Sharply Reduced Biofilm Formation from Cobetia marina and in Black Sea Water on Modified Siloxane Coatings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Coating Compositions
2.2. Coated Test-Samples
2.3. Water Contact Angle (WCA) and Surface Energy (γ)
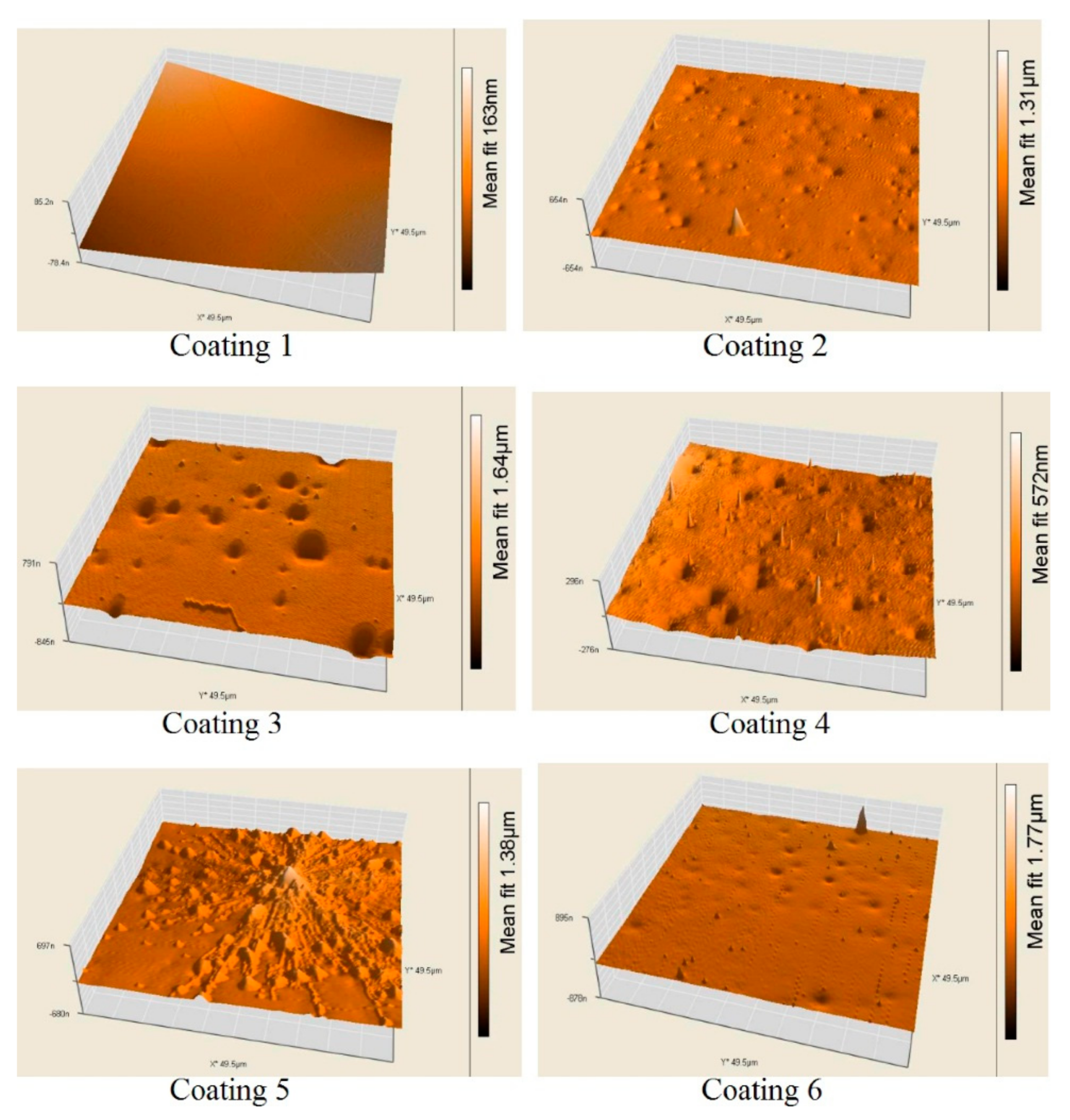
2.4. Atomic Force Microscopy (AFM)
2.5. Depth Sensing Indentation (DSI)
2.6. Elastic Modulus (E)
2.7. Characteristics of Single-Species Biofilms Formed by C. marina
Bacterial Adhesion Test
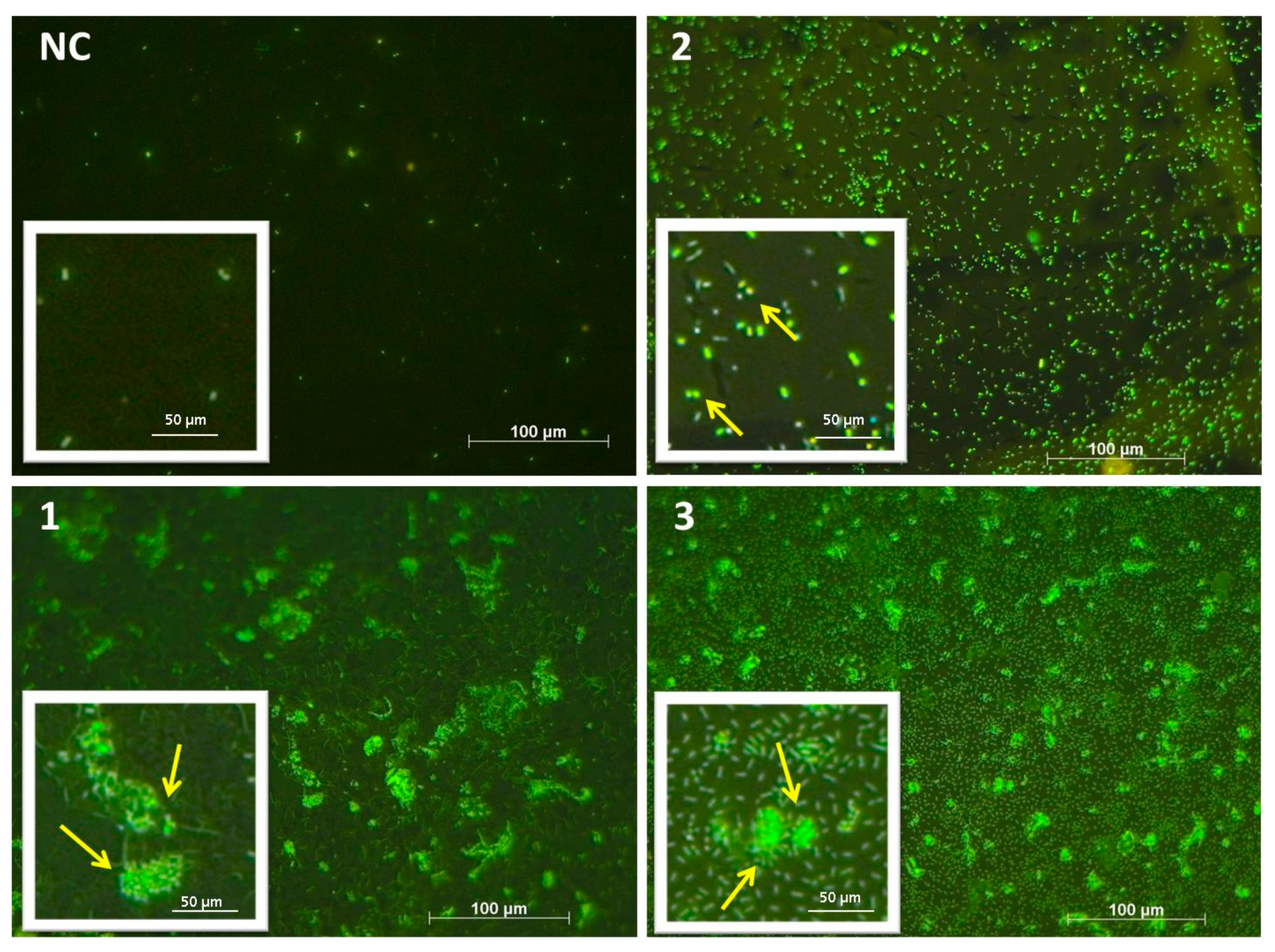
2.8. Bacterial Biofilm Visualization (Fluorescence Microscopy–Live/Dead Staining)
2.9. Quantitative Estimation the Bacterial Biofilm
2.10. Multi-Species Biofilms
Multi-Species Biofilm Formation in a Black Sea Aquarium (BSA)
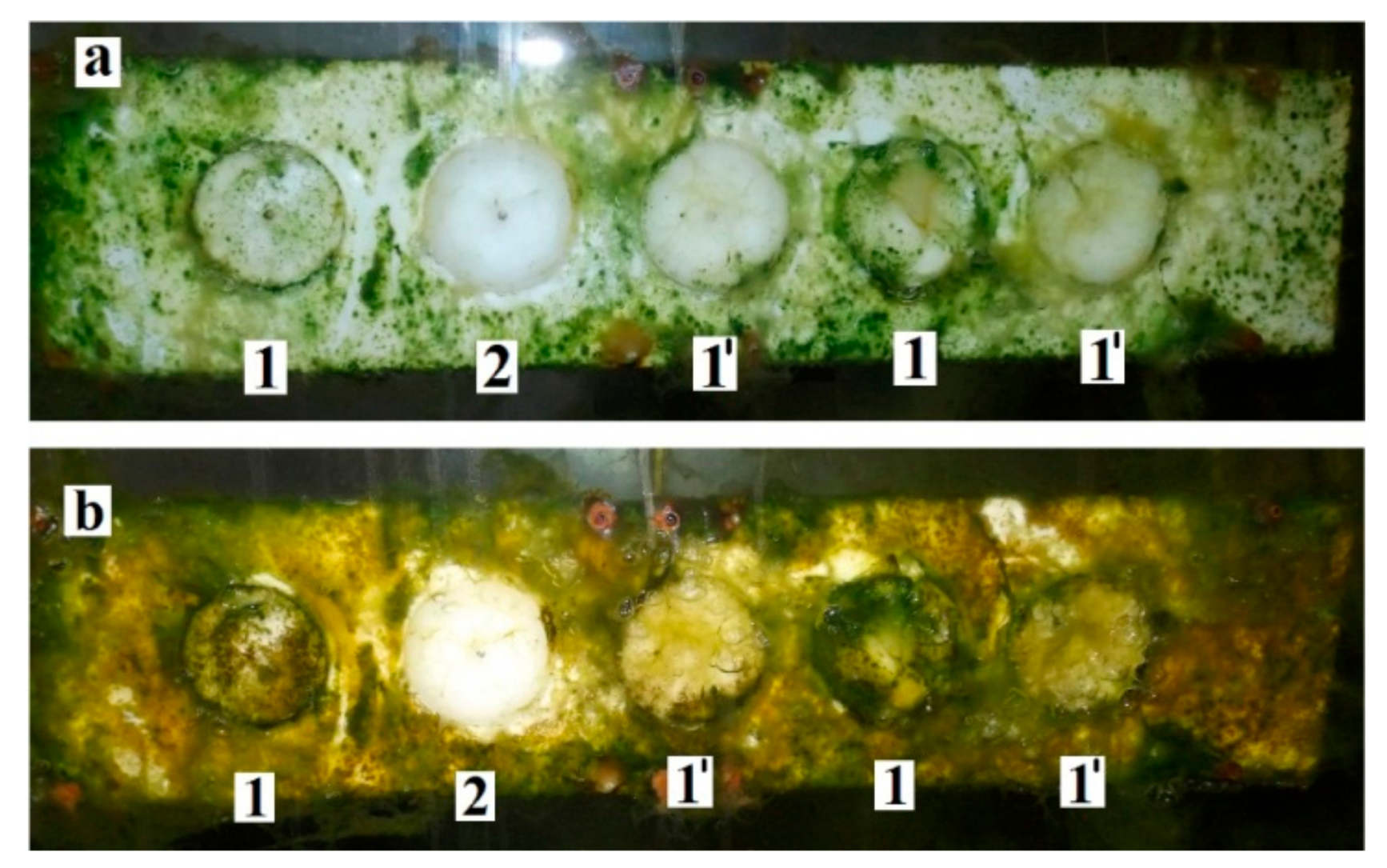
2.11. Biofilm Formation in a Field Experiment and Its Characterization
2.12. Total Chlorophyll (A and B) and Carotenoids (Carotenes and Xanthophylls)
2.13. Biofilm Dry Mass
3. Results
3.1. Characteristics of the Test Surfaces
3.2. Bacterial Biofilms
3.2.1. Bacterial Adhesion of C. marina
3.2.2. Adenosine Triphosphate Bioluminescence
3.2.3. Sea Biofouling and Multi-Species Biofilms Formation
4. Discussion
4.1. Single Species Biofilms of C. marina
4.2. Multi-Species Biofilms
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Milne, A. Coated Marine Surfaces. UK Patent 1470465 A, 24 April 1977. [Google Scholar]
