1. Introduction
The technological and scientific advance of humanity is linked to the evolution of materials, although there are different kinds of materials such as ceramics, polymers and composites [
1], those that are specifically used for the development of medical devices have been traditionally called biomaterials [
2]. There are many definitions of biomaterials, any current definition of biomaterials is neither perfect nor complete, such as “any substance or combination of substances, other than drugs, synthetic or natural, which augments or replaces partially or totally any tissue, organ or function of the body, in order to maintain or improve the quality of life of the individual” [
3,
4] or any non-drug material that can be used to treat, enhance or replace any tissue, organ or function in an organism [
5]; however, the term biomaterials also includes materials derived from biological sources [
6].
Almost from the birth of the field of polymer science, polymers have been widely used in medicine [
6] because of their chemical versatility and biocompatibility [
7]. There are several factors that should be considered before using a polymer for biomedical applications, such as its chemical composition, physical characteristics [
8], molecular weight, solubility, shape, structure, hydrophilicity/hydrophobicity [
7], and biocompatibility. This last one, biocompatibility, “is very important for the development of biomaterials and is described as the ability of a material to perform with an appropriate host response a specific function” [
8].
Many medical devices such as catheters, prostheses, sutures,
etc. are made using synthetic polymers like poly(methyl methacrylate), polypropylene, poly(vinyl chloride), polyethylene, and silicone rubber (SR) [
8]. Solid surfaces, including those made out of synthetic polymers, are susceptible to the bacterial adhesion [
1,
9] phenomenon, which can lead to undesirable effects like hospital-acquired infections (HAIs). According to Gabrani
et al. (2015), HAIs are a leading threat, as 5%–10% of hospitalized patients succumb, leading to approximately 90,000 deaths per year [
10]. The most frequent pathogens causing HAIs are the Coagulase Negative Staphylococci (CoNS) genus [
9,
11], specifically
Staphylococcus epidermis [
12,
13].
SR is a synthetic polymer, with a backbone of alternating silicon and oxygen atoms, that has been used as a biomaterial since the early 1950s and is regarded as one of the most biocompatible materials [
14]. SR is widely used in medicine not only because it is not toxic or irritant and has good mechanical properties, but also because of its relative inertness and stability when in contact with tissues in living organisms [
15]. Some medical devices based on SR are contact lenses, artificial skins, cardiac pacemakers and catheters [
15]. However, there are many reports indicating that there is susceptibility for SR to be colonized by different pathogens [
15,
16,
17,
18]. A possible solution to avoid the formation of biofilms on SR is to coat it with antimicrobial polymers, such as those bearing imidazolium groups, with one possible candidate being poly-(N-vinylimidazole) (PNVIM) [
19].
Smart polymers, also known as stimuli-responsive polymers, are defined as those polymers that respond to changes (called stimuli) in the environment where they are immersed. Common stimuli include changes in the electric field, magnetic field, pH, temperature,
etc., and the responses can be diverse and can include phase separation, as well as changes in the shape surface, mechanical, optical and electrical properties [
20]. However, one of the most significant problems that plague biomaterials, including the polymeric ones, is their susceptibility to bacterial adhesion [
1]. Thermo- and pH-responsive delivery systems have drawn much attention because some diseases manifest themselves by a change in the temperature and/or pH [
21]. In accordance with Ferraz
et al. (2014), among water soluble polymers with lower critical solution temperature (LCST), poly(N-vinylcaprolactam) (PNVCL) stands out based on the fact that it is not only ionic, water-soluble, nontoxic and thermos-sensitive, but also biocompatible [
22]. The biocompatibility of PVCL is attributed to the fact that the amide group in PVCL is directly bounded to the hydrophobic backbone, thus its hydrolysis will not result in the production of cytotoxic small amide compounds [
23]. Additionally, PVCL shows a dissolution/precipitation transition in water at temperatures close to physiological temperatures (30–40 °C), which opens perspectives for applications in biochemistry and medicine [
24].
Polymerization via γ-ray radiation offers many advantages over conventional methods, principally because is not necessary to add catalysts or additives to initiate the reaction [
25]. The aim of this work is to determinate the appropriate conditions for the synthesis of the binary graft copolymer (SR-g-NVIM-co-NVCL) via γ-ray radiation. Surface functionalization with stimulus–responsiveness is being evaluated for the preparation of drug eluting medical devices, with promising results that drug-eluting coatings could be useful to fight this problem. The bioactive compounds can chemically interact through reversible bonds with the modified surfaces, becoming trapped in a three-dimensional polymer network from which they can be released in a controlled way by certain physiological variables, such temperature or pH [
26].
