Hydrocolloid-Based Coatings are Effective at Reducing Acrylamide and Oil Content of French Fries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Coating Solutions
2.3. French Fry Preparation and Frying Process
2.4. Acrylamide Standard Preparation
2.5. Extraction of Acrylamide from the Fried Potato Strips
2.6. HPLC-UV Analysis
2.7. Daily Intake (DI) and Margin of Exposure (MOE) of Acrylamide Risk Assessment
2.8. Oil Content
2.9. Water Content Analysis
2.10. Statistical Analysis
3. Results and Discussion
3.1. Effect of Hydrocolloid Coating Solutions on the Acrylamide Content
3.2. Effect of Coating to Acrylamide Risk Assessment
3.3. Effect of Coating Materials on the Water and Oil Content
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tareke, E.; Rydberg, P.; Carlson, P.; Eriksson, S.; Törnqvist, M. Analysis of acrylamide, a carcinogen formed in heated foodstuffs. J. Agric. Food Chem. 2002, 50, 4998–5006. [Google Scholar] [CrossRef] [PubMed]
- Mottram, D.S.; Wedzicha, B.L.; Dodson, A.T. Food chemistry: Acrylamide is formed in the Maillard reaction. Nature 2002, 419, 448–449. [Google Scholar] [CrossRef] [PubMed]
- IARC. Acrylamide. In Monographs on the Evaluation of Carcinogenic Risks to Humans Some Industrial Chemicals; IARC: Lyon, France, 1994; pp. 389–433. [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain). Scientific opinion on acrylamide in food. EFSA J. 2015, 13, 4104. [Google Scholar] [CrossRef] [Green Version]
- Medeiros, R.; Mestdagh, F.; De Meulenaer, B. Acrylamide formation in fried potato product present and future a critical review on mitigation strategies. J. Food Chem. 2011, 50, 8–11. [Google Scholar] [CrossRef]
- Mestdagh, F.; De Wilde, T.; Castelein, P.; Ne’meth, O.; Van Peteghem, C.; De Meulenaer, B. Impact of the reducing sugars on the relationship between acrylamide and Maillard browning in French fries. Eur. Food Res. Technol. 2008, 227, 69–76. [Google Scholar] [CrossRef]
- Gӧkmen, V.; Palazoğlu, T.K.; Şenyuva, H.Z. Relation between the acrylamide formation and time history of surface and core regions of French fries. J. Food Eng. 2006, 77, 972–976. [Google Scholar] [CrossRef]
- Pedreschi, F.; Kaack, K.; Granby, K. Acrylamide content and color development in fried potato strips. Food Res. Int. 2006, 39, 40–46. [Google Scholar] [CrossRef]