- Milne, A. Antifouling Marine Compositions. U.S. Patent 4025693, 24 May 1978. [Google Scholar]
- Vladkova, T.G.; Dineff, P.D.; Zlatanov, I.; Katiroly, V.; Venkatezan, R.; Murthy, S. Composition Coating for Biofouling Protection. WO Patent 074102 A1, 26 June 2008. [Google Scholar]
- Vladkova, T.G.; Akuzov, D.T.; Klöppel, A.; Brümmer, F. Current approaches to reduction of marine biofilm formation. J. Chem. Technol. Metall. 2014, 49, 345–355. [Google Scholar]
- Becherer, T.; Vieira Nascimento, M.; Sindram, J.; Noeske, P.L.M.; Wei, Q.; Haag, R.; Grunwald, I. Fast and easily applicable glycerol-based spray coating. Prog. Org. Coat. 2015, 87, 146–154. [Google Scholar] [CrossRef]
- Akuzov, D.T.; Brümmer, F.; Vladkova, T.G. Some possibilities to reduce the biofilm formation on transparent siloxane coatings. Colloids Surf. B Biointerfaces 2013, 104, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Jansen, K. Antioxidant coatings could help reduce marine biofouling. Chemical & Engineering News, 29 September 2016. [Google Scholar]
- Del Grosso, C.A.; McCarthy, T.M.; Clark, C.L.; Cloud, J.L.; Wilker, J.J. Managing the redox chemistry to deter marine biological adhesion. Chem. Mater. 2016, 28, 6791–6796. [Google Scholar] [CrossRef]