3. Results and Discussion
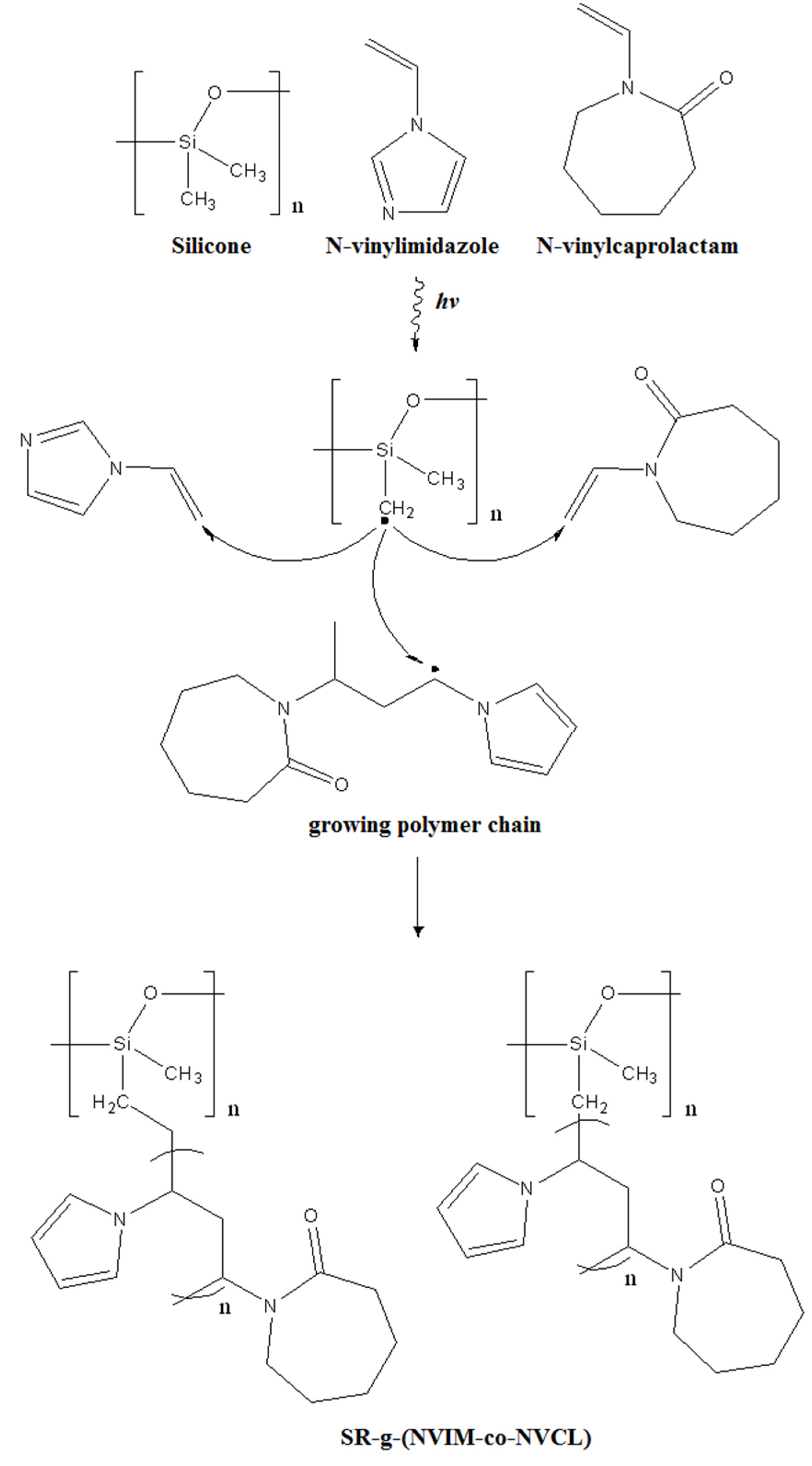
Gamma radiation is a form of ionizing radiation that is able to generate radical species by extracting electrons from the molecules by means of its high energy. The polymerization reaction to graft the binary-copolymer via the direct γ-ray radiation method is shown in
Scheme 1. First, γ-rays generate free radicals on the monomers (NVIM and NVCL) and SR; the radicals generated on the SR initiate the grafting process by reacting with the vinyl substituent of the monomers. Activation of NVIM and NVCL results in the formation of growing random copolymer chains that can react with the SR free radicals to form the binary graft copolymer.