- Mesías, M.; Morales, F.J. Acrylamide in commercial potato crisps from Spanish market: Trends from 2004 to 2014 and assessment of the dietary exposure. Food Chem. Toxicol. 2015, 81, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Esposito, F.; Nardone, A.; Fasano, E.; Triassi, M.; Cirillo, T. Determination of acrylamide levels in potato crisps and other snacks and exposure risk assessment through a Margin of Exposure approach. Food Chem. Toxicol. 2017, 108, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Krishnakumar, T.; Visvanathan, R. Acrylamide in food products: A Review. J. Food Process. Technol. 2014, 5, 344. [Google Scholar] [CrossRef]
- Rydberg, P.; Eriksson, S.; Tareke, E.; Karlsson, P.; Ehrenberg, L.; Tornqvist, M. Investigations of factors that influence the acrylamide content of heated foodstuffs. J. Agric. Food Chem. 2003, 51, 7012–7018. [Google Scholar] [CrossRef] [PubMed]
- Mestdagh, F.; Maertens, J.; Cucu, T.; Delporte, K.; Van Peteghem, C.; De Meulenaer, B. Impact of additives to lower the formation of acrylamide in a potato model system through pH reduction and other mechanisms. Food Chem. 2008, 107, 26–31. [Google Scholar] [CrossRef]
- Pedreschi, F.; Granby, K.; Risum, J. Acrylamide mitigation in potato chips by using NaCl. Food Bioprocess Technol. 2010, 3, 917–921. [Google Scholar] [CrossRef]
- Xu, F.; Oruna-Concha, M.-J.; Elmore, J.S. The use of asparaginase to reduce acrylamide levels in cooked food. Food Chem. 2016, 210, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Suyatma, N.E.; Ulfah, K.; Prangdimurti, E.; Ishikawa, Y. Effect of blanching and pectin coating as pre-frying treatments to reduce acrylamide formation in banana chips. Int. Food. Res. J. 2015, 22, 936–942. [Google Scholar]
- Zeng, X.; Cheng, K.-W.; Du, Y.; Kong, R.; Lo, C.; Chu, I.K.; Chen, F.; Wang, M. Activities of hydrocolloids as inhibitors of acrylamide formation in model systems and fried potato strips. Food Chem. 2010, 121, 424–428. [Google Scholar] [CrossRef]
- Sansano, M.; Castello, M.L.; Heredia, A.; Andres, A. Protective effect of chitosan on acrylamide formation in model and batter systems. Food Hydrocoll. 2016, 60, 1–6. [Google Scholar] [CrossRef]
- BeMiller, J.N. Hydrocolloids. In Gluten-Free Cereal Products and Beverage, 1st ed.; Arendt, E.K., Dal Bello, F., Eds.; Elsevier Academic Press: Burlington, NJ, USA, 2008; pp. 203–215. [Google Scholar]
