Preparation and Photothermal Antimicrobial Performance of Triple Linkage Hydrogels
Abstract
:1. Introduction
2. Materials
2.1. Synthesis of TNT Nanoparticles
2.2. Synthesis of TNT@MPDA Nanoparticles
2.3. Synthesis of TNT@MPDA@Au Nanoparticles
2.4. Synthesis of TNT@MPDA@Au@PVA/PEG Composite Hydrogel
2.5. Photothermal Performance Test
- h—heat transfer coefficient;
- A808—absorbance of the sample at 808 nm;
- S—specific surface area of the container;
- I—laser power (2 W);
- ms—the quality of the water;
- Cs—heat capacity of water (4.2 J/g °C);
- ΔT—difference between the temperature at time t and the ambient temperature;
- ΔTmax—difference between maximum temperature and ambient temperature.
2.6. In Vitro Cytotoxicity Test
2.7. Bacteriostasis Experiments
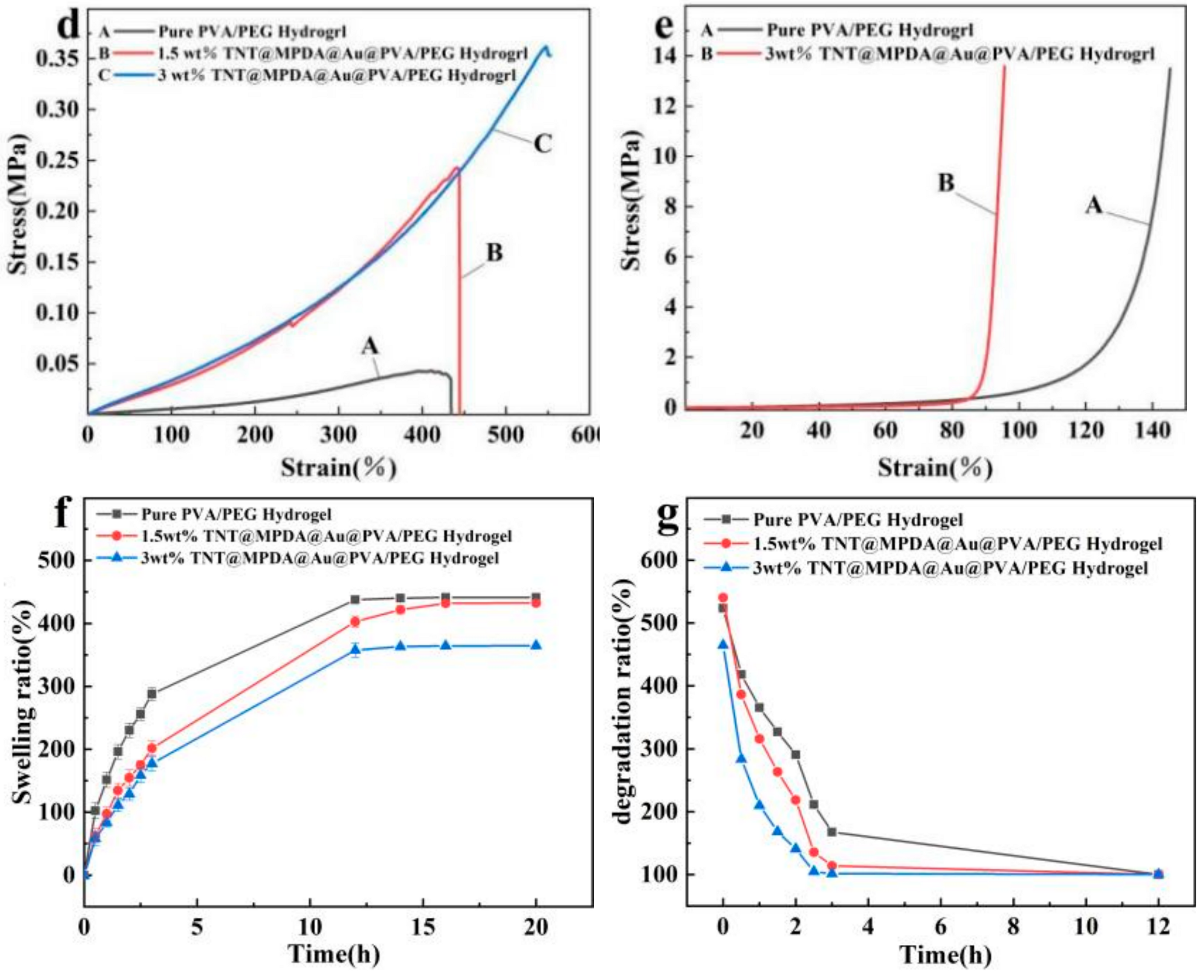
2.8. Swelling and Degradation Behaviour of Hydrogels
2.9. Mechanical Properties of Hydrogels
3. Results and Discussion
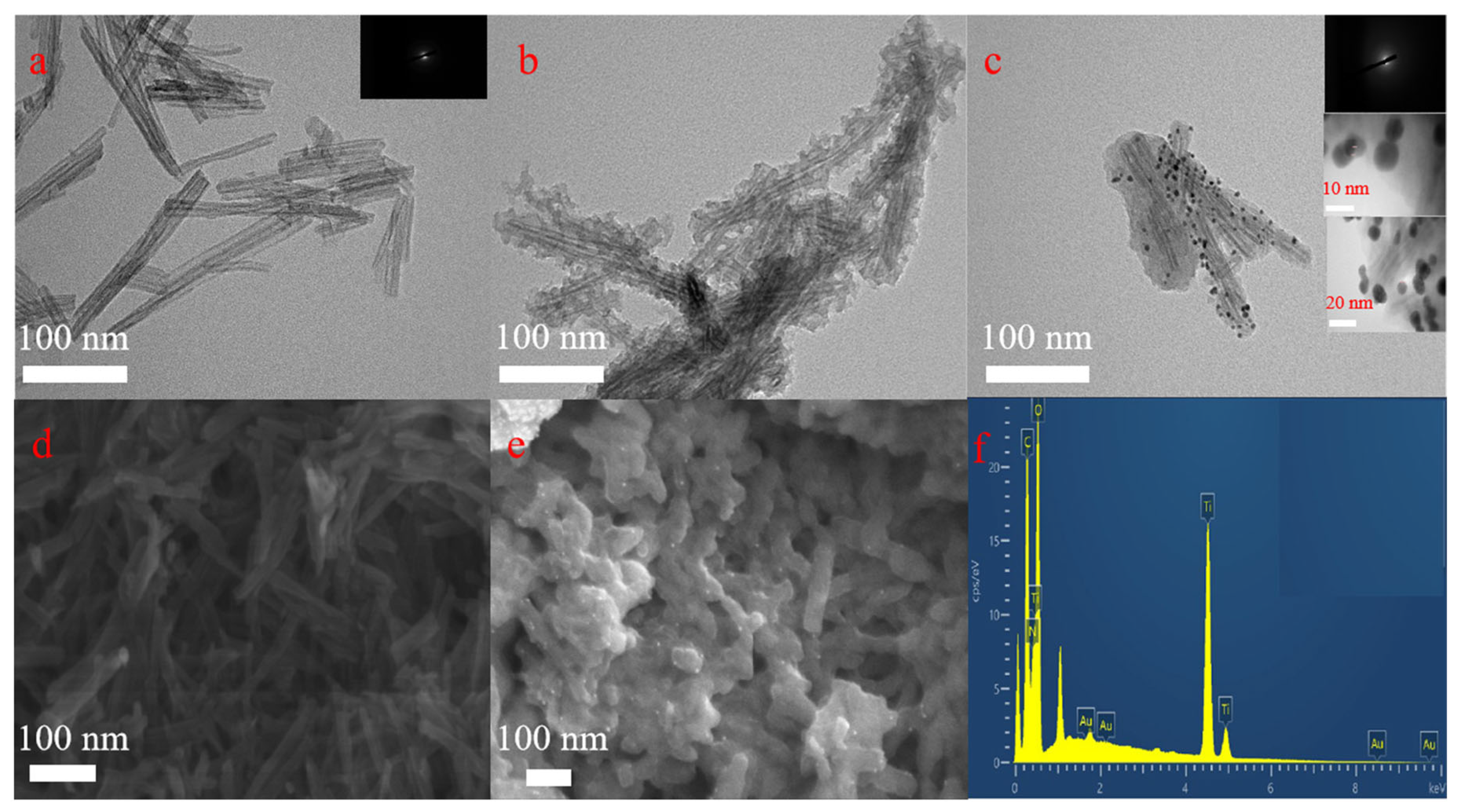
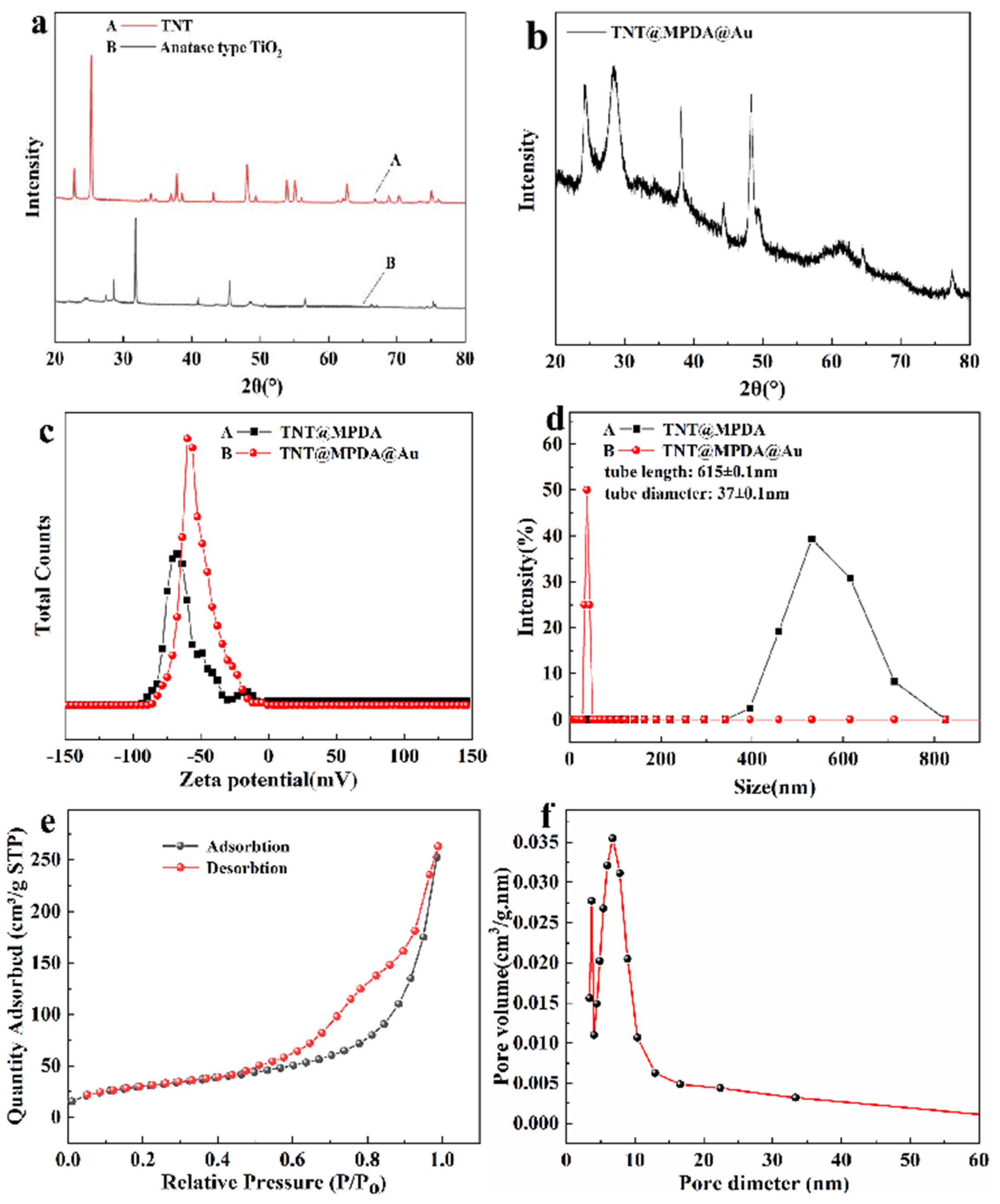
3.1. Morphology and Composition Characterization of TiO2TNTs@MPDA@Au Nanotubes Antibacterial Material
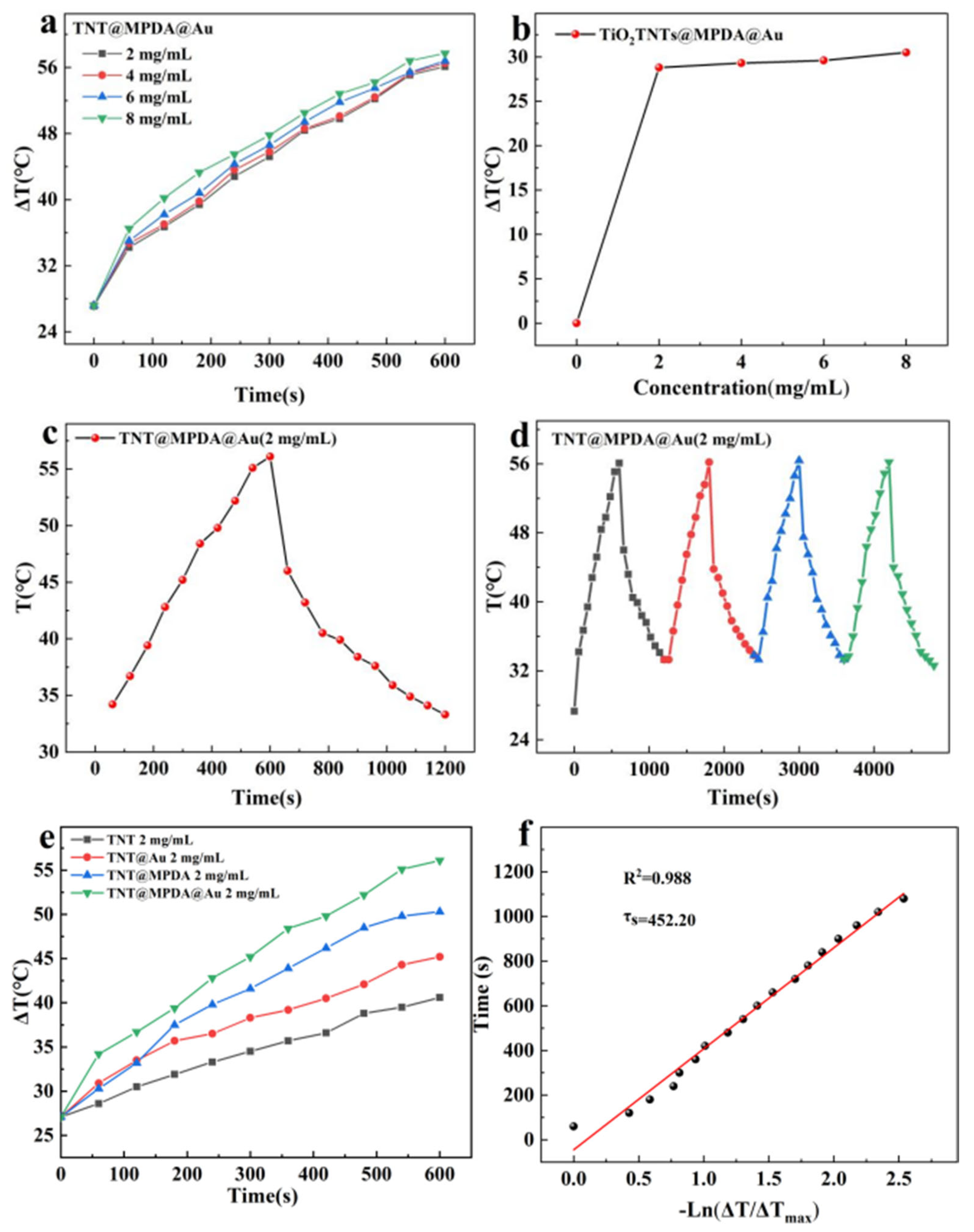
3.2. Photothermal Performance
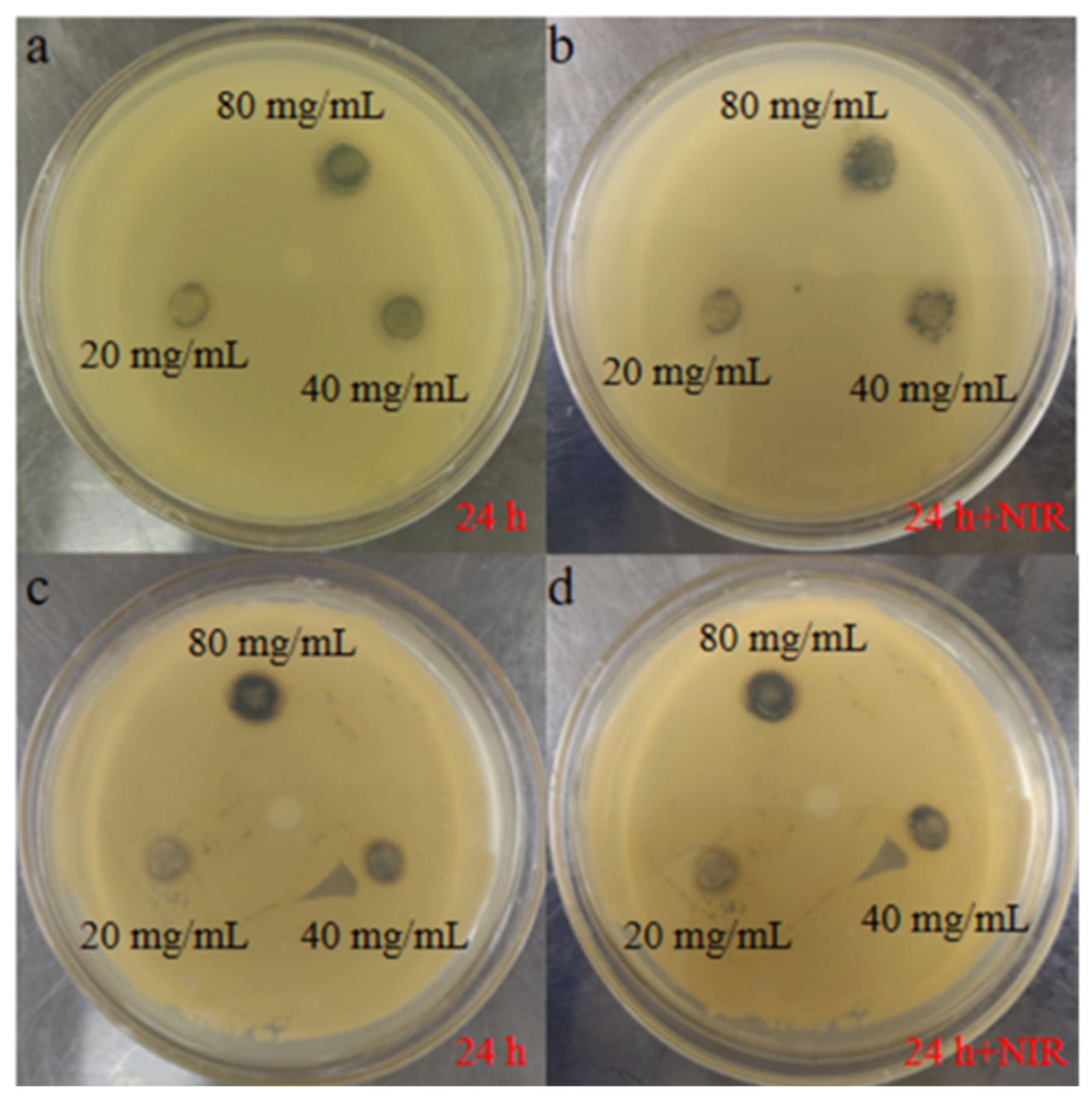
3.3. In Vitro Antibacterial Properties
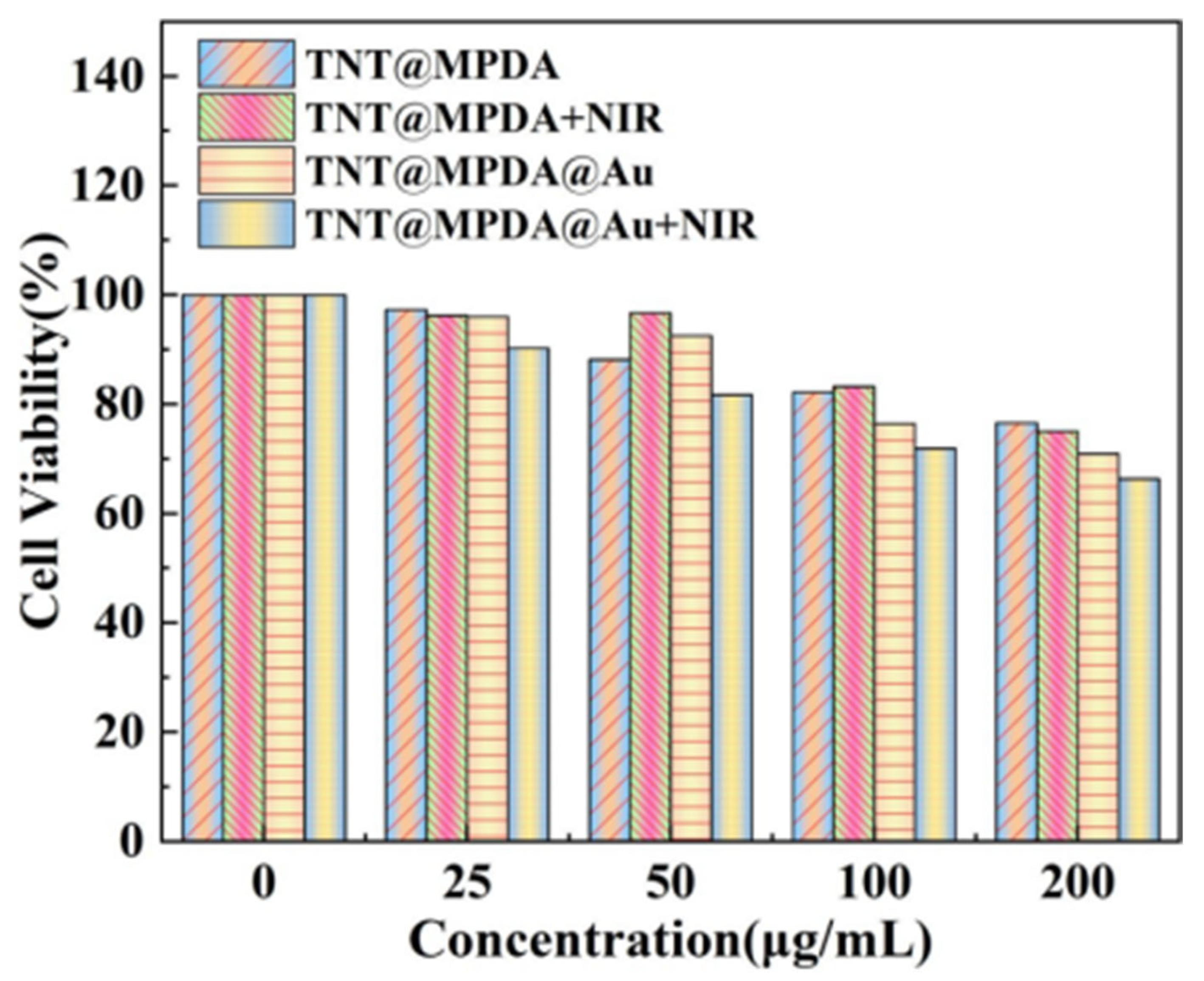
3.4. In Vitro Cytotoxicity
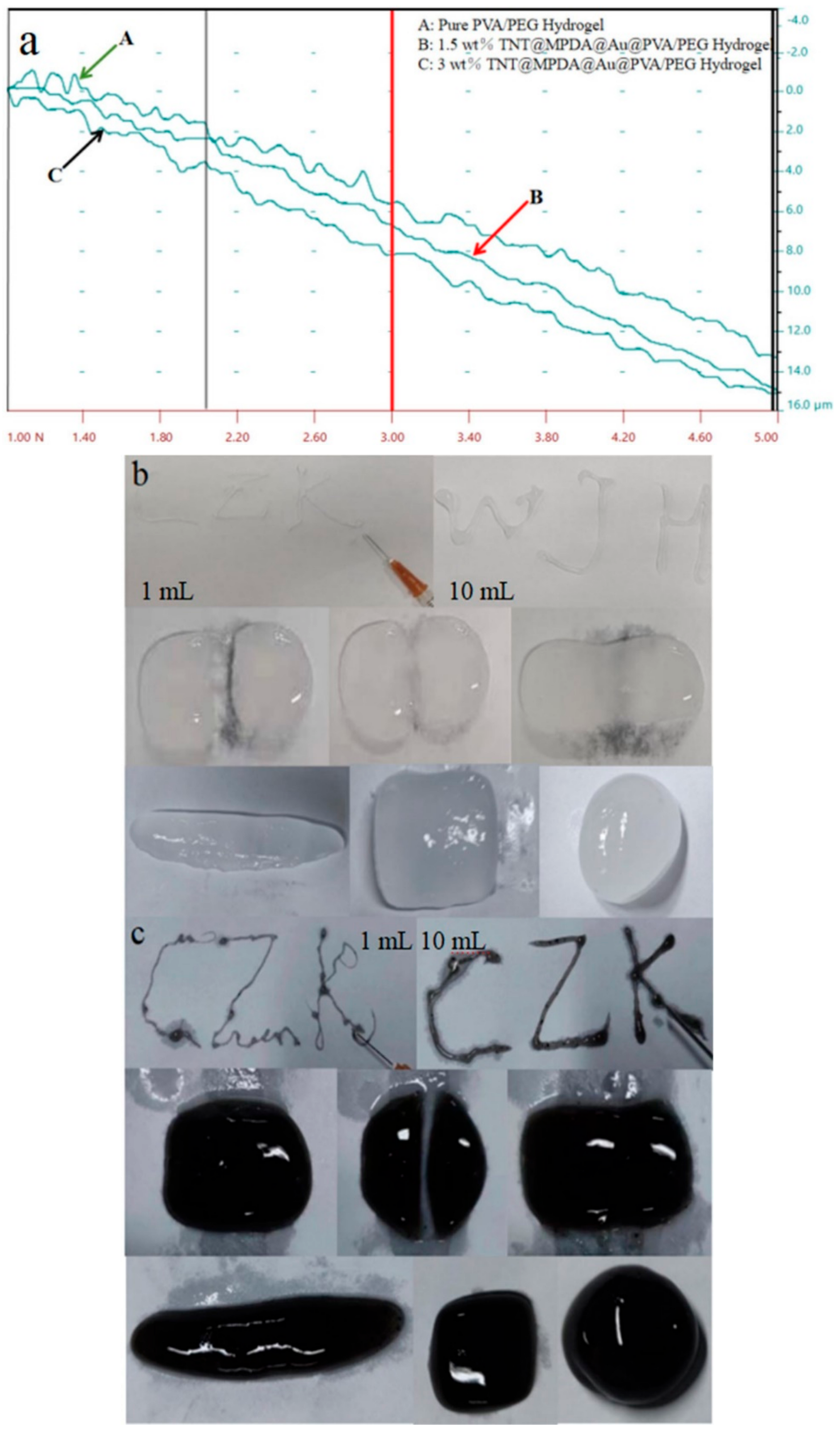
3.5. Mechanical Properties of Hydrogels
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Unnithan, A.R.; Nejad, A.G.; Sasikala, A.R.K.; Thomas, R.G.; Jeong, Y.Y.; Murugesan, P.; Nasseri, S.; Wu, D.-M.; Park, C.H.; Kim, C.S. Electrospun zwitterionic nanofibers with in situ decelerated epithelialization property for non-adherent and easy removable wound dressing application. Chem. Eng. J. 2016, 287, 640–648. [Google Scholar] [CrossRef]
- Luo, X.-G.; Zhang, H.; Cao, Z.-N.; Cai, N.; Xue, Y.-A.; Yu, F.-Q. A simple route to develop transparent doxorubicin-loaded nanodiamonds/cellulose nanocomposite membranes as potential wound dressings. Carbohydr. Polym. 2016, 143, 231–238. [Google Scholar] [CrossRef]
- Xu, R.; Luo, G.-X.; Xia, H.-S.; He, W.-F.; Zhao, J.; Liu, B.; Tan, J.-L.; Zhou, J.-Y.; Liu, D.-S.; Wang, Y.-Z.; et al. Novel bilayer wound dressing composed of silicone rubber with particular micropores enhanced wound re-epithelialization and contraction. Biomaterials 2015, 40, 1–11. [Google Scholar] [CrossRef]
