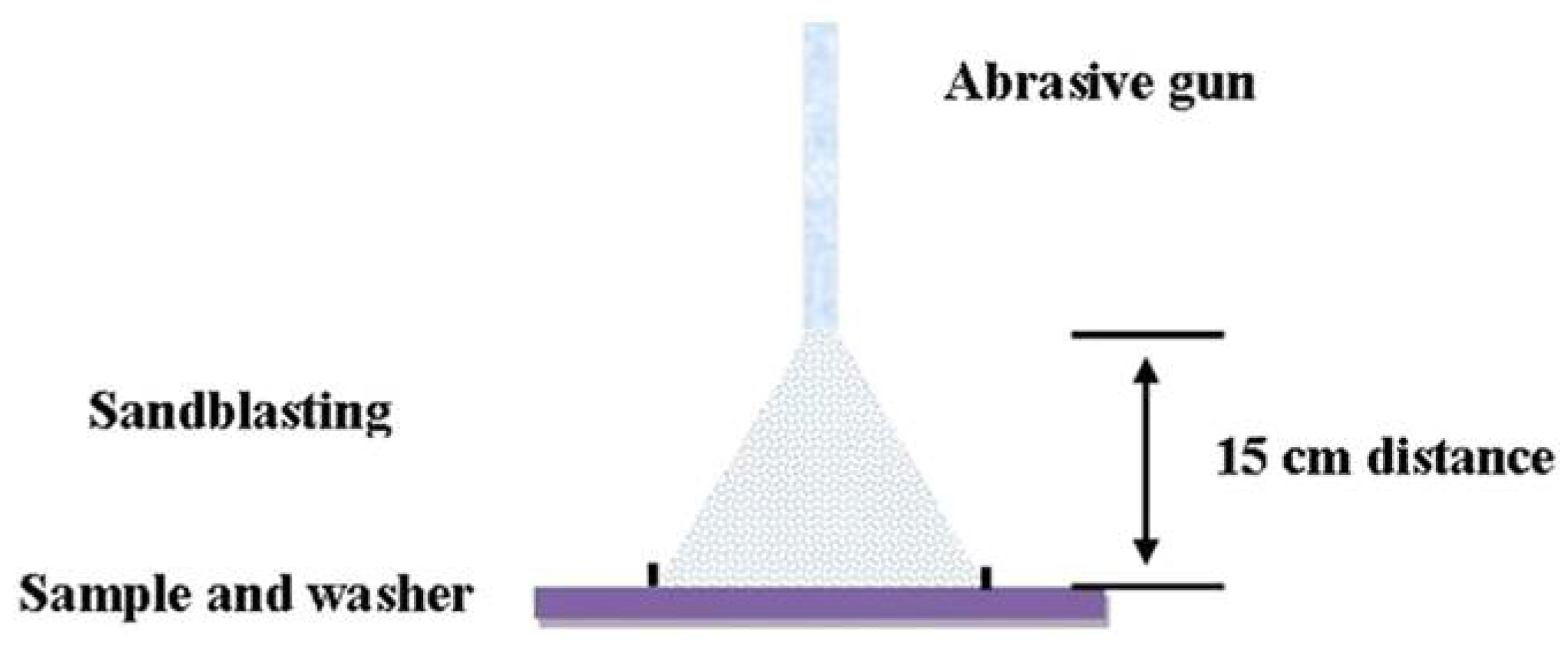
7.3. Sand Blasting Test
7.4. Tape Peeling Test
7.5. Nano-Indentation Test
8. Effectiveness of Various Coatings
9. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Tsutsumi, Y.; Nishikata, A.; Tsuru, T. Pitting Corrosion Mechanism of Type 304 Stainless Steel under a Droplet of Chloride Solutions. Corros. Sci. 2007, 49, 1394–1407. [Google Scholar] [CrossRef]
- Zhang, R.; Castel, A.; François, R. Concrete Cover Cracking with Reinforcement Corrosion of RC Beam during Chloride-Induced Corrosion Process. Cem. Concr. Res. 2010, 40, 415–425. [Google Scholar] [CrossRef]
- Garcia, B.; Armand, M. Aluminium Corrosion in Room Temperature Molten Salt. J. Power Sources 2004, 132, 206–208. [Google Scholar] [CrossRef]
- Vanboven, G.; Chen, W.; Rogge, R. The Role of Residual Stress in Neutral Ph Stress Corrosion Cracking of Pipeline Steels. Part I: Pitting and Cracking Occurrence. Acta Mater. 2007, 55, 29–42. [Google Scholar] [CrossRef]
- Melchers, R. Mathematical Modelling of the Diffusion Controlled Phase in Marine Immersion Corrosion of Mild Steel. Corros. Sci. 2003, 45, 923–940. [Google Scholar] [CrossRef]
- Johnston, J. The Mechanism of Corrosion. Ind. Eng. Chem. 1923, 15, 904–905. [Google Scholar] [CrossRef]
- Davy, H. On the Corrosion of Copper Sheeting by Sea Water, and on Methods of Preventing This Effect; And on Their Application to Ships of War and Other Ships. Philos. Trans. R. Soc. Lond. 1824, 114, 151–158. [Google Scholar] [CrossRef]
- Ganjaee Sari, M.; Ramezanzadeh, B.; Shahbazi, M.; Pakdel, A. Influence of Nanoclay Particles Modification by Polyester-Amide Hyperbranched Polymer on the Corrosion Protective Performance of the Epoxy Nanocomposite. Corros. Sci. 2015, 92, 162–172. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, D.; Lu, Z. Advantage of Super-Hydrophobic Surface as a Barrier against Atmospheric Corrosion Induced by Salt Deliquescence. Corros. Sci. 2015, 90, 23–32. [Google Scholar] [CrossRef]
- Ramezanzadeh, B.; Niroumandrad, S.; Ahmadi, A.; Mahdavian, M.; Moghadam, M. Enhancement of Barrier and Corrosion Protection Performance of an Epoxy Coating through Wet Transfer of Amino Functionalized Graphene Oxide. Corros. Sci. 2016, 103, 283–304. [Google Scholar] [CrossRef]
- Graziani, G.; Sassoni, E.; Scherer, G.; Franzoni, E. Resistance to Simulated Rain of Hydroxyapatite- and Calcium Oxalate-Based Coatings for Protection of Marble Against Corrosion. Corros. Sci. 2017, 127, 168–174. [Google Scholar] [CrossRef]
- Aguirre-Guerrero, A.; Robayo-Salazar, R.; de Gutiérrez, R. A Novel Geopolymer Application: Coatings to Protect Reinforced Concrete against Corrosion. Appl. Clay Sci. 2017, 135, 437–446. [Google Scholar] [CrossRef]
- Mrad, M.; Ben Amor, Y.; Dhouibi, L.; Montemor, M. Corrosion Prevention of AA2024-T3 Aluminum Alloy with A Polyaniline/Poly(Γ-Glycidoxypropyltrimethoxysilane) Bi-Layer Coating: Comparative Study with Polyaniline Mono-Layer Feature. Surf. Coat. Technol. 2018, 337, 1–11. [Google Scholar] [CrossRef]
- Aneja, K.; Böhm, H.; Khanna, A.; Böhm, S. Functionalised Graphene as a Barrier Against Corrosion. FlatChem 2017, 1, 11–19. [Google Scholar] [CrossRef]
- Figueira, R.; Silva, C.; Pereira, E. Organic–Inorganic Hybrid Sol–Gel Coatings for Metal Corrosion Protection: A Review of Recent Progress. J. Coat. Technol. Res. 2014, 12, 1–35. [Google Scholar] [CrossRef]
- Liu, T.; Chen, S.; Cheng, S.; Tian, J.; Chang, X.; Yin, Y. Corrosion behavior of super-hydrophobic surface on copper in seawater. Electrochim. Acta 2007, 52, 8003–8007. [Google Scholar] [CrossRef]