- Kohajdova, Z.; Karovicova, J. Application of hydrocolloids as baking improvers. Chem. Pap. 2009, 63, 26–38. [Google Scholar] [CrossRef]
- Albert, S.; Mittal, G.S. Comparative evaluation of edible coatings to reduce fat uptake in a deep-fried cereal product. Food Res. Int. 2002, 35, 445–458. [Google Scholar] [CrossRef]
- Pedroso, R.A.; Demiate, I.M. Evaluation of the influence of starch and carrageenan on physicochemical and sensory characteristics of cooked turkey ham. Cienc. Tecnol. Aliment. 2008, 28, 24–31. [Google Scholar] [CrossRef]
- Ramos, N.A.G.; Farias, M.E.; Almada, C.; Crivaro, N. Stability of sausages with emulsifiers and hydrocolloids. Inf. Tecnol. 2004, 15, 91–94. [Google Scholar]
- Mariniello, L.; Di Pierro, P.; Esposito, C.; Sorrentino, A.; Masi, P.; Porta, R. Preparation and mechanical properties of edible pectin-soy flour films obtained in the absence or presence of transglutaminase. J. Biotechnol. 2003, 102, 191–198. [Google Scholar] [CrossRef]
- Di Pierro, P.; Chico, B.; Villalonga, R.; Mariniello, L.; Damiao, A.E.; Masi, P.; Porta, R. Chitosan-whey protein edible films produced in the absence or presence of transglutaminase: Analysis of their mechanical and barrier properties. Biomacromolecules 2006, 7, 744–749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porta, R.; Mariniello, L.; Di Pierro, P.; Sorrentino, A.; Giosafatto, C.V.L. Transglutaminase crosslinked pectin-and chitosan-based edible films: A review. Crit. Rev. Food Sci. Nutr. 2011, 51, 223–238. [Google Scholar] [CrossRef] [PubMed]
- Giosafatto, C.V.L.; Di Pierro, P.; Gunning, P.; Mackie, A.; Porta, R.; Mariniello, L. Characterization of citrus pectin edible films containing transglutaminase-modified phaseolin. Carbohydr. Polym. 2014, 106, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Porta, R.; DiPierro, P.; Sabbah, M.; Regalado-Gonzales, C.; Mariniello, L.; Kadivar, M.; Arabestani, A. Blend films of pectin and bitter vetch (Vicia ervilia) proteins: Properties and effect of transglutaminase. Innov. Food Sci. Emerg. Technol. 2016, 36, 245–251. [Google Scholar] [CrossRef]
- Rossi Marquez, G.; Di Pierro, P.; Esposito, M.; Mariniello, L.; Porta, R. Application of transglutaminase-crosslinked whey protein/pectin films as water barrier coatings in fried and baked foods. Food Bioprocess Technol. 2013, 7, 447–455. [Google Scholar] [CrossRef]