- Zhao, X.; Liang, Y.; Huang, Y.; He, J.-H.; Han, Y.; Guo, B.-L. Physical double-network hydrogel adhesives with rapid shape adaptability, fast self-healing, antioxidant and NIR/pH stimulus-responsiveness for multidrug-resistant bacterial infection and removable wound dressing. Adv. Funct. Mater. 2020, 30, 1910748–1910765. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, H.; Guo, B.-L.; Dong, R.-N.; Qiu, Y.-S.; Peter, X.M. Antibacterial antioxidant electroactive injectable hydrogel as self-healing wound dressing with hemostasis and adhesiveness for cutaneous wound healing. Biomaterials 2017, 122, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Li, F.-X.; Wang, X.-L.; Yu, J.-Y.; Wu, D.-Q. Hyaluronic acid and polyethylene glycol hybrid hydrogel encapsulating nanogel with hemostasis and sustainable antibacterial property for wound healing. ACS Appl. Mater. Interfaces 2018, 10, 13304–13316. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, M.-R.; Zhao, Z. Facile fabrication of self-healing, injectable and antimicrobial cationic guar gum hydrogel dressings driven by hydrogen bonds. Carbohydr. Polym. 2023, 310, 120723–120732. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-A.; Wang, J.-M.; Yang, Y.-Q.; Shi, J.; Zhang, H.-Y.; Yao, X.-H.; Chen, W.-Y.; Zhang, X.-Y. A rose bengal/graphene oxide/PVA hybrid hydrogel with enhanced mechanical properties and light-triggered antibacterial activity for wound treatment. Mater. Sci. Eng. C 2021, 118, 111447–111460. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Liu, Z.; Abubaker, M.A.; Ding, L.; Zhang, J.; Yang, S.-R.; Fan, Z.-J. Antibacterial polyvinyl alcohol/bacterial cellulose/nano-silver hydrogels that effectively promote wound healing. Mater. Sci. Eng. C 2021, 126, 112171–112183. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-L.; Cheng, J.-W.; Ran, L.-X.; Yu, K.; Lu, B.-T.; Lan, G.-Q.; Dai, F.-Y.; Lu, F. An injectable self-healing hydrogel with adhesive and antibacterial properties effectively promotes wound healing. Carbohydr. Polym. 2018, 201, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Renganathan, R.; Subha, V.; Sathyaa, R.; Ravindran, R.S.E. Silver nanoparticles from methanolic stem extract of Gymnema sylvestre and its characterisation studies. Int. J. Nano Biomater. 2017, 7, 1752–8933. [Google Scholar] [CrossRef]