- Akuzov, D.T.; Vladkova, T.G.; Zamfirova, G.; Gaydarov, V.; Naszimento, M.; Szeglat, N.; Grunwald, I. Polydimetthyl siloxane coatings with superior antifouling efficiency in laboratory and marine conditions. Prog. Org. Coat. 2017, 103, 126–134. [Google Scholar] [CrossRef]
- Chen, H.; Brook, M.; Chen, Y.; Sheardown, H. Surface properties of PEO-silicone composites: Reducing protein adsorption. J. Biomater. Sci. Polym. Ed. 2005, 16, 531–548. [Google Scholar] [CrossRef] [PubMed]
- Murthy, R.; Cox, C.; Hahn, M.; Grunlan, M. Protein-resistant silicones: Incorporation of poly(ethylene oxide) via siloxane tethers. Biomacromolecules 2007, 8, 3244–3255. [Google Scholar] [CrossRef] [PubMed]
- Murthy, R.; Shell, C.; Grunlan, M. The influence of poly(ethylene oxide) grafting via siloxane tethers on protein adsorption. Biomaterials 2009, 30, 2433–2439. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, M.; Grunlan, M. The protein resistance of silicones prepared with a PEO-silane amphiphile. J. Mater. Chem. 2012, 22, 19540–19546. [Google Scholar] [CrossRef]
- Rufin, M.; Barry, M.; Adair, P.; Hawkins, M.; Raymond, J.; Grunlan, M. Protein resistance efficacy of PEO-silane amphiphiles: Dependence on PEO-segment length and concentration in silicone. Acta Biomater. 