- Huang, Y.; Sarkar, D.K.; Gallant, D.; Chen, X.-G. Corrosion resistance properties of superhydrophobic copper surfaces fabricated by one-step electrochemical modification process. Appl. Surf. Sci. 2013, 282, 689–694. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, D.; Qiu, R.; Wan, Y.; Wu, J. Green approach to fabrication of a super-hydrophobic film on copper and the consequent corrosion resistance. Corros. Sci. 2014, 80, 366–373. [Google Scholar] [CrossRef]
- Liu, Y.; Li, S.; Zhang, J.; Liu, J.; Han, Z.; Ren, L. Corrosion inhibition of biomimetic super-hydrophobic electrodeposition coating on copper substrate. Corros. Sci. 2015, 94, 190–196. [Google Scholar] [CrossRef]
- Thakur, V.; Kessler, M. Self-Healing Polymer Nanocomposite Materials: A Review. Polymer 2015, 69, 369–383. [Google Scholar] [CrossRef]
- Ulaeto, S.; Rajan, R.; Pancrecious, J.; Rajan, T.; Pai, B. Developments in Smart Anticorrosive Coatings with Multifunctional Characteristics. Prog. Org. Coat. 2017, 111, 294–314. [Google Scholar] [CrossRef]
- Asri, N.; Husaini, T.; Sulong, A.; Majlan, E.; Daud, W. Coating of Stainless Steel and Titanium Bipolar Plates for Anticorrosion In PEMFC: A Review. Int. J. Hydrogen Energy 2017, 42, 9135–9148. [Google Scholar] [CrossRef]
- Gao, X.; Liu, H.; Cheng, F.; Chen, Y. Thermoresponsive Polyaniline Nanoparticles: Preparation, Characterization, and Their Potential Application in Waterborne Anticorrosion Coatings. Chem. Eng. J. 2016, 283, 682–691. [Google Scholar] [CrossRef]
- Sørensen, P.; Kiil, S.; Dam-Johansen, K.; Weinell, C. Anticorrosive Coatings: A Review. J. Coat. Technol. Res. 2009, 6, 135–176. [Google Scholar] [CrossRef]
- Zheludkevich, M.; Shchukin, D.; Yasakau, K.; Möhwald, H.; Ferreira, M. Anticorrosion Coatings with Self-Healing Effect Based on Nanocontainers Impregnated with Corrosion Inhibitor. Chem. Mater. 2007, 19, 402–411. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, L.; Qian, H.; Li, X. Superhydrophobic Surfaces for Corrosion Protection: A Review of Recent Progresses and Future Directions. J. Coat. Technol. Res. 2015, 13, 11–29. [Google Scholar] [CrossRef]
- Milionis, A.; Loth, E.; Bayer, I. Recent Advances in the Mechanical Durability of Superhydrophobic Materials. Adv. Colloid Interface Sci. 2016, 229, 57–79. [Google Scholar] [CrossRef] [PubMed]
- Puliyalil, H.; Filipič, G.; Cvelbar, U. Recent Advances in the Methods for Designing Superhydrophobic Surfaces. Surf. Energy 2015. [Google Scholar] [CrossRef]
- Darmanin, T.; Guittard, F. Superhydrophobic and Superoleophobic Properties in Nature. Mater. Today 2015, 18, 273–285. [Google Scholar] [CrossRef]
- Zhou, C.; Chen, Z.; Yang, H.; Hou, K.; Zeng, X.; Zheng, Y.; Cheng, J. Nature-Inspired Strategy Toward Superhydrophobic Fabrics for Versatile Oil/Water Separation. ACS Appl. Mater. Interfaces 2017, 9, 9184–9194. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.; Abdullah, A.; Younan, N. Corrosion Behavior of Superhydrophobic Surfaces: A Review. Arab. J. Chem. 2015, 8, 749–765. [Google Scholar] [CrossRef]
- Lin, Y.; Shen, Y.; Liu, A.; Zhu, Y.; Liu, S.; Jiang, H. Bio-Inspiredly Fabricating the Hierarchical 3D Porous Structure Superhydrophobic Surfaces for Corrosion Prevention. Mater. Des. 2016, 103, 300–307. [Google Scholar] [CrossRef]
- Ou, J.; Hu, W.; Xue, M.; Wang, F.; Li, W. Superhydrophobic Surfaces on Light Alloy Substrates Fabricated by A Versatile Process and Their Corrosion Protection. ACS Appl. Mater. Interfaces 2013, 5, 3101–3107. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Li, Y.; Zhang, P.; Ma, J.; Jia, S. Washable and Wear-Resistant Superhydrophobic Surfaces with Self-Cleaning Property by Chemical Etching of Fibers and Hydrophobization. ACS Appl. Mater. Interfaces 2014, 6, 10153–10161. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Liu, Y.; Ma, M.; Zhu, J. Facile Preparation of Robust and Superhydrophobic Materials for Self-Cleaning and Oil/Water Separation. Colloids Surf. A Physicochem. Eng. Asp. 2017, 529, 18–25. [Google Scholar] [CrossRef]