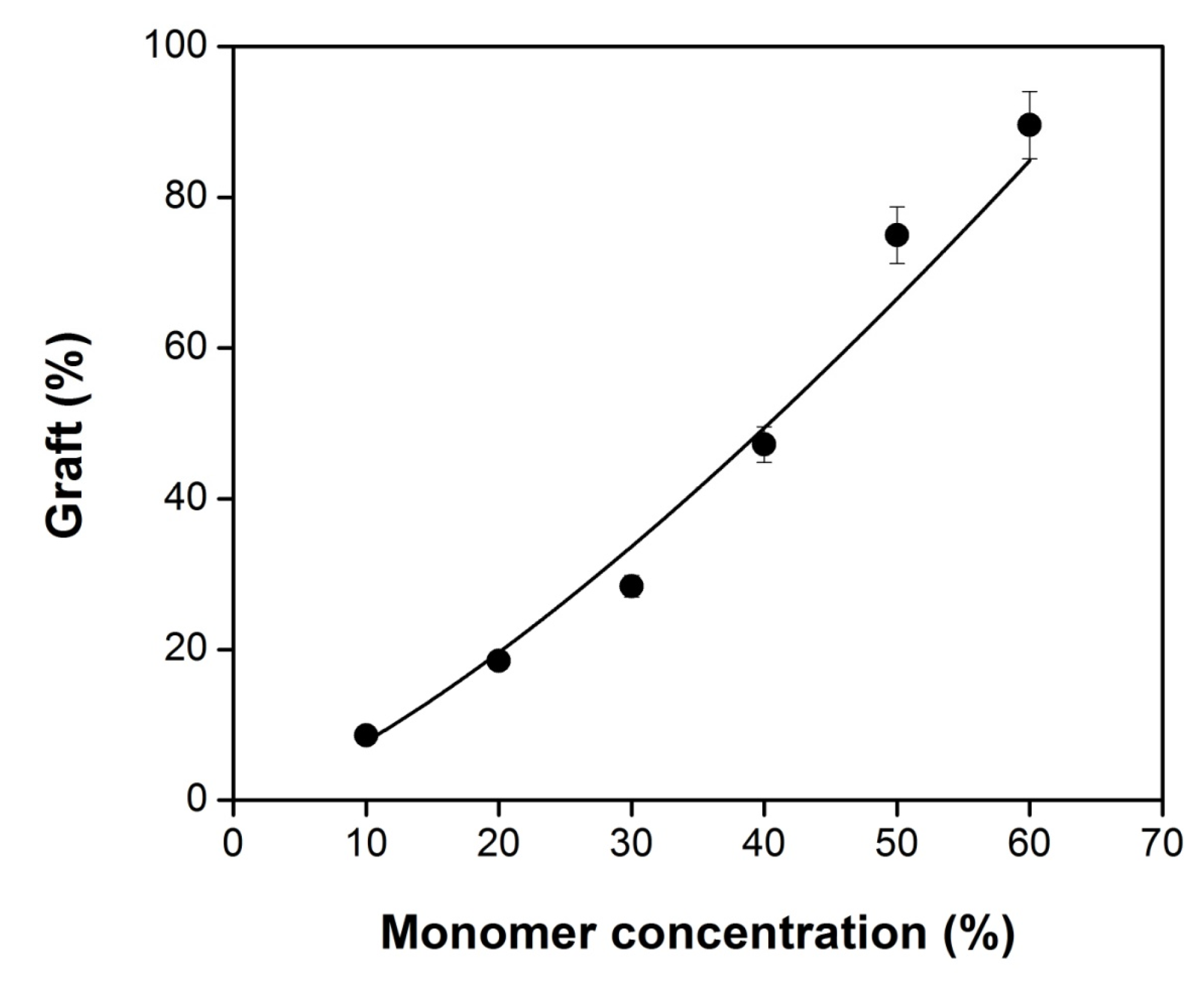
Figure 1 shows an efficient grafting of NVIM and NVCL onto SR. As the figure shows, the grafting percentage increases with increasing monomer concentration, reaching a maximum of 90% for a 60% monomer concentration. From the figure, it can also be gathered that a higher monomer concentration favors “the gel effect” and crosslinking, which results in a slower termination step due to the lack of mobility of the growing chains. It should be noted that at the working conditions the grafting process was not difficult to extract homopolymer from grafted SR up to 50 vol% of monomer concentration.
Scheme 1.
Grafting of SR-g-(NVCL-co-NVIM) by means of gamma radiation.
Scheme 1.
Grafting of SR-g-(NVCL-co-NVIM) by means of gamma radiation.
Figure 1.
Grafting yield by one step method of poly(N-vinylcaprolactam) (PNVCL) and poly-(N-vinylimidazole) (PNVIM) onto silicone rubber (SR) films as function of monomer concentration in toluene, dose fixed of 50 kGy and dose rate of 9.3 kGy h−1.
Figure 1.
Grafting yield by one step method of poly(N-vinylcaprolactam) (PNVCL) and poly-(N-vinylimidazole) (PNVIM) onto silicone rubber (SR) films as function of monomer concentration in toluene, dose fixed of 50 kGy and dose rate of 9.3 kGy h−1.
The effect of the irradiation dose on the grafting yield was examined by performing graft polymerizations at three different monomer concentrations (
Figure 2), the number of latent initiating sites is expected to increase with increasing radiation dose, although not necessarily in a proportional manner. It was found that a directly proportional relationship between dose and grafting yield exits for this system. This is because the greater the exposure to gamma radiation, more active sites will be generated because there will be more free radicals in the reaction medium. Using a 50 kGy dose, the grafting percentage was of 50%, 58%, and 75% for a 50% monomer (NVCL/NVIM 1/1, vol%), 50% monomer (NVCL/NVIM 7/3, vol%), and 100% monomer (NVCL/NVIM 1/1, vol%) concentration, respectively.
Figure 2.