- Song, J.-W.; Yuan, C.-Q.; Jiao, T.-F.; Xing, R.-R.; Yang, M.-Y.; Adams, D.J.; Yan, X.-H. Multifunctional antimicrobial biometallohydrogels based on amino acid coordinated self-assembly. Small 2020, 16, 1907309–1907318. [Google Scholar] [CrossRef]
- Tortorella, S.; Inzalaco, G.; Dapporto, F.; Maturi, M.; Sambri, L.; Vetri Buratti, V.; Chiariello, M.; Franchini, M.C.; Locatelli, E. Biocompatible pectin-based hybrid hydrogels for tissue engineering applications. New J. Chem. 2021, 45, 22386–22395. [Google Scholar] [CrossRef]
- Gherardini, L.; Vetri Buratti, V.; Maturi, M.; Inzalaco, G.; Locatelli, E.; Sambri, L.; Gargiulo, S.; Barone, V.; Bonente, D.; Bertelli, E.; et al. Loco-regional treatment with temozolomide-loaded thermogels prevents glioblastoma recurrences in orthotopic human xenograft models. Sci. Rep. 2023, 13, 4630. [Google Scholar] [CrossRef]
- Heiba, Z.K.; Mohamed, M.B.; Ahmed, S.I. Exploring the physical properties of PVA/PEG polymeric material upon doping with nano gadolinium oxide. Alex. Eng. J. 2022, 61, 3375–3383. [Google Scholar] [CrossRef]
- Wang, Z.-H.; Ye, Q.-Z.; Yu, S.; Akhavan, B. Poly Ethylene Glycol (PEG)-based Hydrogels for Drug Delivery in Cancer Therapy: A Comprehensive Review. Adv. Healthc. Mater. 2023, 12, 2300105–2300150. [Google Scholar] [CrossRef] [PubMed]
- Casettari, L.; Vllasaliu, D.; Castagnino, E.; Stolnik, C.; Howdle, S.; Illum, L. PEGylated chitosan derivatives: Synthesis, characterizations and pharmaceutical applications. Prog. Polym. Sci. 2012, 37, 659–685. [Google Scholar] [CrossRef]
- Fang, H.; Wang, J.-H.; Li, L.; Xu, L.-Q.; Wu, Y.-X.; Wang, Y.; Xu, F.; Tian, J.; Yao, L. A novel high-strength poly (ionic liquid)/PVA hydrogel dressing for antibacterial applications. Chem. Eng. J. 2019, 365, 153–164. [Google Scholar] [CrossRef]
- Bo, Y.-Y.; Zhang, L.-H.; Wang, Z.-F.; Shen, J.-F.; Zhou, Z.-W.; Yang, Y.; Wang, Y.; Qin, J.-L.; He, Y.-N. Antibacterial Hydrogel with Self-Healing Property for Wound-Healing Applications. ACS Biomater. Sci. Eng. 2021, 7, 5135–5143. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-Y.; Yang, H.-T.; Liang, H.-B.; Xu, Y.-M.; Zhou, J.-P.; Peng, H.-X.; Zhong, J.; Xi, W.-X. Polydopamine particles reinforced poly(vinyl alcohol) hydrogel composites with fast self healing behavior. Prog. Org. Coat. 2020, 143, 105636–105644. [Google Scholar] [CrossRef]