- Kumar, D.; Li, L.; Chen, Z. Mechanically Robust Polyvinylidene Fluoride (PVDF) Based Superhydrophobic Coatings for Self-Cleaning Applications. Prog. Org. Coat. 2016, 101, 385–390. [Google Scholar] [CrossRef]
- Wang, P.; Chen, M.; Han, H.; Fan, X.; Liu, Q.; Wang, J. Transparent and Abrasion-Resistant Superhydrophobic Coating with Robust Self-Cleaning Function in Either Air or Oil. J. Mater. Chem. A 2016, 4, 7869–7874. [Google Scholar] [CrossRef]
- Sun, K.; Yang, H.; Xue, W.; He, A.; Zhu, D.; Liu, W.; Adeyemi, K.; Cao, Y. Anti-Biofouling Superhydrophobic Surface Fabricated by Picosecond Laser Texturing of Stainless Steel. Appl. Surf. Sci. 2018, 436, 263–267. [Google Scholar] [CrossRef]
- Zhou, X.; Lee, Y.; Chong, K.; He, C. Superhydrophobic And Slippery Liquid-Infused Porous Surfaces Formed by The Self-Assembly of a Hybrid ABC Triblock Copolymer and Their Antifouling Performance. J. Mater. Chem. B 2018, 6, 440–448. [Google Scholar] [CrossRef]
- Feng, Y.; Chen, S.; Cheng, Y.F. Stearic Acid Modified Zinc Nano-Coatings with Superhydrophobicity and Enhanced Antifouling Performance. Surf. Coat. Technol. 2018, 340, 55–65. [Google Scholar] [CrossRef]
- Liu, Y.; Su, Y.; Zhao, X.; Li, Y.; Zhang, R.; Jiang, Z. Improved Antifouling Properties of Polyethersulfone Membrane by Blending the Amphiphilic Surface Modifier with Crosslinked Hydrophobic Segments. J. Membr. Sci. 2015, 486, 195–206. [Google Scholar] [CrossRef]
- Spasova, M.; Manolova, N.; Markova, N.; Rashkov, I. Superhydrophobic PVDF and PVDF-HFP Nanofibrous Mats with Antibacterial and Anti-Biofouling Properties. Appl. Surf. Sci. 2016, 363, 363–371. [Google Scholar] [CrossRef]
- Geraldi, N.; Dodd, L.; Xu, B.; Wells, G.; Wood, D.; Newton, M.; McHale, G. Drag Reduction Properties of Superhydrophobic Mesh Pipes. Surf. Topogr. Metrol. Prop. 2017, 5, 034001. [Google Scholar] [CrossRef]
- Taghvaei, E.; Moosavi, A.; Nouri-Borujerdi, A.; Daeian, M.; Vafaeinejad, S. Superhydrophobic Surfaces with A Dual-Layer Micro- and Nanoparticle Coating for Drag Reduction. Energy 2017, 125, 1–10. [Google Scholar] [CrossRef]
- Aziz, H.; Tafreshi, H. Role of Particles Spatial Distribution in Drag Reduction Performance of Superhydrophobic Granular Coatings. Int. J. Multiph. Flow 2018, 98, 128–138. [Google Scholar] [CrossRef]
- Cai, C.; Sang, N.; Teng, S.; Shen, Z.; Guo, J.; Zhao, X.; Guo, Z. Superhydrophobic Surface Fabricated by Spraying Hydrophobic R974 Nanoparticles and The Drag Reduction in Water. Surf. Coat. Technol. 2016, 307, 366–373. [Google Scholar] [CrossRef]
- Eduok, U.; Suleiman, R.; Khaled, M.; Akid, R. Enhancing Water Repellency and Anticorrosion Properties of a Hybrid Silica Coating on Mild Steel. Prog. Org. Coat. 2016, 93, 97–108. [Google Scholar] [CrossRef]
- Latthe, S.; Terashima, C.; Nakata, K.; Fujishima, A. Superhydrophobic Surfaces Developed by Mimicking Hierarchical Surface Morphology of Lotus Leaf. Molecules 2014, 19, 4256–4283. [Google Scholar] [CrossRef] [PubMed]
- Alexander, S.; Eastoe, J.; Lord, A.; Guittard, F.; Barron, A. Branched Hydrocarbon Low Surface Energy Materials for Superhydrophobic Nanoparticle Derived Surfaces. ACS Appl. Mater. Interfaces 2015, 8, 660–666. [Google Scholar] [CrossRef] [PubMed]
- Boscher, N.; Vaché, V.; Carminati, P.; Grysan, P.; Choquet, P. A Simple and Scalable Approach towards the Preparation of Superhydrophobic Surfaces–Importance of the Surface Roughness Skewness. J. Mater. Chem. A 2014, 2, 5744–5750. [Google Scholar] [CrossRef]
- Bharathidasan, T.; Kumar, S.; Bobji, M.; Chakradhar, R.; Basu, B. Effect of Wettability and Surface Roughness on Ice-Adhesion Strength of Hydrophilic, Hydrophobic and Superhydrophobic Surfaces. Appl. Surf. Sci. 2014, 314, 241–250. [Google Scholar] [CrossRef] [Green Version]