Grafting yield by one step method of PNVCL and PNVIM onto SR films in toluene as function of irradiation dose, at dose rate of 9.3 kGy h−1 and different monomer concentration: 50% monomer in toluene (NVCL/NVIM 1/1, vol%) (●); 50% monomer in toluene (NVCL/NVIM 7/3, vol%) (□); and 100% monomer (NVCL/NVIM 1/1, vol%) (△).
Figure 2.
Grafting yield by one step method of PNVCL and PNVIM onto SR films in toluene as function of irradiation dose, at dose rate of 9.3 kGy h−1 and different monomer concentration: 50% monomer in toluene (NVCL/NVIM 1/1, vol%) (●); 50% monomer in toluene (NVCL/NVIM 7/3, vol%) (□); and 100% monomer (NVCL/NVIM 1/1, vol%) (△).
The swelling behavior of SR-g-(NVIM-co-NVCL) as a function of time was studied between 15 and 350 min (
Figure 3). Grafted SR films were immersed in water at 25 °C and the swelling percentage was calculated using gravimetry. The water uptake was high during the first 60 min, but leveled off regardless of the particular system, clearly indicating that equilibrium was reached at around 150 min. It was also observed that swelling increases with higher grafting percentages, which in turn depend on monomer concentration and radiation dose. SR-g-(NVIM-co-NVCL) showed 8%, 45%, and 170% swelling for a 29%, 75%, and 106% graft, respectively. Regarding the swelling degree of the SR-g-(NVIM-co-NVCL), the incorporation of poly(NVIM-co-NVCL) caused the swelling percentage to increase twofold, because of the high capacity of poly(NVIM-co-NVCL) to absorb water.
Figure 3 shows essential contribution of the grafted poly(NVIM-co-NVCL) concentration in the swelling increase, and the equilibrium swelling was reached in less than 150 min for all samples.
Figure 3.
Swelling kinetics of PNVCL and PNVIM onto SR films graft contents of SR alone (□), SR-g-NVCL 29% graft (▲), SR-g-(NVCL-co-NVIM) 75% graft (◆), and SR-g-NVIM 106% graft (●) immersed in water at 25 °C.
Figure 3.
Swelling kinetics of PNVCL and PNVIM onto SR films graft contents of SR alone (□), SR-g-NVCL 29% graft (▲), SR-g-(NVCL-co-NVIM) 75% graft (◆), and SR-g-NVIM 106% graft (●) immersed in water at 25 °C.
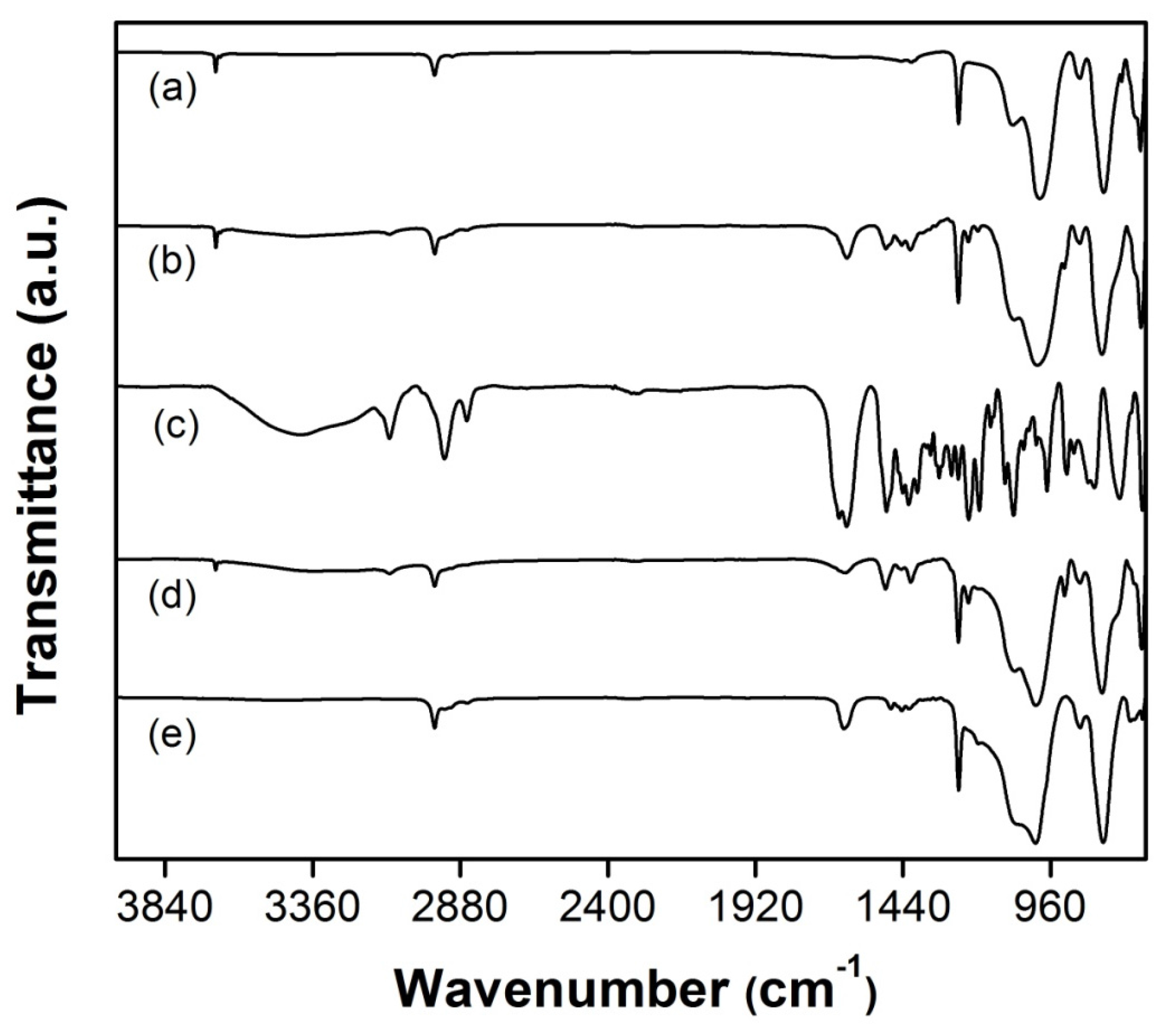
FT-IR spectra of SR and graft copolymer SR-g-VIM, SR-g-NVCL, and SR-g-(NVCL-co-NVIM) at different grafting yields are shown in
Figure 4. The spectrum of pristine SR film showed a band at 1005 cm
−1 due to stretching vibrations of the Si–O–Si bond, and signals at 2963 and 1258 cm
