The High-Temperature Resistance Properties of Polysiloxane/Al Coatings with Low Infrared Emissivity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Polysiloxane/Al Coatings
2.3. Heat Treatment
2.4. Characterization
3. Results and Discussion
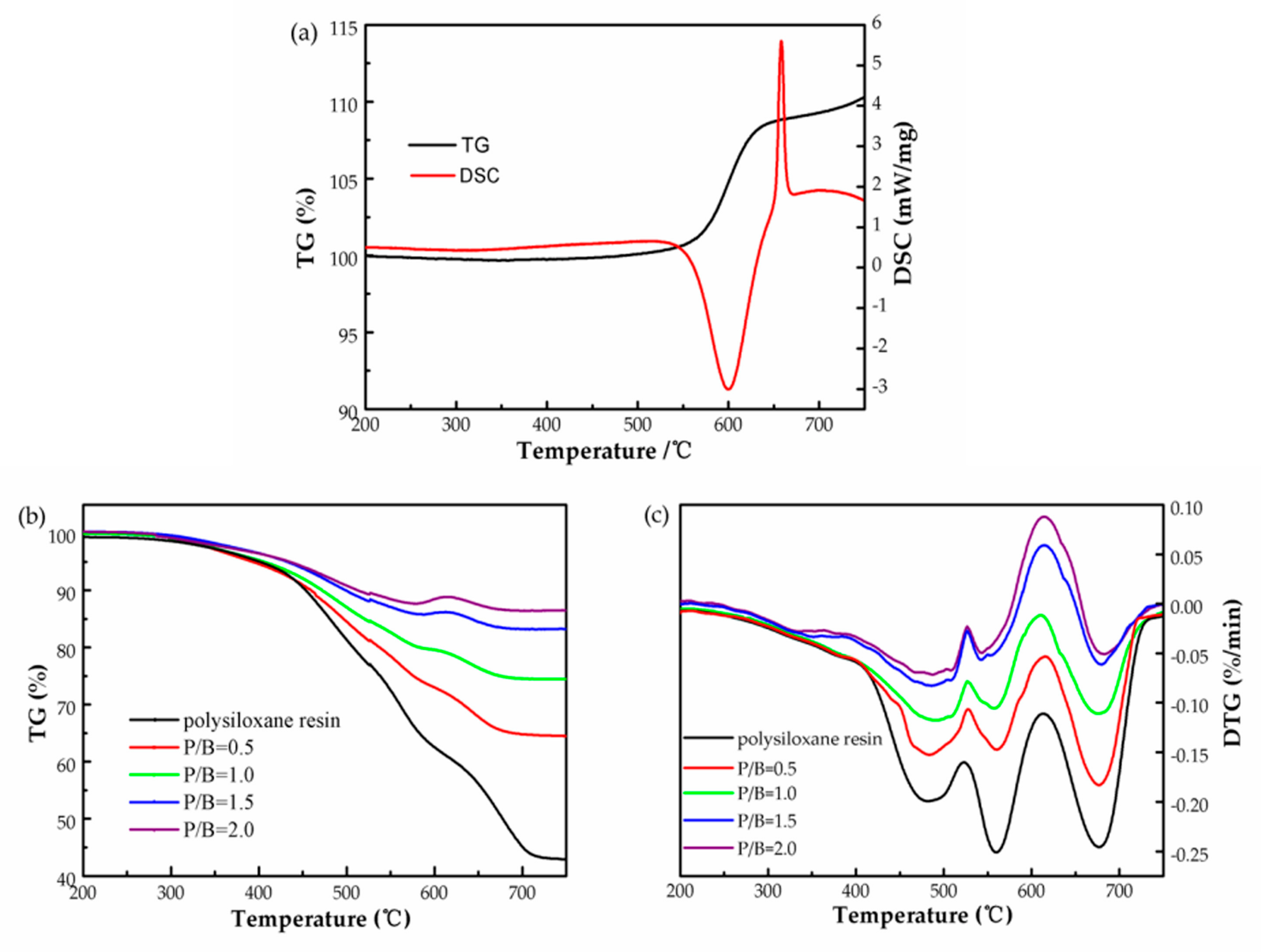
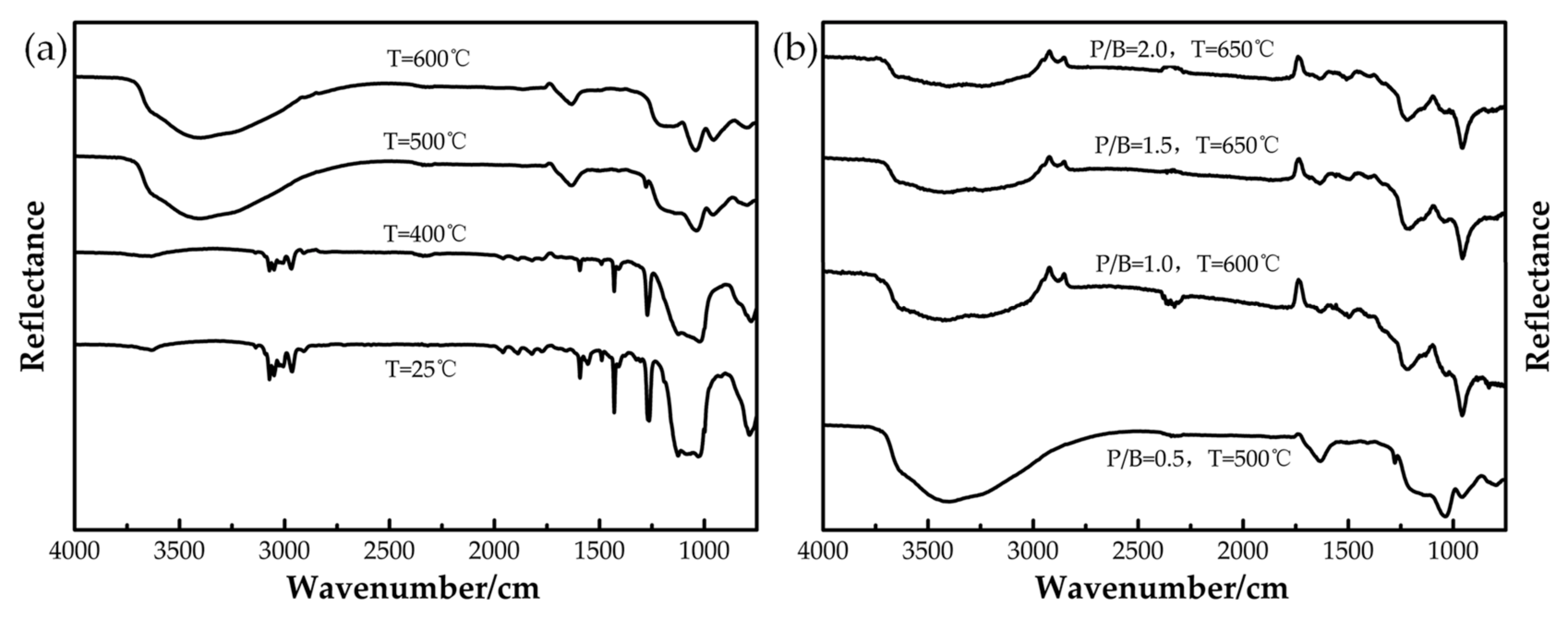
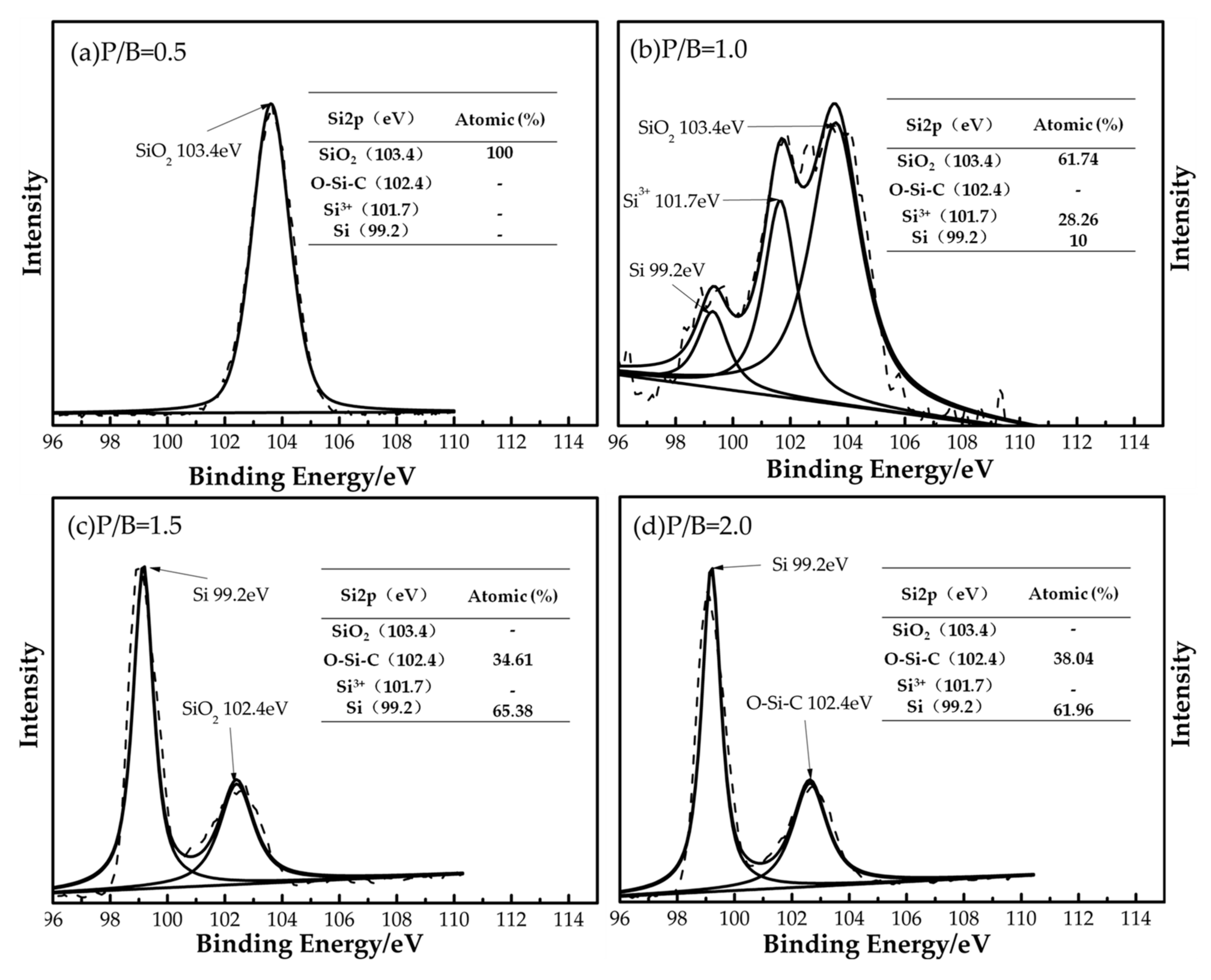
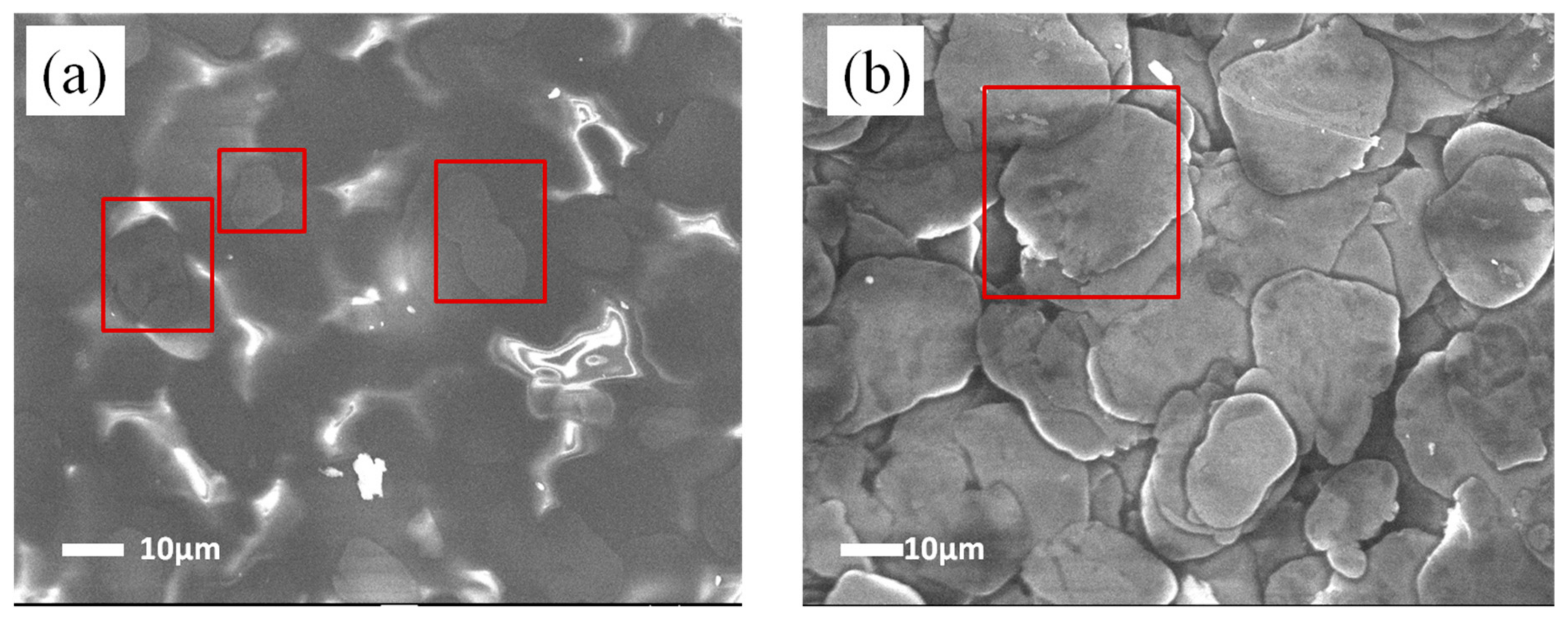
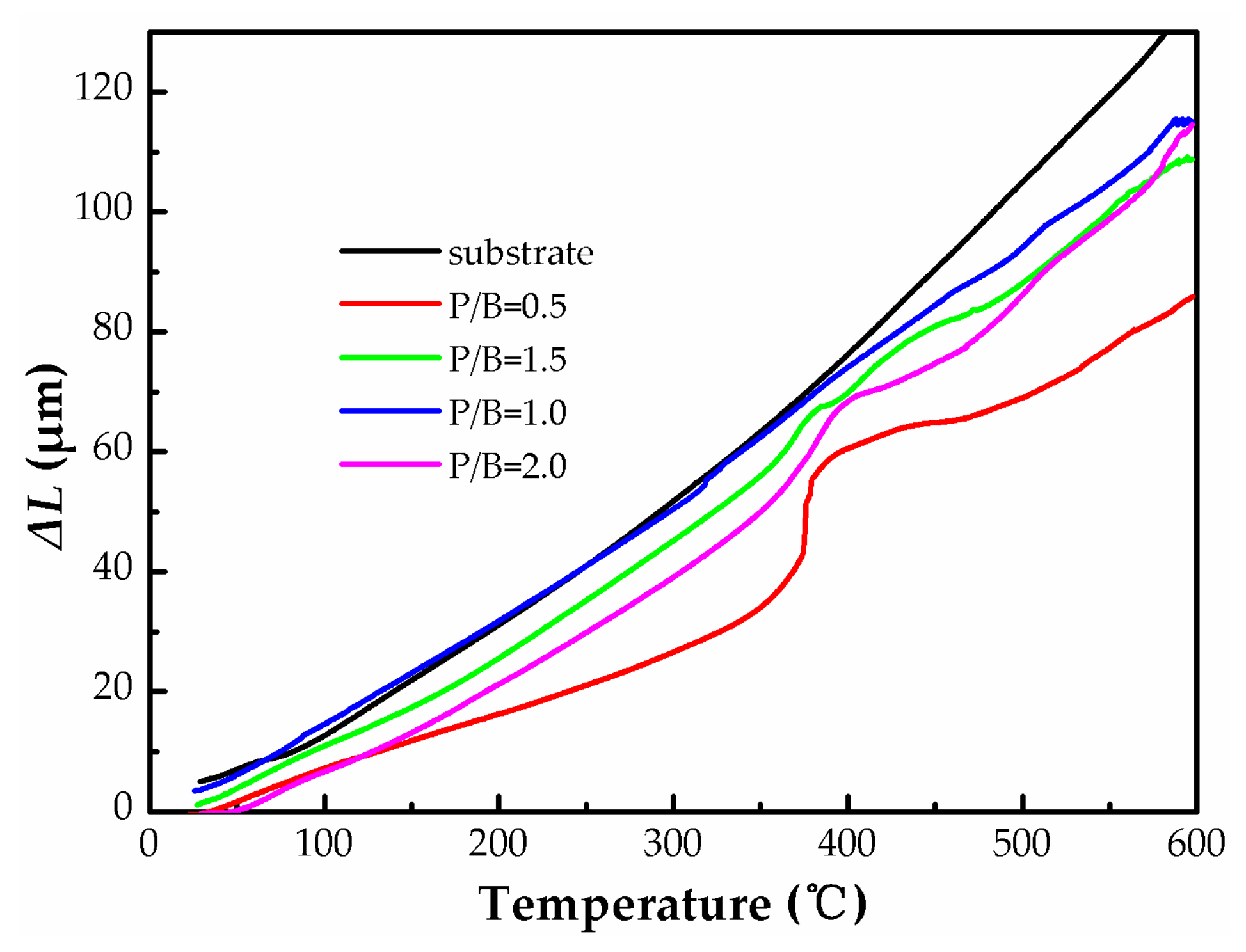
3.1. Heat-Resistance Properties of the Polysiloxane/Al Coatings
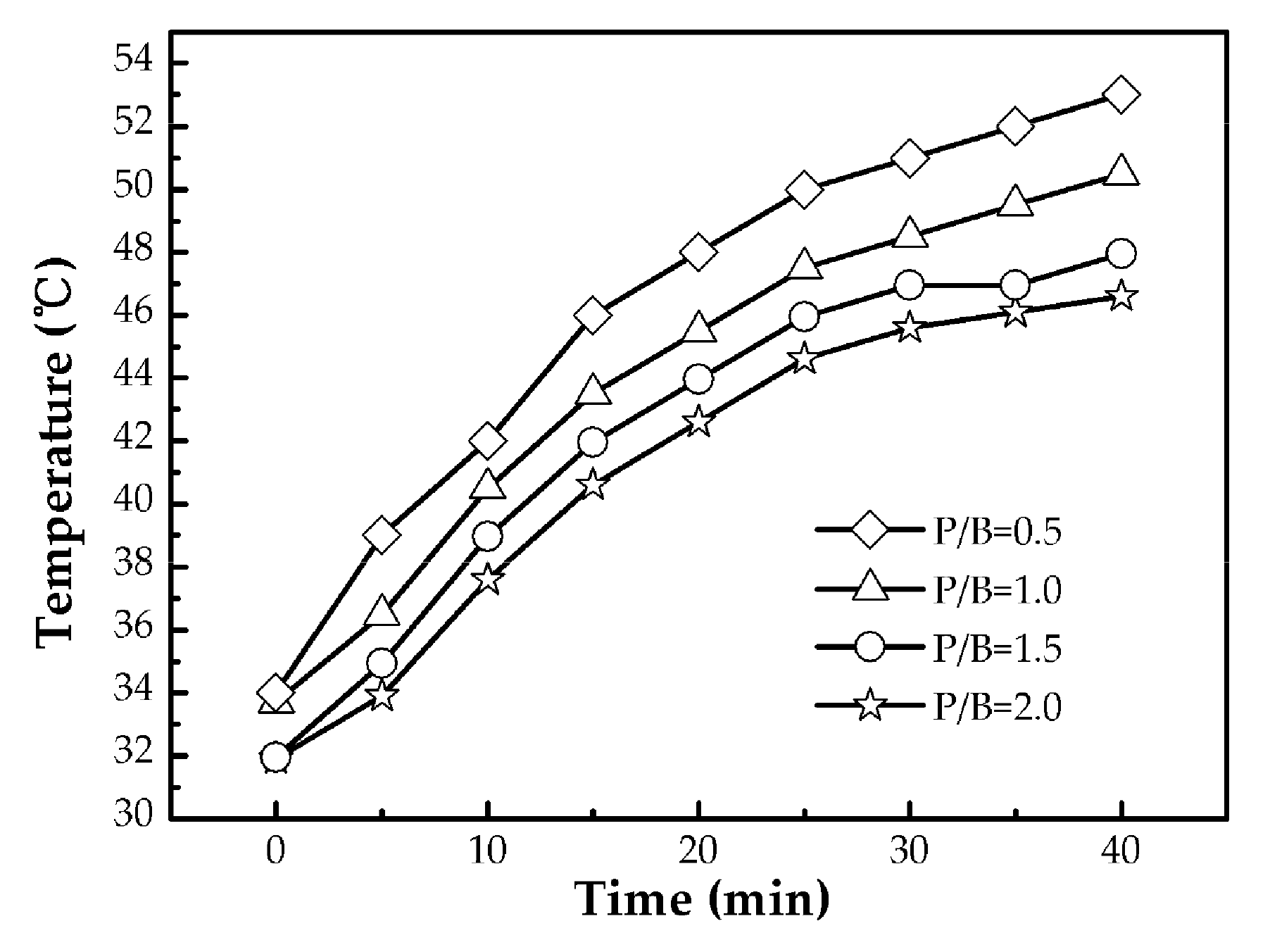
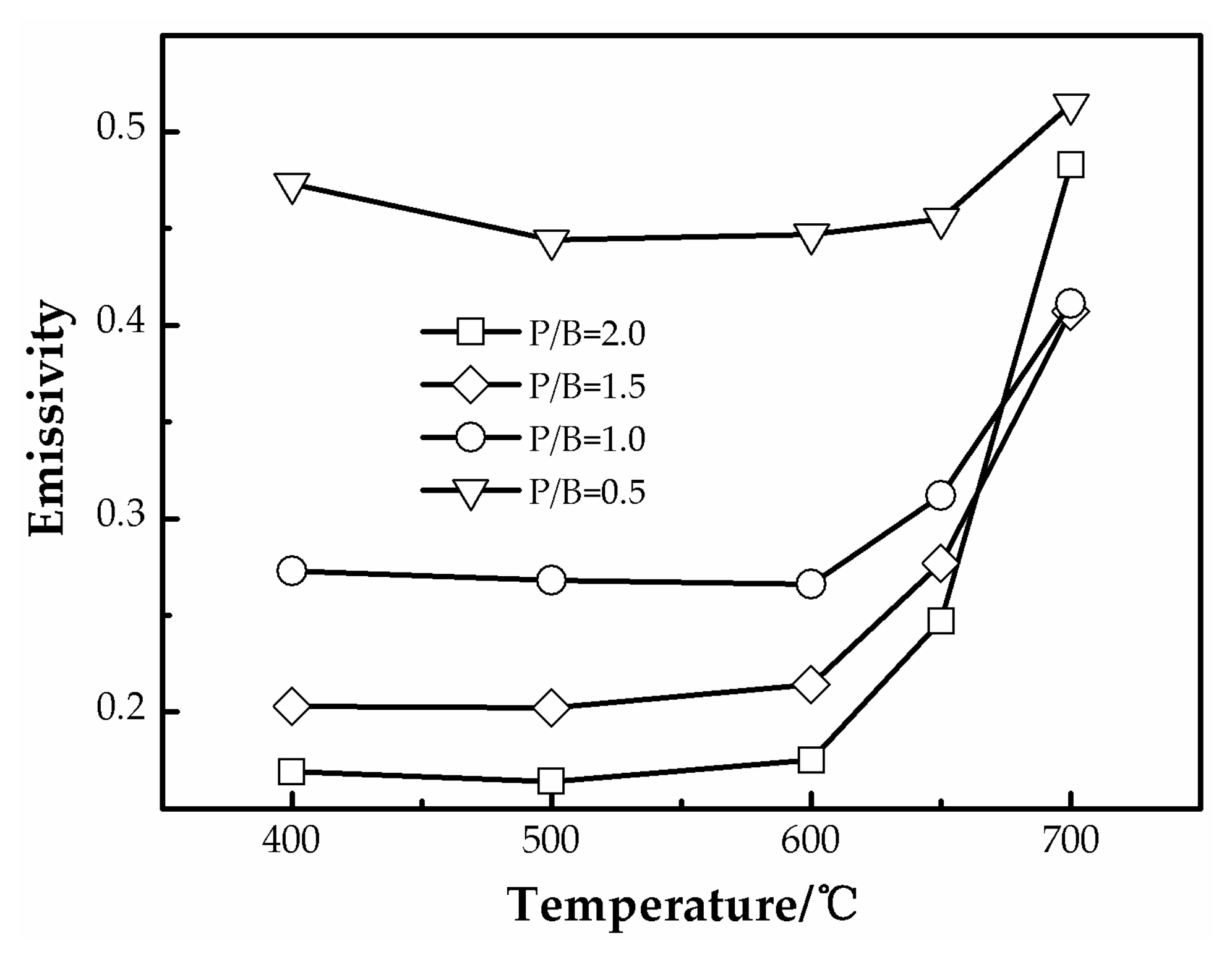
3.2. Emissivity Properties of Polysiloxane/Al Coatings
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yuan, L.; Weng, X.L.; Deng, L.J. Influence of binder viscosity on the control of infrared emissivity in low emissivity coating. Infrared Phys. Technol. 2013, 56, 25–29. [Google Scholar] [CrossRef]