- Bormashenko, E. Progress in Understanding Wetting Transitions on Rough Surfaces. Adv. Colloid Interface Sci. 2015, 222, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Kwok, D.; Neumann, A. Contact Angle Measurement and Contact Angle Interpretation. Adv. Colloid Interface Sci. 1999, 81, 167–249. [Google Scholar] [CrossRef]
- Cassie, A.; Baxter, S. Wettability of Porous Surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Wenzel, R. Resistance of Solid Surfaces to Wetting by Water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- El Dessouky, W.; Abbas, R.; Sadik, W.; El Demerdash, A.; Hefnawy, A. Improved Adhesion of Superhydrophobic Layer on Metal Surfaces Via One Step Spraying Method. Arab. J. Chem. 2017, 10, 368–377. [Google Scholar] [CrossRef]
- Tu, K.; Wang, X.; Kong, L.; Chang, H.; Liu, J. Fabrication of Robust, Damage-Tolerant Superhydrophobic Coatings on Naturally Micro-Grooved Wood Surfaces. RSC Adv. 2016, 6, 701–707. [Google Scholar] [CrossRef]
- Gu, C.; Ren, H.; Tu, J.; Zhang, T. Micro/Nanobinary Structure of Silver Films on Copper Alloys with Stable Water-Repellent Property Under Dynamic Conditions. Langmuir 2009, 25, 12299–12307. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhang, X.; Hu, J. Corrosion Protection of Mild Steel by One-Step Electrodeposition of Superhydrophobic Silica Film. Corros. Sci. 2014, 85, 482–487. [Google Scholar] [CrossRef]
- Calabrese, L.; Bonaccorsi, L.; Caprì, A.; Proverbio, E. Adhesion Aspects of Hydrophobic Silane Zeolite Coatings for Corrosion Protection of Aluminium Substrate. Prog. Org. Coat. 2014, 77, 1341–1350. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Q.; Guo, Z.; Shi, T.; Yu, J.; Tang, M.; Huang, X. Fabrication of Superhydrophobic Surface with Improved Corrosion Inhibition on 6061 Aluminum Alloy Substrate. Appl. Surf. Sci. 2015, 342, 76–83. [Google Scholar] [CrossRef]
- Jeeva Jothi, K.; Palanivelu, K. Facile Fabrication of Core–Shell Pr6o11-Zno Modified Silane Coatings for Anti-Corrosion Applications. Appl. Surf. Sci. 2014, 288, 60–68. [Google Scholar] [CrossRef]
- Cho, E.; Chang-Jian, C.; Chen, H.; Chuang, K.; Zheng, J.; Hsiao, Y.; Lee, K.; Huang, J. Robust multifunctional superhydrophobic coatings with enhanced water/oil separation, self-cleaning, anti-corrosion, and anti-biological adhesion. Chem. Eng. J. 2017, 314, 347–357. [Google Scholar] [CrossRef]
- Gao, X.; Guo, Z. Mechanical Stability, Corrosion Resistance of Superhydrophobic Steel and Repairable Durability of Its Slippery Surface. J. Colloid Interface Sci. 2018, 512, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.; Hsu, M.; Lu, H.; Lai, M.; Liu, P.; Hsu, C.; Ji, W.; Chuang, T.; Wei, Y.; Yeh, J.; et al. Room-Temperature Cured Hydrophobic Epoxy/Graphene Composites as Corrosion Inhibitor for Cold-Rolled Steel. Carbon 2014, 66, 144–153. [Google Scholar] [CrossRef]
- Glover, C.; Richards, C.; Baker, J.; Williams, G.; McMurray, H. In-Coating Graphene Nano-Platelets for Environmentally-Friendly Corrosion Protection of Iron. Corros. Sci. 2017, 114, 169–172. [Google Scholar] [CrossRef]
- Wang, H.; Gao, D.; Meng, Y.; Wang, H.; Wang, E.; Zhu, Y. Corrosion-Resistance, Robust and Wear-Durable Highly Amphiphobic Polymer Based Composite Coating Via a Simple Spraying Approach. Prog. Org. Coat. 2015, 82, 74–80. [Google Scholar] [CrossRef]
- Zhang, Z.; Ge, B.; Men, X.; Li, Y. Mechanically Durable, Superhydrophobic Coatings Prepared by Dual-Layer Method for Anti-Corrosion and Self-Cleaning. Colloids Surf. A Physicochem. Eng. Asp. 2016, 490, 182–188. [Google Scholar] [CrossRef]
- Huang, T.; Yeh, L.; Lai, G.; Huang, B.; Yang, T.; Hsu, S.; Lo, A.; Yeh, J. Advanced Superhydrophobic Electroactive Fluorinated Polyimide and Its Application in Anticorrosion Coating. Int. J. Green Energy 2016, 14, 113–120. [Google Scholar] [CrossRef]