−1, which corresponded to C–H groups. On the other hand, SR-g-VIM showed a peak at 3105 cm
−1 due to the stretching vibration of C–H from the imidazole ring, a signal at 1657 cm
−1 assigned to the stretching vibration of aromatic C=C, bands, peaks at 1495 and 1416 cm
−1 corresponding to the stretching vibration of aromatic C=N and C–N bonds, and a signal at 1226 cm
−1 due to the N–C–N bond. The graft copolymer for SR-g-NVCL also showed peaks at 1260 and 1160 cm
−1 that belong to the C–N stretching vibrations from NVCL, and two bands at 2914 and 2848 cm
−1 that correspond to the C–H stretching vibrations. The characteristic signals of PNVIM and PNVCL are present in grafted SR.
Figure 4.
FTIR-ATR spectra for SR (a), SR-g-(NVCL-co-NVIM) 75% graft (b), poly(NVCL-co-NVIM) (c), SR-g-NVIM 106% graft (d), and SR-g-NVCL 29% graft (e).
Figure 4.
FTIR-ATR spectra for SR (a), SR-g-(NVCL-co-NVIM) 75% graft (b), poly(NVCL-co-NVIM) (c), SR-g-NVIM 106% graft (d), and SR-g-NVCL 29% graft (e).
The thermal behavior of SR and SR-(NCVL-co-NVIM) (
Figure 5) was characterized by thermogravimetic analysis in a nitrogen atmosphere using a temperature range from 25 to 800 °C and a heat ramp of 10 °C min
−1.The SR sample showed one change due to the polymer decomposition. The 10% weight-loss temperature of SR, SR-g-NVCL 29% graft, SR-g-NVIM 106% graft and SR-g-(NVCL-co-NVIM) 75% graft was found at 501 °C, 411 °C, 403 °C, and 397 °C, respectively, with the decomposition temperature of poly(NVCL-co-NVIM) occurring at 237 °C; the TGA data for grafted SR are similar, with a difference of only 14 °C with thermal stability close to 400 °C.
Figure 5.
Thermogravimetric curves for SR alone (a), SR-g-VCL 29% graft (b), SR-g-VIM 106% graft (c), SR-g-(VCL-co-VIM) 75% (d), and poly(NVCL-co-NVIM) (e).
Figure 5.
Thermogravimetric curves for SR alone (a), SR-g-VCL 29% graft (b), SR-g-VIM 106% graft (c), SR-g-(VCL-co-VIM) 75% (d), and poly(NVCL-co-NVIM) (e).
The ability to simultaneously respond to both temperature and pH offers an additional control over the graft copolymer phase behavior. In this regard, a highly diverse set of smart materials can be prepared with the purpose of mimicking the behavior of responsive macromolecules found in nature. Indeed, there is a growing interest in obtaining smart copolymer onto SR films whose aqueous solution swelling properties can abruptly and reversibly change in response to simultaneous pH and temperature changes in the physiological range.