- Tao, C.-H.; Ma, F.-S.; Chen, T.-D.; Li, X.-Q.; Guan, W.-J.; Zhang, A.-Q. Facile synthesis and performance studies of BSA and PDA@Ag hollow microcapsules using SiO2 microspheres as the templates. J. Alloys Compd. 2017, 715, 154–160. [Google Scholar] [CrossRef]
- Zeng, J.-K.; Wang, Y.-T.; Suna, Z.-Y.; Chang, H.-H.; Cao, M.; Zhao, J.; Lin, K.; Xie, Y.-Z. A novel biocompatible PDA/IR820/DAP coating for antibiotic/photodynamic/photothermal triple therapy to inhibit and eliminate Staphylococcus aureus biofilm. Chem. Eng. J. 2020, 394, 125017–125029. [Google Scholar] [CrossRef]
- Liu, J.-Y.; Liu, Y.-W.; Cao, Y.-D.; Sang, S.-H.; Guan, L.; Wang, Y.-Y.; Wang, J. Preparation of Fe3O4@PDA@Au@GO Composite as SERS Substrate and Its Application in the Enrichment and Detection for Phenanthrene. Micromachines 2022, 13, 128–141. [Google Scholar] [CrossRef] [PubMed]
- Maturi, M.; Locatelli, E.; Sambri, L.; Tortorella, S.; Šturm, S.; Kostevšek, N.; Comes Franchini, M. Synthesis of ultrasmall single-crystal gold-silver alloy nanotriangles and their application in photothermal therapy. Nanomaterials 2021, 11, 912. [Google Scholar] [CrossRef]
- Vines, J.B.; Yoon, J.H.; Ryu, N.E.; Lim, D.J.; Park, H. Gold nanoparticles for photothermal cancer therapy. Front. Chem. 2019, 7, 167. [Google Scholar] [CrossRef]
- Li, H.-Y.; Lin, L.-L.; Su, S.-S.; Wen, X.-Y.; Yan, R.; Liu, H.-M.; Tao, C.-H. Enhanced photothermal effect of functionalized HMPDA@AuNPs microcapsules for near-infrared theranostic treatment of tumor. J. Mater. Sci. 2022, 57, 7694–7705. [Google Scholar] [CrossRef]
- Jiang, K.; Smith, D.A.; Pinchuk, A. Size-dependent photothermal conversion efficiencies of plasmonically heated gold nanoparticles. J. Phys. Chem. C 2013, 117, 27073–27080. [Google Scholar] [CrossRef]
- Cao, F.; Wei, C.; Ma, G.; Hou, L.; Zhang, R.; Mei, L.; Qin, Q. Synthesis of photothermal antimicrobial cotton gauze using AuNPs as photothermal transduction agents. RSC Adv. 2021, 11, 25976–25982. [Google Scholar] [CrossRef]
- Liu, Y.-H.; Li, Z.-W.; Yin, Z.-B.; Zhang, H.-X.; Gao, Y.; Huo, G.-Y.; Wu, A.-J.; Zeng, L.-Y. Amplified photoacoustic signal and enhanced photothermal conversion of poly-dopamine-coated gold nanobipyramids for phototheranostics and synergistic chemotherapy. ACS Appl. Mater. Interfaces 2020, 12, 14866–14875. [Google Scholar] [CrossRef]