- Chen, S.; Yuan, L.; Weng, X.L.; Deng, L.J. Modeling emissivity of low-emissivity coating containing horizontally oriented metallic flake particles. Infrared Phys. Technol. 2014, 67, 377–381. [Google Scholar] [CrossRef]
- Wu, G.; Yu, D. Preparation and characterization of a new low infrared-emissivity coating based on modified aluminum. Prog. Org. Coat. 2013, 76, 107–112. [Google Scholar] [CrossRef]
- Tomas, H.; Tiina, N.; Eva, H.K.; Salonen, P.S.; Christina, N.; Anna, J. Development of Low-Emissive Camouflage Paint: Final Report; Sensor Technology, Swedish Defence Research Agency: Stockholm, Sweden, 2005. [Google Scholar]
- Leftheriotis, G.; Yianoulis, P. Characterization and stability of low-emittance multiple coatings for glazing applications. Energy Mater. Sol. Cells 1999, 58, 185–197. [Google Scholar] [CrossRef]
- Sun, K.; Zhou, W.; Tang, X.; Luo, F. Application of indium tin oxide (ITO) thin film as a low emissivity film on Ni-based alloy at high temperature. Infrared Phys. Technol. 2016, 78, 156–161. [Google Scholar] [CrossRef]
- Xiao, S.R.; Ding, H.Y.; Wang, Z.Y.; Zhang, T.; Liu, P.R. Thermal stability of a low infrared emissivity coating. Aeroengine 2014, 40, 15–18. [Google Scholar]
- Hu, C.; Xu, G.Y.; Shen, X.M.; Shao, C.; Yan, X. The epoxy-siloxane/Al composite coatings with low infrared emissivity for high temperature applications. Appl. Surf. Sci. 2010, 256, 3459–3463. [Google Scholar] [CrossRef]
- Guo, T.; Xu, G.Y.; Chen, Y.; Hu, C.; Wang, Y. Preparation of heat resistant coatings with low infrared emissivity and mechanism. Mater. Rev. 2011, 25, 96–99. [Google Scholar]