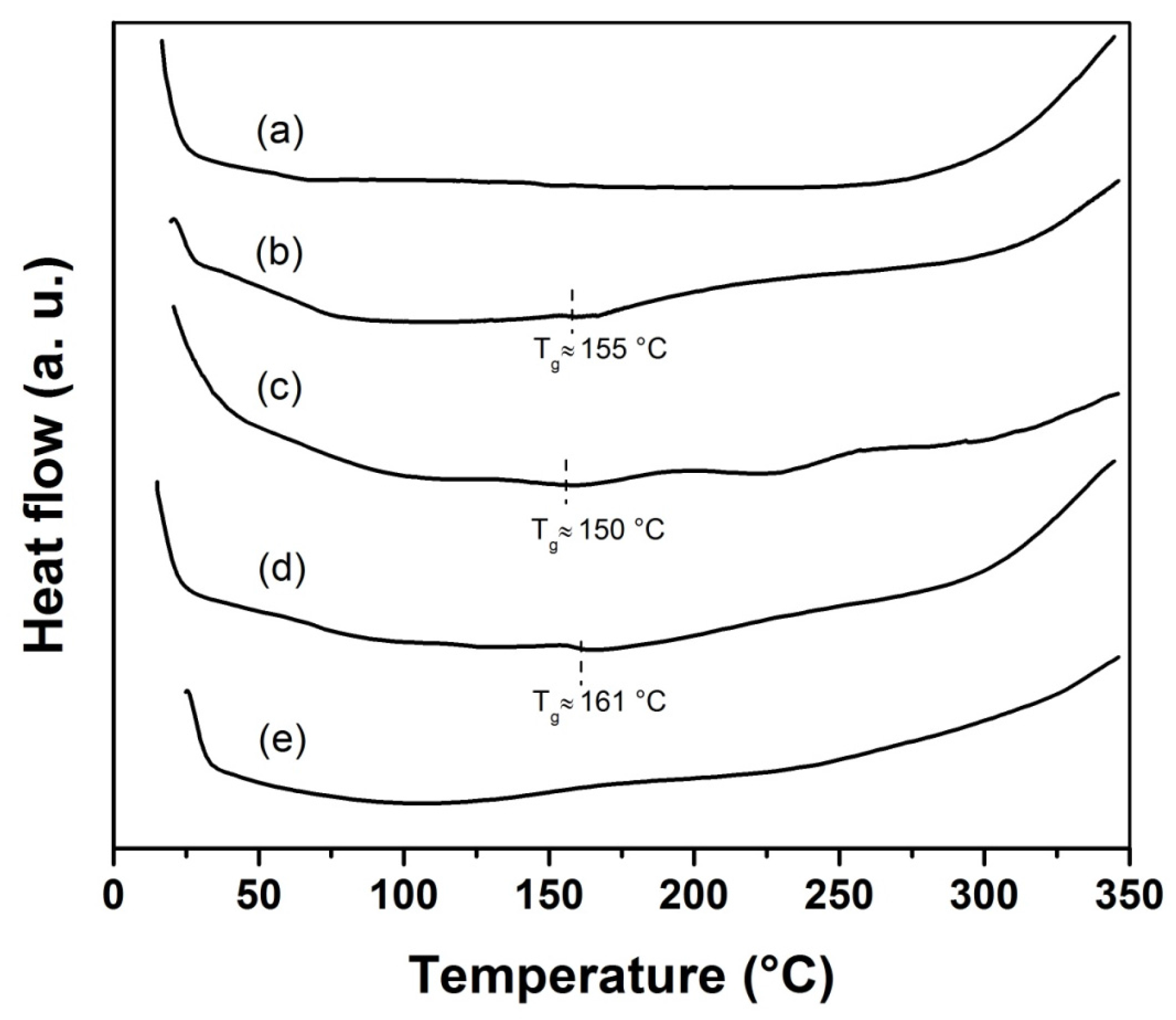
Figure 6 shows the DSC thermographs of SR-g-(NVCL-co-NVIM) SR and poly(NVCL-co-NVIM), where it is observed that the glass transition temperature (
Tg) of SR-g-(NVCL-co-NVIM) was found at 155 °C, that of poly(NVCL-co-NVIM) at 150 °C, and the
Tg for SR-g-NVIM was at 161 °C; no transitions were observed for SR and SR-g-NVCL. These results confirm that PNVIM and PNVCL are grafted onto SR films being thermally stable at around 150 °C.
Figure 6.
DSC thermograms of SR (a), SR-g-(NVCL-co-NVIM) 75% graft (b), poly(NVCL-co-NVIM) (c), SR-g-NVIM 90% graft (d), and SR-g-NVCL 29% graft (e).
Figure 6.
DSC thermograms of SR (a), SR-g-(NVCL-co-NVIM) 75% graft (b), poly(NVCL-co-NVIM) (c), SR-g-NVIM 90% graft (d), and SR-g-NVCL 29% graft (e).