2016, 41, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, M.L.; Faÿ, F.; Réhel, K.; Linossier, I.; Grunlan, M.A. Bacteria and diatom resistance of silicones modified with PEO-silane amphiphiles. Biofouling 2014, 30, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Bioclean ECO. Environmentally-Friendly Biocide-Free Silicone Foul Release Coating System; Chugoku Marine Paints Ltd.: Tokyo, Japan, 2017. [Google Scholar]
- Murthy, R.; Bailey, B.; Valentin-Rodriguez, C.; Ivanisevic, A.; Grunlan, M. Amphiphilic silicones prepared with branched PEO-silanes with siloxane tethers. Polym. Sci. Part A Polym. Chem. 2010, 48, 4108–4119. [Google Scholar] [CrossRef]
- Hawkins, M.; Rufin, M.; Raymond, J.; Grunlan, M. Direct observation of the nanocomplex surface reorganization of antifouling silicones containing a highly mobile PEO-silane amphiphile. J. Mater. Chem. Part B 2014, 2, 5689–5697. [Google Scholar] [CrossRef]
- Rufin, M.; Gruetzner, J.; Hurley, M.; Hawkins, M.L.; Raymond, E.S.; Raymond, J.E.; Grunlan, M.A. Enhancing the protein resistance of silicone via surface-restructuring PEO-silane amphiphiles with variable PEO length. J. Mater. Chem. B 2015, 3, 2816–2825. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.-M. Polymer Surface Modification and Characterisation; Carl Hanser: Munich, Germany; Vienna, Austria; New York, NY, USA, 1993. [Google Scholar]