- Kothary, P.; Dou, X.; Fang, Y.; Gu, Z.; Leo, S.; Jiang, P. Superhydrophobic Hierarchical Arrays Fabricated by A Scalable Colloidal Lithography Approach. J. Colloid Interface Sci. 2017, 487, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, H.; Yao, Q.; Fan, B.; Wang, C.; Xiong, Y.; Jin, C.; Sun, Q. Biomimetic Taro Leaf-Like Films Decorated on Wood Surfaces Using Soft Lithography for Superparamagnetic and Superhydrophobic Performance. J. Mater. Sci. 2017, 52, 7428–7438. [Google Scholar] [CrossRef]
- Wang, F.; Li, S.; Wang, L. Fabrication of Artificial Super-Hydrophobic Lotus-Leaf-Like Bamboo Surfaces through Soft Lithography. Colloids Surf. A Physicochem. Eng. Asp. 2017, 513, 389–395. [Google Scholar] [CrossRef]
- Peng, C.; Chang, K.; Weng, C.; Lai, M.; Hsu, C.; Hsu, S.; Hsu, Y.; Hung, W.; Wei, Y.; Yeh, J. Nano-Casting Technique to Prepare Polyaniline Surface with Biomimetic Superhydrophobic Structures for Anticorrosion Application. Electrochim. Acta 2013, 95, 192–199. [Google Scholar] [CrossRef]
- Hoshian, S.; Kankuri, E.; Ras, R.; Franssila, S.; Jokinen, V. Water and Blood Repellent Flexible Tubes. Sci. Rep. 2017, 7, 16019. [Google Scholar] [CrossRef] [PubMed]
- Park, E.; Yoon, H.; Kim, D.; Kim, Y.; Kim, Y. Preparation of Self-Cleaning Surfaces with a Dual Functionality of Superhydrophobicity and Photocatalytic Activity. Appl. Surf. Sci. 2014, 319, 367–371. [Google Scholar] [CrossRef]
- Faraldos, M.; Kropp, R.; Anderson, M.; Sobolev, K. Photocatalytic Hydrophobic Concrete Coatings to Combat Air Pollution. Catal. Today 2016, 259, 228–236. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, Y.; Zhang, Z.; Zhang, P. Self-Cleaning Superhydrophobic Surface Based on Titanium Dioxide Nanowires Combined with Polydimethylsiloxane. Appl. Surf. Sci. 2013, 284, 319–323. [Google Scholar] [CrossRef]
- Zhang, X.; Jin, M.; Liu, Z.; Tryk, D.; Nishimoto, S.; Murakami, T.; Fujishima, A. Superhydrophobic TiO2 surfaces: Preparation, Photocatalytic Wettability Conversion, and Superhydrophobic−Superhydrophilic Patterning. J. Phys. Chem. C 2007, 111, 14521–14529. [Google Scholar] [CrossRef]
- Hoshian, S.; Jokinen, V.; Franssila, S. Robust hybrid elastomer/metal-oxide superhydrophobic surfaces. Soft Matter 2016, 12, 6526–6535. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Qian, H.; Wang, L.; Wang, Z.; Du, C.; Li, X.; Zhang, D. Superhydrophobic Carbon Nanotubes/Epoxy Nanocomposite Coating by Facile One-Step Spraying. Surf. Coat. Technol. 2018, 341, 15–23. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, H.; Liu, Z.; Zhu, Y.; Wu, S.; Wang, C.; Zhu, Y. Fabrication of Durable Fluorine-Free Superhydrophobic Polyethersulfone (PES) Composite Coating Enhanced by Assembled MMT-SiO2 Nanoparticles. Appl. Surf. Sci. 2017, 396, 1580–1588. [Google Scholar] [CrossRef]
- Chen, X.; Yuan, J.; Huang, J.; Ren, K.; Liu, Y.; Lu, S.; Li, H. Large-Scale Fabrication of Superhydrophobic Polyurethane/Nano-Al2O3 Coatings by Suspension Flame Spraying for Anti-Corrosion Applications. Appl. Surf. Sci. 2014, 311, 864–869. [Google Scholar] [CrossRef]
- Chen, K.; Zhou, S.; Wu, L. Facile Fabrication of Self-Repairing Superhydrophobic Coatings. Chem. Commun. 2014, 50, 11891–11894. [Google Scholar] [CrossRef] [PubMed]
- Nahum, T.; Dodiuk, H.; Dotan, A.; Kenig, S.; Lellouche, J. Superhydrophobic Durable Coating Based on UV-Photoreactive Silica Nanoparticles. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Wang, H.; Chen, E.; Jia, X.; Liang, L.; Wang, Q. Superhydrophobic Coatings Fabricated with Polytetrafluoroethylene and SiO2 Nanoparticles by Spraying Process on Carbon Steel Surfaces. Appl. Surf. Sci. 2015, 349, 724–732. [Google Scholar] [CrossRef]
- Atta, A.; El-Mahdy, G.; Al-Lohedan, H.; Al-Hussain, S. Synthesis of Environmentally Friendly Highly Dispersed Magnetite Nanoparticles Based on Rosin Cationic Surfactants as Thin Film Coatings of Steel. Int. J. Mol. Sci. 2014, 15, 6974–6989. [Google Scholar] [CrossRef] [PubMed]