- Wang, W.-J.; Liu, J.-L.; Feng, W.-J.; Du, S.-L.; Ge, R.; Li, J.; Liu, Y.; Sun, H.-C.; Zhang, D.-Q.; Zhang, H.; et al. Targeting mitochondria with Au-Ag@Polydopamine nanoparticles for papillary thyroid cancer therapy. Biomater. Sci. 2019, 7, 1052–1063. [Google Scholar] [CrossRef]
- Moongraksathuma, B.; Chen, Y.-W. Anatase TiO2 co-doped with silver and ceria for antibacterial application. Catal. Today 2018, 310, 68–74. [Google Scholar] [CrossRef]
- Daghrir, R.; Drogui, P.; Robert, D. Modified TiO2 for environmental photocatalytic applications: A review. Ind. Eng. Chem. Res. 2013, 52, 3581–3599. [Google Scholar] [CrossRef]
- Pichat, P. Are TiO2 nanotubes worth using in photocatalytic purification of air and water? Molecules 2014, 19, 15075–15087. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.; Berger, S.; Schmuki, P. TiO2 nanotubes: Synthesis and applications. Angew. Chem. Int. Edit. 2011, 50, 2904–2939. [Google Scholar] [CrossRef] [PubMed]
- Paramasivam, I.; Jha, H.; Liu, N.; Schmuki, P. A review of photocatalysis using self-organized TiO2 nanotubes and other ordered oxide nanostructures. Small 2012, 8, 3073–3103. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Qi, M.; Li, X.; Liu, X.; Wang, L.; Yu, W.; Liu, M.; Lan, A.; Zhou, Y.; Song, Y. TiO2 nanotubes modified with Au nanoparticles for visible-light enhanced antibacterial and anti-inflammatory capabilities. Electroanal. Chem. 2019, 842, 66–73. [Google Scholar] [CrossRef]
- Camposeco, R.; Castillo, S.; Mejia-Centeno, I.; Navarrete, J.; Gómez, R. Effect of the Ti/Na molar ratio on the acidity and the structure of TiO2 nanostructures: Nanotubes, nanofibers and nanowires. Mater. Charact. 2014, 90, 113–120. [Google Scholar] [CrossRef]
- Chokesawatanakit, N.; Jutakridsada, P.; Boonlue, S.; Knijnenburg, J.; Wright, P.; Sillanpää, M.; Kamwilaisak, K. Ag-doped Cobweb-like structure of TiO2 nanotubes for antibacterial activity against Methicillin-resistant Staphylococcus aureus (MRSA). J. Environ. Chem. Eng. 2021, 9, 105843–105853. [Google Scholar] [CrossRef]
- Seo, H.K.; Kim, G.S.; Ansari, S.G.; Kim, Y.S.; Shin, H.S.; Shim, K.H.; Suh, E.K. A study on the structure/phase transformation of titanate nanotubes synthesized at various hydrothermal temperatures. Sol. Energy Mater. Sol. Cells 2008, 92, 1533–1539. [Google Scholar] [CrossRef]