- Wang, M.; Liang, H.M.; Xu, J. Research on the oxidation characteristics of aluminum flakes of DSC/DTG method. J. Saf. Environ. 2013, 2, 045. [Google Scholar]
- Hu, C.; Xu, G.; Shen, X.M. Preparation and characteristics of thermal resistance polysiloxane/Al composite coatings with low infrared emissivity. J. Appl. Polym. Sci. 2009, 486, 371–375. [Google Scholar] [CrossRef]
- Radhakrishnan, T.S. New method for evaluation of kinetic parameters and mechanism of degradation from pyrolysis-GC studies: Thermal degradation of polydimethylsiloxanes. J. Appl. Polym. Sci. 1999, 73, 441–450. [Google Scholar] [CrossRef]
- Wagner, Ö.; Kenessey, G. A new catalytic method for crosslinking of silicone polymers. J. Appl. Polym. Sci. 1998, 69, 1705–1709. [Google Scholar] [CrossRef]
- Sun, J.T.; Huang, Y.D.; Cao, H.L.; Gong, G.F. Synthesis of heat-resistant silicone resin and studies on its thermal and curing properties. J. Aeronaut. Mater. 2005, 25, 25–29. [Google Scholar]
- Tomer, N.S.; Delorjestin, F.; Frezet, L.; Lacoste, J. Oxidation, chain scission and cross-linking studies of polysiloxanes upon ageings. Open J. Org. Polym. Mater. 2012, 2, 13–22. [Google Scholar] [CrossRef]
- Pham, Q.T.; Chern, C.S. Thermal stability of organofunctional polysiloxanes. Thermochim. Acta 2013, 565, 114–123. [Google Scholar] [CrossRef]
- Jovanovic, J.D.; Govedarica, M.N.; Dvornic, P.R.; Popovic, I.G. The thermogravimetric analysis of some polysiloxanes. Polym. Degrad. Stab 1998, 61, 87–93. [Google Scholar] [CrossRef]
- Nagai, N.; Hashimoto, H. FT-TR-ATR study of depth profile of SiO2 ultra-thin films. Appl. Surf. Sci. 2001, 172, 307–311. [Google Scholar] [CrossRef]
- Cheng, H.S.; Sun, Z.Y.; Shao, J.C. Infrared spectral characteristics of eight different sources of SiO2. Bull. Chin. Ceram. Soc. 2011, 30, 934–937. [Google Scholar]