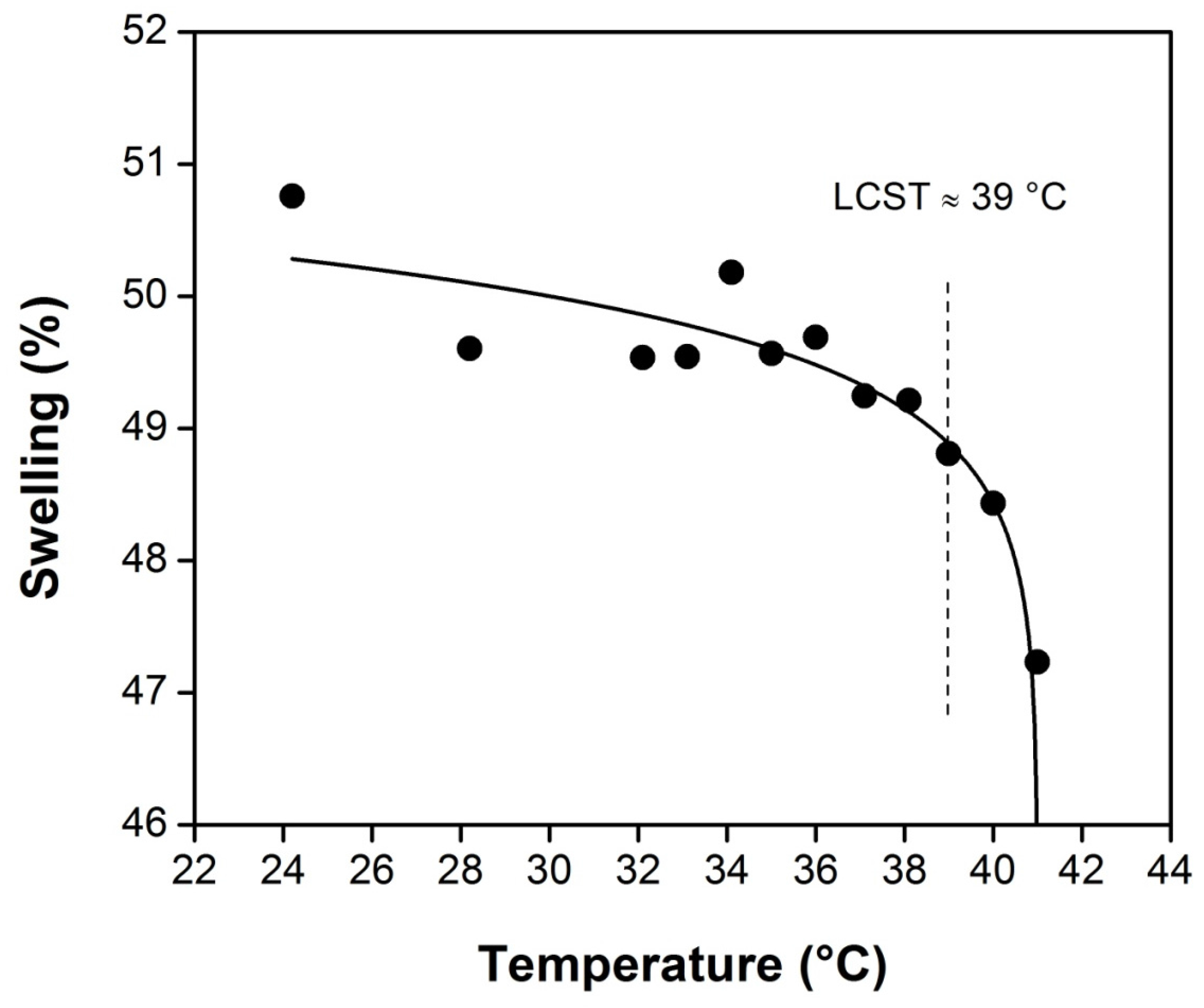
Binary thermo and pH responsive biomaterial showed the typical temperature-dependent swelling for thermosensitive grafted compositions with drastic swelling decrease at 39 °C, due to the LCST of PNVCL; the LCST of SR film-grafted with NVCL/NVIM was determined under different temperatures, as shown in
Figure 7. The grafted film exhibited temperature sensitivity, and the transparency of the copolymer decreased with the increase of temperature, as was observed when the temperature was raised to 40 °C and the grafted film suddenly became opaque. This is due to the hydrophobic interactions between PNVCL groups above the LCST.