- Lin, L.-L.; Li, H.-Y.; Su, S.-S.; Wen, X.-Y.; Yan, R.; Tao, C.-H. Study on the structure and properties of Fe3O4@HMPDA@HA magnetic hollow mesoporous submicron drug-carrying system. Micropor. Mesopor. Mater. 2022, 330, 111582–111592. [Google Scholar] [CrossRef]
- Tao, C.-H.; Chen, T.-D.; Liu, H.; Su, S.-S. Design of biocompatible Fe3O4@MPDA mesoporous core-shell nanospheres for drug delivery. Micropor. Mesopor. Mater. 2020, 293, 109823–109842. [Google Scholar] [CrossRef]
- Yan, P.-F.; Li, M.-Y.; Liu, J.; Hu, Y.-D.; Tang, K.Y. MoS2@PDA@Ag/PVA Hybrid Hydrogel with Excellent Light-Responsive Antibacterial Activity and Enhanced Mechanical Properties for Wound Dressing. Macromol. Mater. Eng. 2021, 307, 2100654–2100666. [Google Scholar] [CrossRef]
- Golabdar, A.; Adelnia, H.; Moshtzan, N.; Gavgani, J.N.; Izadi-Vasafi, H. Anti-bacterial poly(vinyl alcohol) nanocomposite hydrogels reinforced with in situ synthesized silver nanoparticles. Polym. Compos. 2019, 404, 1322–1328. [Google Scholar] [CrossRef]
- Falqi, F.H.; Bin-Dahman, O.A.; Hussain, M.; Al-Harthi, M.A. Preparation of Miscible PVA/PEG Blends and Effect of Graphene Concentration on Thermal, Crystallization, Morphological, and Mechanical Properties of PVA/PEG (10wt%) Blend. Int. J. Polym. Sci. 2018, 10, 8527693–8527703. [Google Scholar] [CrossRef]
- Wang, X.-X.; Cao, D.-W.; Tang, X.-J.; Yang, J.-J.; Jiang, D.-Y.; Liu, M.; He, N.-Y.; Wang, Z.-F. Coating Carbon Nanosphere with Patchy Gold for Production of Highly Efficient Photothermal Agent. ACS Appl. Mater. Interfaces 2016, 8, 19321–19332. [Google Scholar] [CrossRef]
- Su, S.-S.; Lin, L.-L.; Li, H.-Y.; Wen, X.-Y.; Yan, R.; Tao, C.-H. Preparation and properties study of F-SiO2@MPDA-AuNPs drug nanocarriers. Micropor. Mesopor. Mater. 2022, 330, 111571–111581. [Google Scholar] [CrossRef]
- Roper, D.K.; Ahn, W.; Hoepfner, M. Microscale heat transfer transduced by surface plasmon resonant gold nanoparticles. J. Phys. Chem. C 2007, 111, 3636–3641. [Google Scholar] [CrossRef]
- Tao, B.-L.; Lin, C.-C.; Yuan, Z.; He, Y.; Chen, M.-W.; Li, K.; Hu, J.-W.; Yang, Y.-L.; Xia, Z.-Z.-L.; Cai, K.-Y. Near infrared light-trig-gered on-demand currelease from Gel-PDA@Cur composite hydrogel for antibacterial wound healing. Chem. Eng. J. 2020, 403, 126182–126240. [Google Scholar] [CrossRef]