- Ammar, S.; Ramesh, K.; Vengadaesvaran, B.; Ramesh, S.; Arof, A. Amelioration of Anticorrosion and Hydrophobic Properties of Epoxy/PDMS Composite Coatings Containing Nano Zno Particles. Prog. Org. Coat. 2016, 92, 54–65. [Google Scholar] [CrossRef]
- Zhang, X.; Liang, J.; Liu, B.; Peng, Z. Preparation of Superhydrophobic Zinc Coating for Corrosion Protection. Colloids Surf. A Physicochem. Eng. Asp. 2014, 454, 113–118. [Google Scholar] [CrossRef]
- Liu, H.; Wang, X.; Ji, H. Fabrication of Lotus-Leaf-Like Superhydrophobic Surfaces via Ni-Based Nano-Composite Electro-Brush Plating. Appl. Surf. Sci. 2014, 288, 341–348. [Google Scholar] [CrossRef]
- Sun, D.; Brantley, W.; Frankel, G.; Heshmati, R.; Johnston, W. Potentiodynamic Polarization Study of the Corrosion Behavior of Palladium-Silver Dental Alloys. J. Prosthet. Dent. 2018, 119, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Al-Sabagh, A.; Abdou, M.; Migahed, M.; Abd-Elwanees, S.; Fadl, A.; Deiab, A. Investigations Using Potentiodynamic Polarization Measurements, Cure Durability, Ultra Violet Immovability and Abrasion Resistance of Polyamine Cured Ilmenite Epoxy Coating for Oil and Gas Storage Steel Tanks in Petroleum Sector. Egypt. J. Pet. 2017. [Google Scholar] [CrossRef]
- Liu, W.; Xu, Q.; Han, J.; Chen, X.; Min, Y. A Novel Combination Approach for the Preparation of Superhydrophobic Surface on Copper and the Consequent Corrosion Resistance. Corros. Sci. 2016, 110, 105–113. [Google Scholar] [CrossRef]
- Jie, H.; Xu, Q.; Wei, L.; Min, Y. Etching and Heating Treatment Combined Approach for Superhydrophobic Surface on Brass Substrates and the Consequent Corrosion Resistance. Corros. Sci. 2016, 102, 251–258. [Google Scholar] [CrossRef]
- Ishizaki, T.; Kumagai, S.; Tsunakawa, M.; Furukawa, T.; Nakamura, K. Ultrafast Fabrication of Superhydrophobic Surfaces on Engineering Light Metals by Single-Step Immersion Process. Mater. Lett. 2017, 193, 42–45. [Google Scholar] [CrossRef]
- Rezayi, T.; Entezari, M. Toward A Durable Superhydrophobic Aluminum Surface by Etching and Zno Nanoparticle Deposition. J. Colloid Interface Sci. 2016, 463, 37–45. [Google Scholar] [CrossRef] [PubMed]
- McCafferty, E. Validation of Corrosion Rates Measured by the Tafel Extrapolation Method. Corros. Sci. 2005, 47, 3202–3215. [Google Scholar] [CrossRef]
- Shi, Z.; Liu, M.; Atrens, A. Measurement of the Corrosion Rate of Magnesium Alloys Using Tafel Extrapolation. Corros. Sci. 2010, 52, 579–588. [Google Scholar] [CrossRef]
- Elsener, B. Corrosion Rate of Steel in Concrete—Measurements Beyond the Tafel Law. Corros. Sci. 2005, 47, 3019–3033. [Google Scholar] [CrossRef]
- Soleymani, H.; Ismail, M. Comparing Corrosion Measurement Methods to Assess the Corrosion Activity of Laboratory OPC And HPC Concrete Specimens. Cem. Concr. Res. 2004, 34, 2037–2044. [Google Scholar] [CrossRef]
- Ribeiro, D.; Abrantes, J. Application of Electrochemical Impedance Spectroscopy (EIS) To Monitor the Corrosion of Reinforced Concrete: A New Approach. Constr. Build. Mater. 2016, 111, 98–104. [Google Scholar] [CrossRef]
- Ryl, J.; Wysocka, J.; Slepski, P.; Darowicki, K. Instantaneous Impedance Monitoring of Synergistic Effect between Cavitation Erosion and Corrosion Processes. Electrochim. Acta 2016, 203, 388–395. [Google Scholar] [CrossRef]
- Chilcott, T.; Chan, M.; Gaedt, L.; Nantawisarakul, T.; Fane, A.; Coster, H. Electrical Impedance Spectroscopy Characterisation of Conducting Membranes I. Theory. J. Membr. Sci. 2002, 195, 153–167. [Google Scholar] [CrossRef]
- Gaedt, L.; Chilcott, T.; Chan, M.; Nantawisarakul, T.; Fane, A.; Coster, H. Electrical Impedance Spectroscopy Characterisation of Conducting Membranes II. Experimental. J. Membr. Sci. 2002, 195, 169–180. [Google Scholar] [CrossRef]