- Donlan, R.M. Biofilms: Microbial life on surfaces. Emerg. Infect. Dis. 2002, 8, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.C.; Wingender, J. Relevance of microbial extracellular polymeric substances (EPSs)—Part I: Structural and ecological aspects. Water Sci. Technol. 2001, 43, 1–8. [Google Scholar] [PubMed]
- Brady, R.F.; Singer, I.L. Mechanical factors favouring release from fouling release coatings. Biofouling 2000, 15, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Boks, N.; Norde, W.; van der Mei, H.; Busscher, H. Forces involved in bacterial adhesion to hydrophilic and hydrophobic surfaces. Microbiology 2008, 154, 3122–3133. [Google Scholar] [CrossRef] [PubMed]
- Cunliffe, D.; Smart, C.; Alexander, C.; Vulfson, E.N. Bacterial adhesion at synthetic surfaces. Appl. Environ. Microbiol. 1999, 65, 4995–5002. [Google Scholar] [PubMed]
- Altankov, G.P. Interaction of Cells with Biomaterial Surfaces. Ph.D. Thesis, Institute of Biophysics, Bulgarian Academy of Sciences, Sofia, Bulgaria, 2003. [Google Scholar]
- Ikada, Y.; Suzuki, M.; Tamada, Y. Polymer surfaces possessing minimal interaction with blood components. In Polymers as Biomaterials; Shalaby, S.W., Ed.; Plenum Press: New York, NY, USA, 1984. [Google Scholar]
- DL-Alpha Tocopherol, Product Details. Hangzhou Toyond Biotech Co. Ltd. Available online: http://product.lookchem.com/item/315/dl-alpha-tocopherol.html (accessed on 9 April 2018).