- Li, L.-L.; Lin, L.-L.; Yan, R.; Chen, Z.-K.; Wen, X.-Y.; Zeng, X.-W.; Tao, C.-H. Multi-functional Fe3O4@HMPDA@G5-Au core-releasable satellite nano drug carriers for multimodal treatment of tumor cells. Eur. Pol. J. 2022, 181, 111647–111659. [Google Scholar] [CrossRef]
- Shumbula, N.P.; Nkabinde, S.S.; Ndala, Z.B.; Mpelane, S.; Shumbula, M.P.; Mdluli, P.S.; Njengele-Tetyana, Z.; Tetyana, P.; Hlatshwayo, P.; Mlambo, M.; et al. Evaluating the antimicrobial activity and cytotoxicity of polydopamine capped silver and silver/polydopamine core-shell nanocomposites. Arab. J. Chem. 2022, 15, 103798–103810. [Google Scholar] [CrossRef]
- Zeng, Q.-K.; Qian, Y.-N.; Huang, Y.-J.; Ding, F.; Qi, X.-L.; Shen, J.-L. Polydopamine nanoparticle-dotted food gum hydrogel with excellent antibacterial activity and rapid shape adaptability for accelerated bacteria-infected wound healing. Bioact. Mater. 2021, 6, 2647–2657. [Google Scholar] [CrossRef] [PubMed]
- Cui, K.-X.; Yan, B.; Xie, Y.-J.; Qian, H.; Wang, X.-G.; Huang, Q.-X.; He, Y.-H.; Jin, S.-M.; Zeng, H.-B. Regenerable urchin-like Fe3O4@PDA-Ag hollow microspheres as catalyst and adsorbent for enhanced removal of organic dyes. J. Hazard. Mater. 2018, 350, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Tao, C.-H.; Chen, T.-D.; Liu, H.; Su, S.-S. Preparation and adsorption performance research of large-volume hollow mesoporous polydopamine microcapsules. MRS Commun. 2019, 9, 744–749. [Google Scholar] [CrossRef]
- Vargas, M.A.; Rodríguez-Páez, J.E. Amorphous TiO2 nanoparticles: Synthesis and antibacterial capacity. J. Non-Cryst. Solids 2017, 459, 192–205. [Google Scholar] [CrossRef]
- Feng, X.-Y.; Wang, P.-F.; Hou, J.; Qian, J.; Wang, C.; Ao, Y.-H. Oxygen vacancies and phosphorus codoped black titania coated Carbon nano-tube composite photocatalyst with efficient photocatalytic performance for the degradation of acetaminophen under visible light irradiation. Chem. Eng. J. 2018, 352, 947–956. [Google Scholar] [CrossRef]
- Arab, M.; Jallab, M.; Ghaffari, M.; Moghbelli, E.; Saeb, M.R. Synthesis, rheological characterization, and antibacterial activity of polyvinyl alcohol (PVA)/zinc oxide nanoparticles wound dressing, achieved under electron beam irradiation. Iran. Polym. J. 2021, 30, 1019–1028. [Google Scholar] [CrossRef]