- Voevodin, N.; Balbyshev, V.; Donley, M. Investigation of Corrosion Protection Performance of Sol–Gel Coatings on AA2024-T3. Prog. Org. Coat. 2005, 52, 28–33. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Li, S.; Wang, Y.; Han, Z.; Ren, L. Fabrication of a Superhydrophobic Graphene Surface with Excellent Mechanical Abrasion and Corrosion Resistance on an Aluminum Alloy Substrate. RSC Adv. 2014, 4, 45389–45396. [Google Scholar] [CrossRef]
- Watson, T.; Gordillo, M.; Ernst, A.; Bedard, B.; Aindow, M. Salt Fog Corrosion Behavior in a Powder-Processed Icosahedral-Phase-Strengthened Aluminum Alloy. Corros. Sci. 2017, 121, 133–138. [Google Scholar] [CrossRef]
- Arunnellaiappan, T.; Arun, S.; Hariprasad, S.; Gowtham, S.; Ravisankar, B.; Rameshbabu, N. Fabrication of Corrosion Resistant Hydrophobic Ceramic Nanocomposite Coatings on PEO Treated AA7075. Ceram. Int. 2018, 44, 874–884. [Google Scholar] [CrossRef]
- Zhang, D.; Qian, H.; Wang, L.; Li, X. Comparison of Barrier Properties for a Superhydrophobic Epoxy Coating under Different Simulated Corrosion Environments. Corros. Sci. 2016, 103, 230–241. [Google Scholar] [CrossRef]
- Dhawan, S.K.; Kumar, A.; Bhandari, H.; Bisht, B.M.S.; Khatoon, F. Development of Highly Hydrophobic and Anticorrosive Conducting Polymer Composite Coating for Corrosion Protection in Marine Environment. Am. J. Polym. Sci. 2015, 5, 7–17. [Google Scholar] [CrossRef]
- Ammar, S.; Ramesh, K.; Vengadaesvaran, B.; Ramesh, S.; Arof, A. A Novel Coating Material That Uses Nano-Sized Sio 2 Particles to Intensify Hydrophobicity and Corrosion Protection Properties. Electrochim. Acta 2016, 220, 417–426. [Google Scholar] [CrossRef]
- Chen, T.; Liu, H.; Yang, H.; Yan, W.; Zhu, W.; Liu, H. Biomimetic Fabrication of Robust Self-Assembly Superhydrophobic Surfaces with Corrosion Resistance Properties on Stainless Steel Substrate. RSC Adv. 2016, 6, 43937–43949. [Google Scholar] [CrossRef]
- Su, F.; Yao, K. Facile Fabrication of Superhydrophobic Surface with Excellent Mechanical Abrasion and Corrosion Resistance on Copper Substrate by a Novel Method. ACS Appl. Mater. Interfaces 2014, 6, 8762–8770. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Kang, Z. Fabrication of Corrosion Resistant Superhydrophobic Surface with Self-Cleaning Property on Magnesium Alloy and Its Mechanical Stability. Surf. Coat. Technol. 2014, 253, 205–213. [Google Scholar] [CrossRef]
- Zhang, Y.; Ge, D.; Yang, S. Spray-Coating of Superhydrophobic Aluminum Alloys with Enhanced Mechanical Robustness. J. Colloid Interface Sci. 2014, 423, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Sundaramali, G.; Jeeva, P.A.; Karthikeyan, S. Surface properties of Pressure Die Cast (PDC) AL 7075 using laser texturing and electrodeposition zinc-nickel alloy coatings. Int. J. ChemTech Res. 2016, 9, 707–711. [Google Scholar]
- Smolyakov, G.; Pruvost, S.; Cardoso, L.; Alonso, B.; Belamie, E.; Duchet-Rumeau, J. AFM Peakforce QNM Mode: Evidencing Nanometre-Scale Mechanical Properties of Chitin-Silica Hybrid Nanocomposites. Carbohydr. Polym. 2016, 151, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Trotsenko, O.; Koestner, R.; Roiter, Y.; Tokarev, A.; Minko, S. Probing Rough Composite Surfaces with Atomic Force Microscopy: Nafion Ionomer in Fuel Cell Electrodes. Polymer 2016, 102, 396–403. [Google Scholar] [CrossRef]
- Zhao, B.; Wang, X.; Song, Y.; Hu, J.; Lü, J.; Zhou, X.; Tai, R.; Zhang, X.; Zhang, L. Stiffness and Evolution of Interfacial Micropancakes Revealed by AFM Quantitative Nanomechanical Imaging. Phys. Chem. Chem. Phys. 2015, 17, 13598–13605. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Li, C.; Fu, Q.; Hu, W.; Xiang, T.; Wang, Q.; Du, M.; Liu, X.; Chen, Z. Development of Stable Superhydrophobic Coatings on Aluminum Surface for Corrosion-Resistant, Self-Cleaning, and Anti-Icing Applications. Mater. Des. 2016, 93, 261–270. [Google Scholar] [CrossRef]