- Molina-Manso, D.; Gómez-Barrena, E.; Esteban, J.; Adames, H.; Martínez, M.; Cordero, J.; Fernández-Roblas, R.; Puértolas, J.A. Bacterial adherence on UHMWPE doped with vitamin E: An in vitro study. J. Phys. Conf. Ser. 2010, 252, 012014. [Google Scholar] [CrossRef]
- Banche, G.; Allizond, V.; Bracco, P.; Bistolfi, A.; Boffano, M.; Cimino, A.; Brach del Prever, E.M.; Cuffini, A.M. Interplay between surface properties of standard, vitamin E blended and oxidised ultra high molecular weight polyethylene used in total joint replacement and adhesion of Staphylococcus aureus and Escherichia coli. Bone Joint J. 2014, 96B, 497–501. [Google Scholar] [CrossRef]
- Banche, J.; Bracco, P.; Bistolfi, A.; Allizond, V.; Boffano, M.; Costa, L.; Cimino, A.; Cuffini, A.M.; Brach del Prever, E.M. Vitamin E blended UHMWPE may have the potential to reduce bacterial adhesive ability entered the right name of the paper. Orthop. Res. 2011, 29, 1662–1669. [Google Scholar] [CrossRef] [PubMed]
- Campoccia, D.; Visai, L.; Renò, F.; Cangini, I.; Rizzi, M.; Poggi, A.; Montanaro, L.; Rimondini, L.; Arciola, C.R. Bacterial adhesion to poly-(d,l)lactic acid blended with vitamin E: Toward gentle anti-infective biomaterials. J. Biomed. Mater. Res A 2015, 103, 1447–1458. [Google Scholar] [CrossRef] [PubMed]
- Al-Salih, D.; Jehad, M.; Aziz, F.; Mshimesh, B. Antibacterial effects of vitamin E: In vitro study. J. Biotechnol. Res. Cent. 2013, 7, 17–23. [Google Scholar]
- Thomas, X. Silicone Adhesives in Healthcare Application; Dow Corning Corporation: Midland, NM, USA, 2003. [Google Scholar]
- Vroman, I. Effect of adsorbed proteins on the wettability of hydrophylic and hydrophobic solids. Nature 1962, 196, 476–477. [Google Scholar] [CrossRef] [PubMed]
- Beech, I.B. Corrosion of technical materials in the presence of biofilms—Current understanding and state-of-the art methods of study. Int. Biodeterior. Biodegrad. 2004, 53, 177–196. [Google Scholar] [CrossRef]
- Mayer, C.; Moritz, R.; Kirschner, C.; Borchard, W.; Maibaum, R.; Wingender, J.; Flemming, H.-C. The role of intermolecular interactions: Studies on model systems for bacterial biofilms. Int. J. Biol. Macromol. 1999, 26, 3–16. [Google Scholar] [CrossRef]
- Ahimou, F.; Semmens, M.J.; Haugstad, G.; Novak, P.J. Effect of protein, polysaccharide, and oxygen concentration profiles on biofilm cohesiveness. Appl. Environ. Microbiol. 2007, 73, 2905–2910. [Google Scholar] [CrossRef] [PubMed]
- Gehrke, T.; Telegdi, J.; Thierry, D.; Sand, W. Importance of extracellular polymeric substances from Thiobacillus ferrooxidans for bioleaching. Appl. Environ. Microbiol. 1998, 64, 2743–2747. [Google Scholar] [PubMed]
- Harneit, K.; Goksel, A.; Kock, D.; Klock, J.H.; Gehrke, T.; Sand, W. Adhesion to metal sulfide surfaces by cells of Acidithiobacillus ferrooxidans, Acidithiobacillus thiooxidans and Leptospirillum ferrooxidans. Hydrometallurgy 2006, 83, 245–254. [Google Scholar] [CrossRef]
- Sutherland, I.W. Biofilm exopolysaccharides: A strong and sticky framework. Microbiology 2001, 147, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Shea, C.; Nunley, J.W.; Williamson, J.C.; Smithsomerville, H.E. Comparison of the adhesion properties of Deleya marina and the exopolysaccharide-defective mutant strain DMRt. Appl. Environ. Microbiol. 1991, 57, 3107–3113. [Google Scholar] [PubMed]