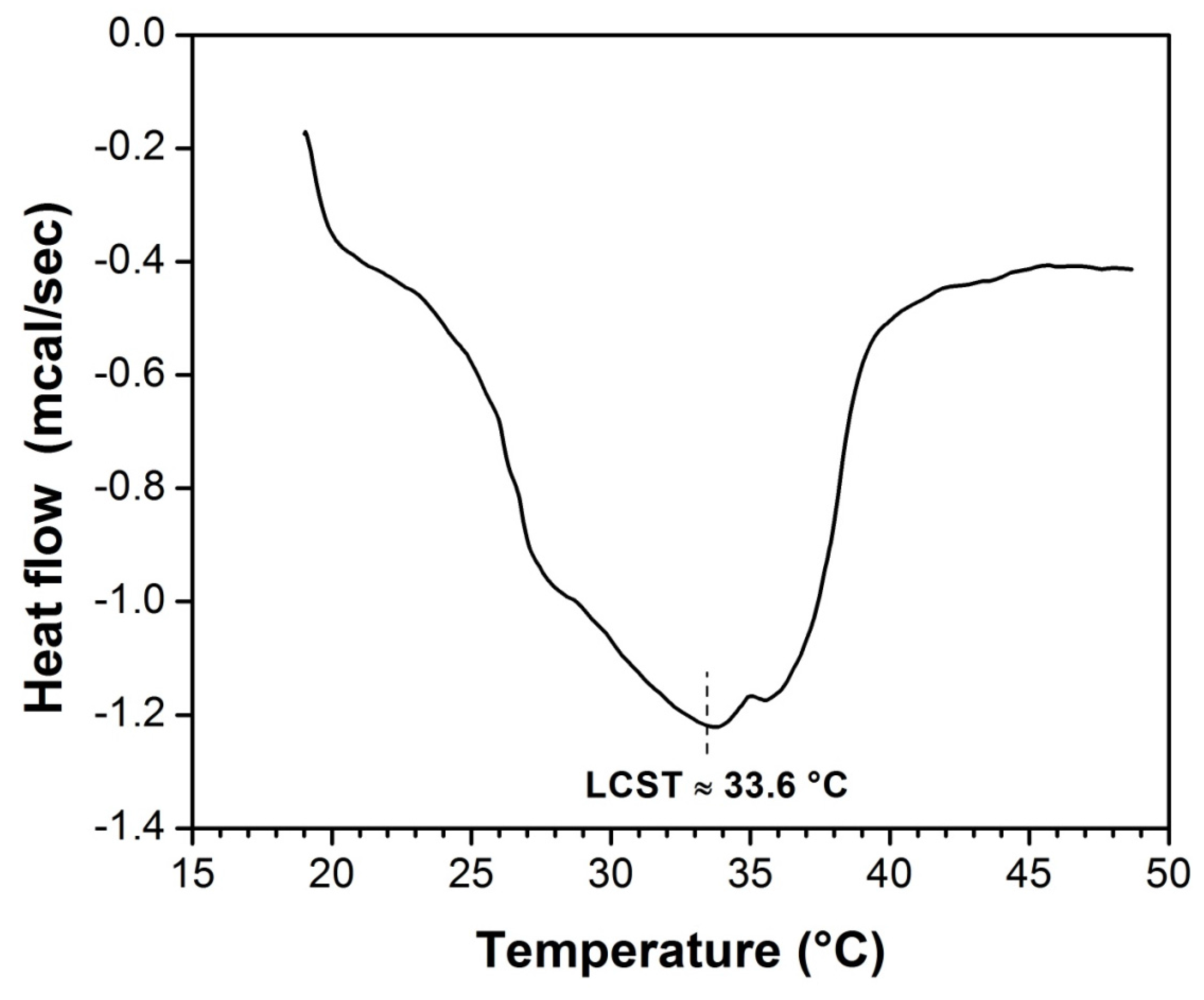
Figure 8 exhibits the DSC thermograms of the grafted NVCL and NVIM onto SR, which was swelled in distilled water. The onset point of the endothermal peak, determined by the intersecting point of two tangent lines from the baseline and slope of the endothermal peak, was used to determine LCST. SR-g-(NVCL-co-NVIM) shows a LCST at °33.6 °C.
Figure 9 shows the film’s equilibrium swelling behavior as a function of pH (from 2 to 12) at 25 °C. As can be seen, the critical pH point is reached at °8.5. Films were compact at pH values greater than 10, and at lower pH values a maximum swelling value was reached. Although the pH-dependent swelling equilibrium of different compositions of PNVCL and PNVIM grafted onto SR presented the same critical pH point (8.5), with an exception for the 24% graft, which showed a critical pH value of 8, the water uptake varied for different grafting degrees, ranging from 50% to 59%. The swelling percent for of SR-g-(NVCL-co-NVIM) changed dramatically around 8 which is close to the pKa value of the imidazole group. At pH below 7, the tertiary amine present in the ring imidazole behaves as a weak base and therefore it becomes protoned. On the other hand, when pH is increased above 7, amine moieties become neutral, diminishing its capacity to interact with water molecules.
Figure 7.
Temperature dependence of the swelling ratio in water as a function of temperature for poly(NVCL-co-NVIM)-grafted SR film (90% graft).
Figure 7.
Temperature dependence of the swelling ratio in water as a function of temperature for poly(NVCL-co-NVIM)-grafted SR film (90% graft).
Figure 8.
DSC thermograms of swelled SR-g-(NVCL-co-NVIM) with distilled water at a heating rate of 1 °C min−1 from 20 to 50 °C. LCST is indicated.
Figure 8.
DSC thermograms of swelled SR-g-(NVCL-co-NVIM) with distilled water at a heating rate of 1 °C min−1 from 20 to 50 °C. LCST is indicated.
Figure 9.
Dependence of swelling degree with respect to pH for SR-g-(NVCL-co-NVIM) at different grafting yield: 7.5% graft (a), 24% graft (b), 50% graft (c), 52% graft (d), and 59% graft (e).
Figure 9.
Dependence of swelling degree with respect to pH for SR-g-(NVCL-co-NVIM) at different grafting yield: 7.5% graft (a), 24% graft (b), 50% graft (c), 52% graft (d), and 59% graft (e).