- Kumar, D.; Wu, X.; Fu, Q.; Ho, J.; Kanhere, P.; Li, L.; Chen, Z. Hydrophobic Sol–Gel Coatings Based on Polydimethylsiloxane for Self-Cleaning Applications. Mater. Des. 2015, 86, 855–862. [Google Scholar] [CrossRef]
- Cholewinski, A.; Trinidad, J.; McDonald, B.; Zhao, B. Bio-Inspired Polydimethylsiloxane-Functionalized Silica Particles-Epoxy Bilayer as A Robust Superhydrophobic Surface Coating. Surf. Coat. Technol. 2014, 254, 230–237. [Google Scholar] [CrossRef]
- Su, Y.; de Rooij, M.; Grouve, W.; Warnet, L. Characterisation of Metal–Thermoplastic Composite Hybrid Joints by Means of a Mandrel Peel Test. Compos. Part B Eng. 2016, 95, 293–300. [Google Scholar] [CrossRef]
- Villey, R.; Cortet, P.; Creton, C.; Ciccotti, M. In-Situ Measurement of The Large Strain Response of the Fibrillar Debonding Region During the Steady Peeling of Pressure Sensitive Adhesives. Int. J. Fract. 2016, 204, 175–190. [Google Scholar] [CrossRef]
- Geng, Z.; He, J.; Yao, L. Fabrication of Robust High-Transmittance Superamphiphobic Coatings through Dip-Coating Followed by Spray-Coating. RSC Adv. 2015, 5, 89262–89268. [Google Scholar] [CrossRef]
- Latthe, S.; Sudhagar, P.; Devadoss, A.; Kumar, A.; Liu, S.; Terashima, C.; Nakata, K.; Fujishima, A. A Mechanically Bendable Superhydrophobic Steel Surface with Self-Cleaning and Corrosion-Resistant Properties. J. Mater. Chem. A 2015, 3, 14263–14271. [Google Scholar] [CrossRef]
- Zhi, D.; Lu, Y.; Sathasivam, S.; Parkin, I.; Zhang, X. Large-Scale Fabrication of Translucent and Repairable Superhydrophobic Spray Coatings with Remarkable Mechanical, Chemical Durability and UV Resistance. J. Mater. Chem. A 2017, 5, 10622–10631. [Google Scholar] [CrossRef]
- Zhang, X.; He, J. Hydrogen-Bonding-Supported Self-Healing Antifogging Thin Films. Sci. Rep. 2015, 5, 9227. [Google Scholar] [CrossRef] [PubMed]
- Verma, G.; Dhoke, S.; Khanna, A. Polyester Based-Siloxane Modified Waterborne Anticorrosive Hydrophobic Coating on Copper. Surf. Coat. Technol. 2012, 212, 101–108. [Google Scholar] [CrossRef]
- Dey, S.; Chatterjee, S.; Singh, B.; Bhattacharjee, S.; Rout, T.; Sengupta, D.; Besra, L. Development of Superhydrophobic Corrosion Resistance Coating on Mild Steel by Electrophoretic Deposition. Surf. Coat. Technol. 2018, 341, 24–30. [Google Scholar] [CrossRef]
- Liu, T.; Kim, C. Turning a Surface Superrepellent Even to Completely Wetting Liquids. Science 2014, 346, 1096–1100. [Google Scholar] [CrossRef] [PubMed]
- Hoshian, S.; Jokinen, V.; Somerkivi, V.; Lokanathan, A.; Franssila, S. Robust Superhydrophobic Silicon Without a Low Surface-Energy Hydrophobic Coating. ACS Appl. Mater. Interfaces 2014, 7, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Price, T.; Weiss, M.; Gao, D. Super Water- And Oil-Repellent Surfaces on Intrinsically Hydrophilic and Oleophilic Porous Silicon Films. Langmuir 2008, 24, 1640–1643. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, F.; Chen, H.; Chen, D. Superhydrophobic Behavior Achieved from Hydrophilic Surfaces. Appl. Phys. Lett. 2009, 95, 084104. [Google Scholar] [CrossRef]






© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sebastian, D.; Yao, C.-W.; Lian, I. Mechanical Durability of Engineered Superhydrophobic Surfaces for Anti-Corrosion. Coatings 2018, 8, 162. https://doi.org/10.3390/coatings8050162
Sebastian D, Yao C-W, Lian I. Mechanical Durability of Engineered Superhydrophobic Surfaces for Anti-Corrosion. Coatings. 2018; 8(5):162. https://doi.org/10.3390/coatings8050162
Chicago/Turabian StyleSebastian, Divine, Chun-Wei Yao, and Ian Lian. 2018. "Mechanical Durability of Engineered Superhydrophobic Surfaces for Anti-Corrosion" Coatings 8, no. 5: 162. https://doi.org/10.3390/coatings8050162