- Wang, H.-F.; Wei, L.-Y.; Wang, Z.-Q.; Chen, S.-G. Preparation, characterization and long-term antibacterial activity of Ag-poly(dopamine)-TiO2 nanotubes composites. Rsc. Adv. 2016, 6, 14097–14104. [Google Scholar] [CrossRef]




| Instrument Model | Model Number | Manufacturer (of a Product) |
|---|---|---|
| High-resolution transmission electron microscopy (TEM) | FEI Corp TF 20 | Japan JEOL Corporation, Tokyo, Japan |
| High-speed freezing centrifuge | GL-G20-II | Shanghai Anting Instrument Co., Shanghai, China |
| Ultraviolet spectrophotometer (UV-Vis) | NanoDrop 2000c | Tianmei Instrument Co., Shanghai, China |
| X-ray photoelectron spectroscopy (XPS) | pHI-5702 | Phisical Electronics Corporation, Chanhassen, MN, USA |
| Zeta potential and particle size analyser | 90plus Pals | Brookhaven Corporation, Lexington, MA, USA |
| Field emission scanning electron microscope (FESEM) | JSM-6701F | Japan JEOL Corporation, Tokyo, Japan |
| X-ray diffractometer (XRD) | XRD7000, 40 kV/150 mA | Shimadzu Corporation, Kyoto, Japan |
| Fourier transform infrared spectrometer (FTIR) | BrukerIFS66V/S Fourier | Bruker Corporation, Ettlingen, Germany |
| Rotary Bath Oscillator | SHZ-82 | Jiangsu Zhengji Instrument Co., Changzhou, China |
| Magnetic stirrer | 85-1 | Shanghai Silo Instrument Co., Shanghai, China |
| Analytical balance | FA2004 | Shanghai Liangping Instrumentation Co., Shanghai, China |
| Concentration of TNT@MPDA@Au | 0 mg/mL | 20 mg/mL | 40 mg/mL | 80 mg/mL | |
|---|---|---|---|---|---|
| Inhibitory Ring Size of Bacteria | E. coli | 0 cm | 0.22 cm | 0.48 cm | 0.6 cm |
| S. aureus | 0 cm | 0.2 cm | 0.36 cm | 0.54 cm | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Yin, Q.; Xu, L.; Guo, W.; Tao, C. Preparation and Photothermal Antimicrobial Performance of Triple Linkage Hydrogels. Coatings 2024, 14, 363. https://doi.org/10.3390/coatings14030363
Chen Z, Yin Q, Xu L, Guo W, Tao C. Preparation and Photothermal Antimicrobial Performance of Triple Linkage Hydrogels. Coatings. 2024; 14(3):363. https://doi.org/10.3390/coatings14030363
Chicago/Turabian StyleChen, Zekun, Qingyue Yin, Liang Xu, Wenwen Guo, and Caihong Tao. 2024. "Preparation and Photothermal Antimicrobial Performance of Triple Linkage Hydrogels" Coatings 14, no. 3: 363. https://doi.org/10.3390/coatings14030363