- Kurek, M.; Scetar, M.; Gali, K. Edible coatings minimize fat uptake in deep fat fried products: A Review. Food Hydrocoll. 2017, 71, 225–235. [Google Scholar] [CrossRef]
- Daraei Garmakhany, A.; Mirzaei, H.O.; Maghsudlo, Y.; Kashaninejad, M.; Jafari, S.M. Production of low fat French-fries with single and multi-layer hydrocolloid coatings. J. Food Sci. Technol. 2014, 51, 1334–1341. [Google Scholar] [CrossRef] [PubMed]
- Hua, X.; Wang, K.; Yang, R.; Kang, J.; Yang, H. Edible coatings from sunflower head pectin to reduce lipid uptake in fried potato chips. LWT Food Sci. Technol. 2015, 62, 1220–1225. [Google Scholar] [CrossRef]
- Tavera-Quiroz, M.J.; Urriza, M.; Pinotti, A.; Bertola, N. Plasticized methylcellulose coating for reducing oil uptake in potato chips. J. Sci. Food Agric. 2012, 92, 1346–1353. [Google Scholar] [CrossRef] [PubMed]
- Luvielmo, M.M.; De Armas, D.S.; Paiva, F.F.; Krolow, A.C.R.; Ferri, N.M.L. Physicochemical and sensory characteristics of potato chips made from blanched potatoes of the cultivar BRS Ana and coated with methylcellulose. Braz. J. Food Technol. 2015, 18, 211–219. [Google Scholar] [CrossRef]
- Aminlari, M.; Ramezani, R.; Khalili, M.H. Production of protein-coated low-fat potato chips. Food Sci. Technol. Int. 2005, 11, 177–181. [Google Scholar] [CrossRef]
- Coltelli, M.-B.; Wild, F.; Bugnicourt, E.; Cinelli, P.; Lindner, M.; Schmid, M.; Weckel, V.; Müller, K.; Rodriguez, P.; Staebler, A.; et al. State of the art in the development and properties of protein-based films and coatings and their applicability to cellulose based products: An extensive review. Coatings 2016, 6, 1. [Google Scholar] [CrossRef]
- Rannou, C.; Laroque, D.; Renault, E.; Prost, C.; Sérot, T. Mitigation strategies of acrylamide, furans, heterocyclic amines and browning during the Maillard reaction in foods. Food Res. Int. 2016, 90, 154–176. [Google Scholar] [CrossRef] [PubMed]
- Sabbah, M.; Di Pierro, P.; Esposito, M.; Giosafatto, C.V.L.; Mariniello, L.; Porta, R. Stabilization of charged polysaccharide film forming solution by sodium chloride: Nanoparticle Z-average and Zeta-potential monitoring. J. Biotechnol. Biomater. 2016, 6, e128. [Google Scholar] [CrossRef]