- Plisova, E.Y.; Balabanova, L.A.; Ivanova, E.P.; Kozhemyako, V.B.; Mikhailov, V.V.; Agafonova, E.V.; Rasskazov, V.A. A highly active alkaline phosphatase from the marine bacterium cobetia. Mar. Biotechnol. 2005, 7, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Ibacache-Quiroga, C.; Ojeda, J.; Espinoza-Vergara, G.; Olivero, P.; Cuellar, M.; Dinamarca, M.A. The hydrocarbon-degrading marine bacterium Cobetia sp. strain MM1IDA2H-1 produces a biosurfactant that interferes with quorum sensing of fish pathogens by signal hijacking. Microb. Biotechnol. 2013, 6, 394–405. [Google Scholar] [CrossRef] [PubMed]
- Lelchat, F.; Cérantola, S.; Brandily, C.; Colliec-Jouault, S.; Baudoux, A.C.; Ojima, T.; Boisset, C. The marine bacteria Cobetia marina DSMZ 4741 synthesizes an unexpected K-antigen-like exopolysaccharide. Carbohydr. Polym. 2015, 124, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Vladkova, T.G.; Krasteva, N.; Kostadinova, A.; Altankov, G. Preparation of PEG-coated surfaces and a study for their interaction with living cells. J. Biomater. Sci. Polym. Ed. 1999, 10, 609–615. [Google Scholar] [CrossRef] [PubMed]



| Parameter | Sample No. | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| WCA | 104.1 ± 0.3 | 103.0 ± 0.5 | 104.1 ± 0.4 | 51.5 ± 0.6 | 90.6 ± 0.4 | 60.0 ± 0.6 |
| γc, mN/m | 22.4 | 22.8 | 22.1 | 30.5 | 24.4 | 32.1 |
| γd, mN/m | 22 | 22.6 | 22 | 20.1 | 22 | 21 |
| γp, mN/m | 0.4 | 0.7 | 0.1 | 10.4 | 2.3 | 11.1 |
| Ra, nm | 12 ± 4 | 12 ± 6 | 33 ± 9 | 21 ± 6 | 49 ± 10 | 51 ± 10 |
| Rq, nm | 15 ± 7 | 18 ± 7 | 42 ± 9 | 27 ± 2 | 63 ± 16 | 61 ± 6 |
| Sp, nm | 56 ± 15 | 428 ± 28 | 151 ± 21 | 232 ± 31 | 458 ± 58 | 737 ± 73 |
| Sv, nm | −38 ± 12 | −158 ± 16 | −275 ± 11 | −120 ± 13 | −193 ± 14 | 151 ± 18 |
| Sy, nm | 94 ± 11 | 640 ± 48 | 426 ± 26 | 352 ± 58 | 651 ± 64 | 888 ± 83 |
| HMV (N/mm2) | 0.13 ± 0.02 | 0.46 ± 0.03 | 0.45 ± 0.02 | 0.34 ± 0.01 | 0.34 ± 0.02 | 0.27 ± 0.01 |
| HIT (N/mm2) | 0.34 ± 0.01 | 0.82 ± 0.01 | 0.82 ± 0.07 | 0.77 ± 0.03 | 0.63 ± 0.02 | 0.51 ± 0.03 |
| EIT (N/mm2) | 1.76 ± 0.06 | 8.13 ± 0.10 | 7.88 ± 0.10 | 4.99 ± 0.08 | 5.87 ± 0.06 | 4.41 ± 0.02 |
| E (N/mm2) | 0.48 ± 0.01 | 0.31 ± 0.01 | 0.35 ± 0.03 | – | – | – |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akuzov, D.; Franca, L.; Grunwald, I.; Vladkova, T. Sharply Reduced Biofilm Formation from Cobetia marina and in Black Sea Water on Modified Siloxane Coatings. Coatings 2018, 8, 136. https://doi.org/10.3390/coatings8040136
Akuzov D, Franca L, Grunwald I, Vladkova T. Sharply Reduced Biofilm Formation from Cobetia marina and in Black Sea Water on Modified Siloxane Coatings. Coatings. 2018; 8(4):136. https://doi.org/10.3390/coatings8040136
Chicago/Turabian StyleAkuzov, Danail, Lia Franca, Ingo Grunwald, and Todorka Vladkova. 2018. "Sharply Reduced Biofilm Formation from Cobetia marina and in Black Sea Water on Modified Siloxane Coatings" Coatings 8, no. 4: 136. https://doi.org/10.3390/coatings8040136