- Egitto, F.D.; Matienzo, L.J. Transformation of poly (dimethylsiloxane) into thin surface films of SiOx by UV/Ozone treatment. Part I: Factors affecting modification. J. Mater. Sci. 2006, 41, 6362–6373. [Google Scholar] [CrossRef]
- Visser, S.A.; Hewitt, C.E.; Fitzgerald, J.J.; Ferrar, W.T.; Binga, T.D. Effect of filler type on the response of polysiloxane elastomers to cyclic stress at elevated temperatures. J. Appl. Polym. Sci. 1997, 65, 1805–1820. [Google Scholar] [CrossRef]
- Wang, H.Q. Preparation of organic silicone coating with high-temperature resistance. J. Beijing Univ. Chem. Technol. 2006, 33, 59–62. [Google Scholar]
- Patel, M.; Shames, M.; Skinner, A.R.; Stephens, T.S. Stress relaxation and thermogravimetric studies on room temperature vulcanised polysiloxane rubbers. Polym. Degrad. Stab. 2004, 83, 111–116. [Google Scholar] [CrossRef]
- Mirabedini, S.M.; Mohseni, M.; Pazokifard, S.; Esfandeh, M. Effect of TiO2 on the mechanical and adhesion properties of RTV silicone elastomer coatings. Colloids Surf. A Physicochem. Eng. Asp. 2008, 317, 80–86. [Google Scholar] [CrossRef]
- Sato, K. The internal stress of coating films. Prog. Org. Coat. 1980, 8, 143–160. [Google Scholar] [CrossRef]
- Hu, C.; Xu, G.; Shen, X.; Huang, R.; Li, F.L. Thermal ageing studies on low infrared emissivity composite coatings. J. Alloys Compd. 2010, 496, 691–694. [Google Scholar] [CrossRef]

| Samples | Heat-Treatment Temperature | ||||
|---|---|---|---|---|---|
| 400 °C | 500 °C | 600 °C | 650 °C | 700 °C | |
| Polysiloxane resin | * | – | – | – | – |
| P/B = 0.5 | ○ | × | × | × | × |
| P/B = 1.0 | ○ | ○ | ○ | Δ | Δ |
| P/B = 1.5 | ○ | ○ | Δ | Δ | Δ |
| P/B = 2.0 | ○ | ○ | Δ | Δ | Δ |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Luo, W.; Qi, L.; Yuan, L.; Huang, G.; Huang, Y.; Weng, X. The High-Temperature Resistance Properties of Polysiloxane/Al Coatings with Low Infrared Emissivity. Coatings 2018, 8, 125. https://doi.org/10.3390/coatings8040125
Zhao J, Luo W, Qi L, Yuan L, Huang G, Huang Y, Weng X. The High-Temperature Resistance Properties of Polysiloxane/Al Coatings with Low Infrared Emissivity. Coatings. 2018; 8(4):125. https://doi.org/10.3390/coatings8040125
Chicago/Turabian StyleZhao, Jun, Wei Luo, Lun Qi, Le Yuan, Gang Huang, Yan Huang, and Xiaolong Weng. 2018. "The High-Temperature Resistance Properties of Polysiloxane/Al Coatings with Low Infrared Emissivity" Coatings 8, no. 4: 125. https://doi.org/10.3390/coatings8040125