- Baxter, A.; Dillon, M.; Taylor, K.D.A.; Roberts, G.A.F. Improved method for i.r. determination of the degree of N-acetylation of chitosan. Int. J. Biol. Macromol. 1992, 14, 166–169. [Google Scholar] [CrossRef]
- Esposito, M.; Di Pierro, P.; Regalado-Gonzales, C.; Mariniello, L.; Giosafatto, C.V.L.; Porta, R. Polyamines as new cationic plasticizers for pectin-based edible films. Carbohydr. Polym. 2016, 153, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Feng, F.; Guo, Y.; Shuang, S.; Choi, M.M.F. HPLC-UV quantitative analysis of acrylamide in baked and deep-fried Chinese foods. J. Food Compos. Anal. 2013, 31, 7–11. [Google Scholar] [CrossRef]
- Krishna, V.N.; Meyyanathan, S.N.; Karthik, Y.; Hemnath, E.; Satiesh, K.R.; Usha, K. A simple and validated RP HPLC method for the estimation of acrylamide in potato chips. World J. Pharm. Pharm. Sci. 2014, 3, 1468–1476. [Google Scholar]
- Michalak, J.; Gujska, E.; Kuncewicz, A. RP-HPLC-DAD studies on acrylamide in cereal-based baby foods. J. Food Compos. Anal. 2013, 32, 68–73. [Google Scholar] [CrossRef]
- Michalak, J.; Gujska, E.; Klepacka, J. The effect of domestic preparation of some potato products on acrylamide content. Plant Foods Hum. Nutr. 2011, 66, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Leclercq, C.; Arcella, D.; Piccinelli, R.; Sette, S.; Donne, C.L.; Turrini, A. The Italian National Food Consumption Survey INRAN-SCAI 2005-06: Main results in terms of food consumption. Public Health Nutr. 2009, 12, 2504–2532. [Google Scholar] [CrossRef] [PubMed]
- Association of Official Analytical Chemists. Method 960.39. In Official Methods of Analysis, 15th ed.; Helrich, K., Ed.; AOAC International: Arlington, VA, USA, 1990. [Google Scholar]
- Association of Official Analytical Chemists. Method 950.46. In Official Methods of Analysis, 15th ed.; Helrich, K., Ed.; AOAC International: Arlington, VA, USA, 1990. [Google Scholar]
- Sayon-Orea, C.; Bes-Rastrollo, M.; Basterra-Gortari, F.J.; Beunza, J.J.; Guallar-Castillon, P.; de la Fuente-Arrillaga, C.; Martinez-Gonzalez, M.A. Consumption of fried foods and weight gain in a mediterranean cohort: The sun project. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, I.; Sonka, C.; Surendar, J. Study on the effective reduction of oil up-take by the application of edible hydrocolloid coatings on French fries. Int. J. Res. Eng. Adv. Technol. 2014, 2. Available online: http://www.ijreat.org/Papers%202014/Issue9/IJREATV2I3056.pdf (accessed on 6 March 2018).
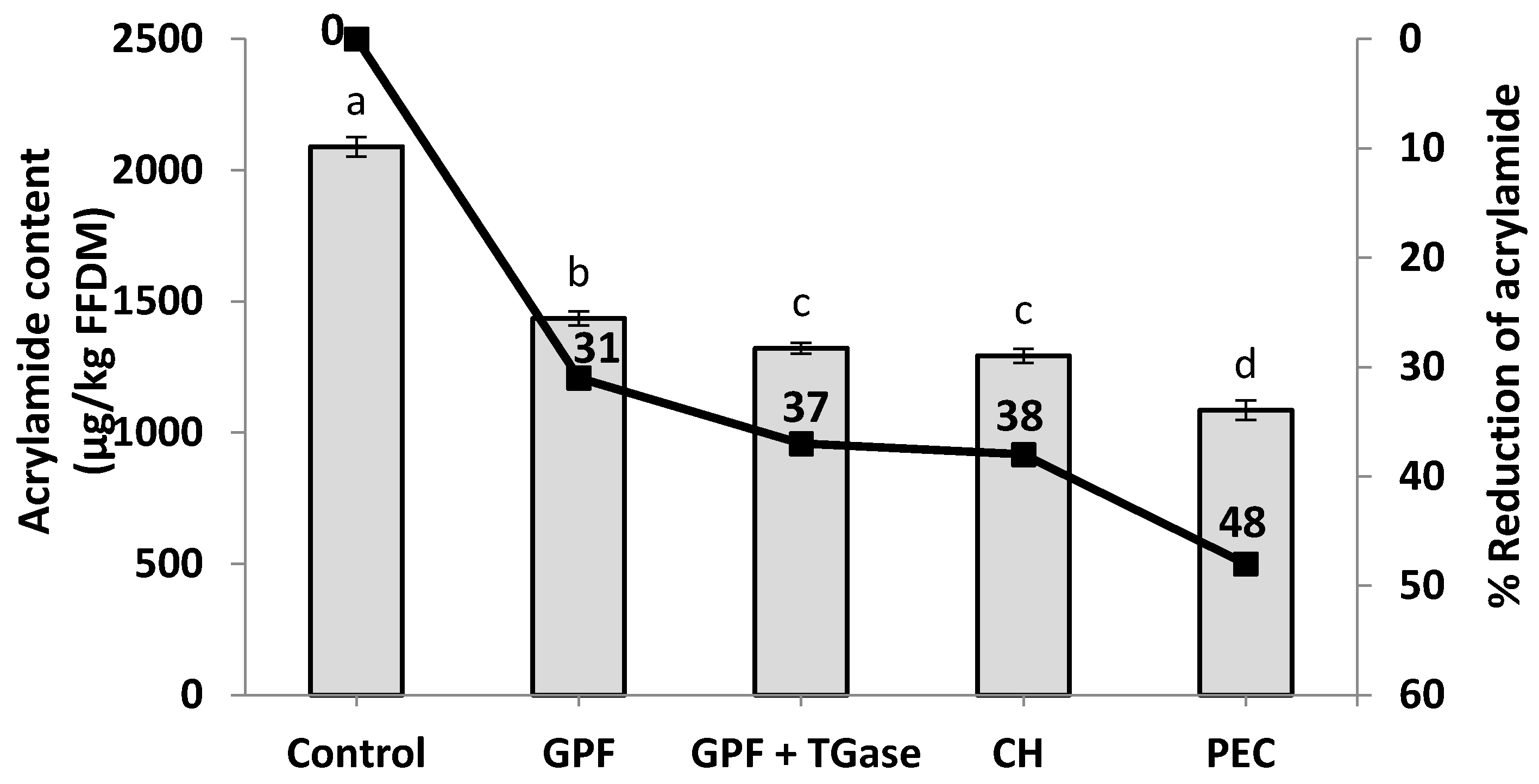
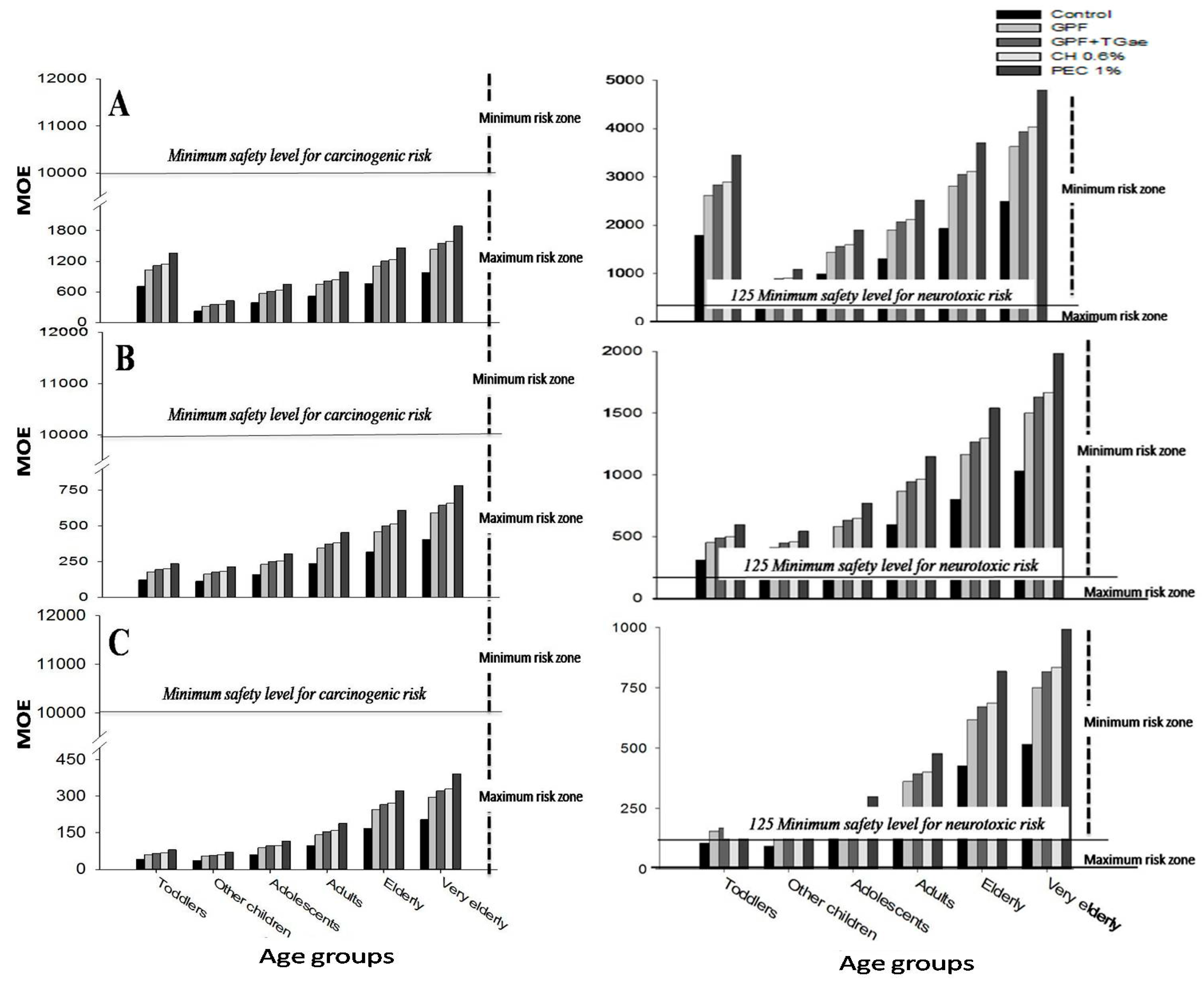
| Fried Potato Samples | Age Groups | Acrylamide Intake (ng (kg body weight)−1 day−1) |
|---|---|---|
| Control | Toddlers | 1387 |
| Other children | 1521 | |
| Adolescents | 1072 | |
| Adults | 719 | |
| Elderly | 536 | |
| Very elderly | 417 | |
| GPF | Toddlers | 952 |
| Other children | 1045 | |
| Adolescents | 737 | |
| Adults | 494 | |
| Elderly | 368 | |
| Very elderly | 287 | |
| GPF + TGase | Toddlers | 877 |
| Other children | 962 | |
| Adolescents | 678 | |
| Adults | 455 | |
| Elderly | 339 | |
| Very elderly | 264 | |
| CH | Toddlers | 858 |
| Other children | 941 | |
| Adolescents | 663 | |
| Adults | 445 | |
| Elderly | 332 | |
| Very elderly | 258 | |
| PEC | Toddlers | 720 |
| Other children | 790 | |
| Adolescents | 557 | |
| Adults | 374 | |
| Elderly | 279 | |
| Very elderly | 217 |
| Coating Solutions | Oil Content (%) | Oil Reducing Due to Coating (%) | Water Content (%) | Water Loss During Frying (%) | Water Retention Due to Coating (%) |
|---|---|---|---|---|---|
| Control | 20.1 ± 0.7 a | – | 41.9 ± 1.1 a | 36.9 ± 1.1 a | – |
| GPF | 18.2 ± 0.4 b | 9.3 ± 1.9 a | 44.9 ± 1.1 b | 33.9 ±1.1 b | 7.1 ± 0.7 a |
| GPF + TGase | 16.5 ± 0.9 b,c | 15.9 ±1.6 b | 49.3 ± 0.7 c | 29.5 ± 0.7 c | 17.5 ± 1.7 b |
| CH | 15.7 ± 0.5 c,d | 21.5 ± 2.7 c | 51.3 ± 1.0 c | 27.4 ± 1.3 c | 22.3 ± 2.4 c |
| PEC | 14.1 ± 0.4 d | 29.4 ± 2.4 d | 56.1 ± 0.6 d | 22.7 ± 0.6 d | 33.8 ± 1.4 d |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Asmar, A.; Naviglio, D.; Giosafatto, C.V.L.; Mariniello, L. Hydrocolloid-Based Coatings are Effective at Reducing Acrylamide and Oil Content of French Fries. Coatings 2018, 8, 147. https://doi.org/10.3390/coatings8040147
Al-Asmar A, Naviglio D, Giosafatto CVL, Mariniello L. Hydrocolloid-Based Coatings are Effective at Reducing Acrylamide and Oil Content of French Fries. Coatings. 2018; 8(4):147. https://doi.org/10.3390/coatings8040147
Chicago/Turabian StyleAl-Asmar, Asmaa, Daniele Naviglio, Concetta Valeria L. Giosafatto, and Loredana Mariniello. 2018. "Hydrocolloid-Based Coatings are Effective at Reducing Acrylamide and Oil Content of French Fries" Coatings 8, no. 4: 147. https://doi.org/10.3390/coatings8040147








