Water Lubrication of Al-Cu Composites Reinforced by Nickel-Coated Si3N4 Particles
Abstract
:1. Introduction
2. Materials and Methods
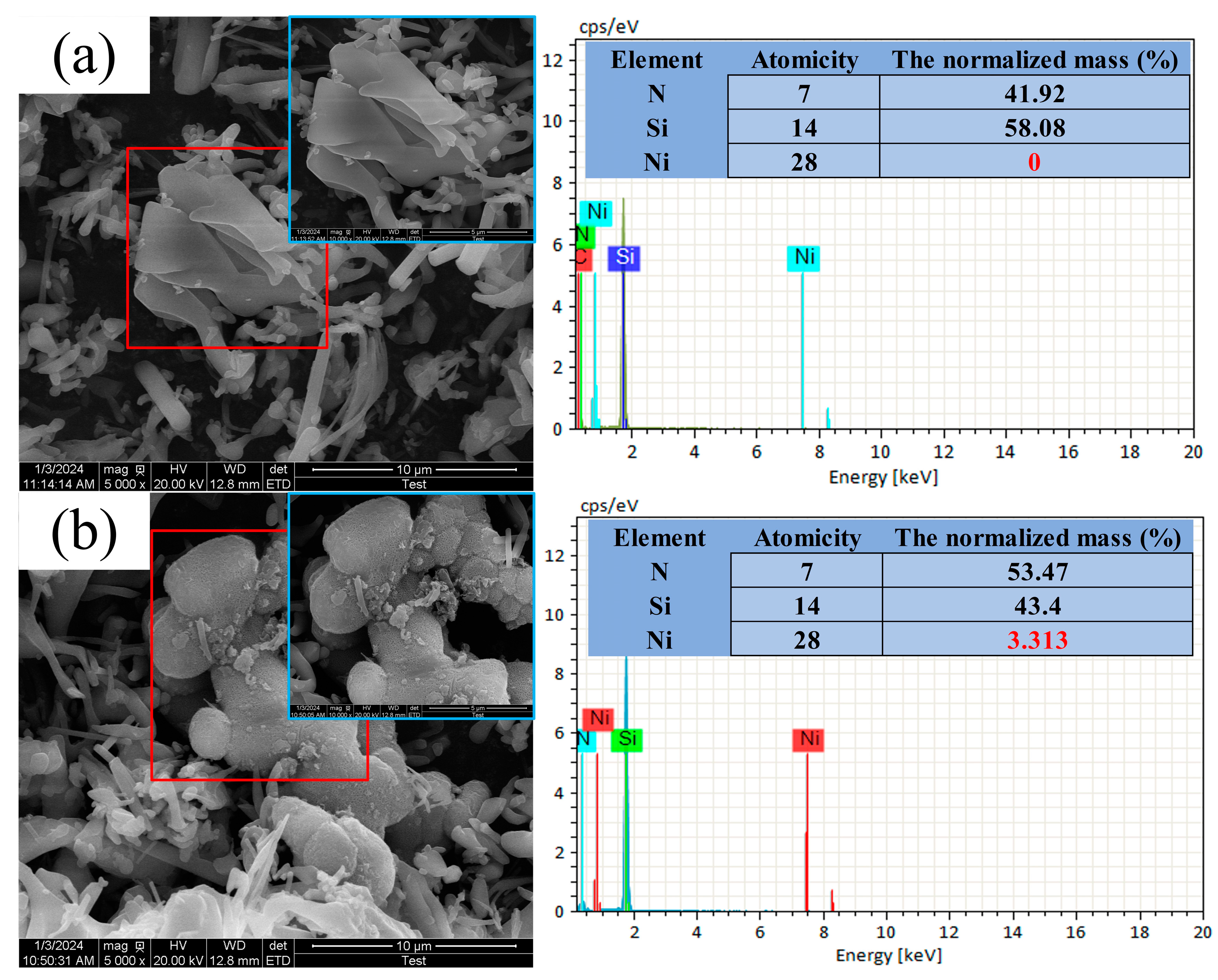
2.1. Preparation of Ni-Coated Si3N4 Particles by Electroless Plating
2.2. Preparation of Al-Cu Alloy Composites Reinforced by Ni-Coated Si3N4
2.3. Friction and Wear Test in Water
3. Results and Discussion
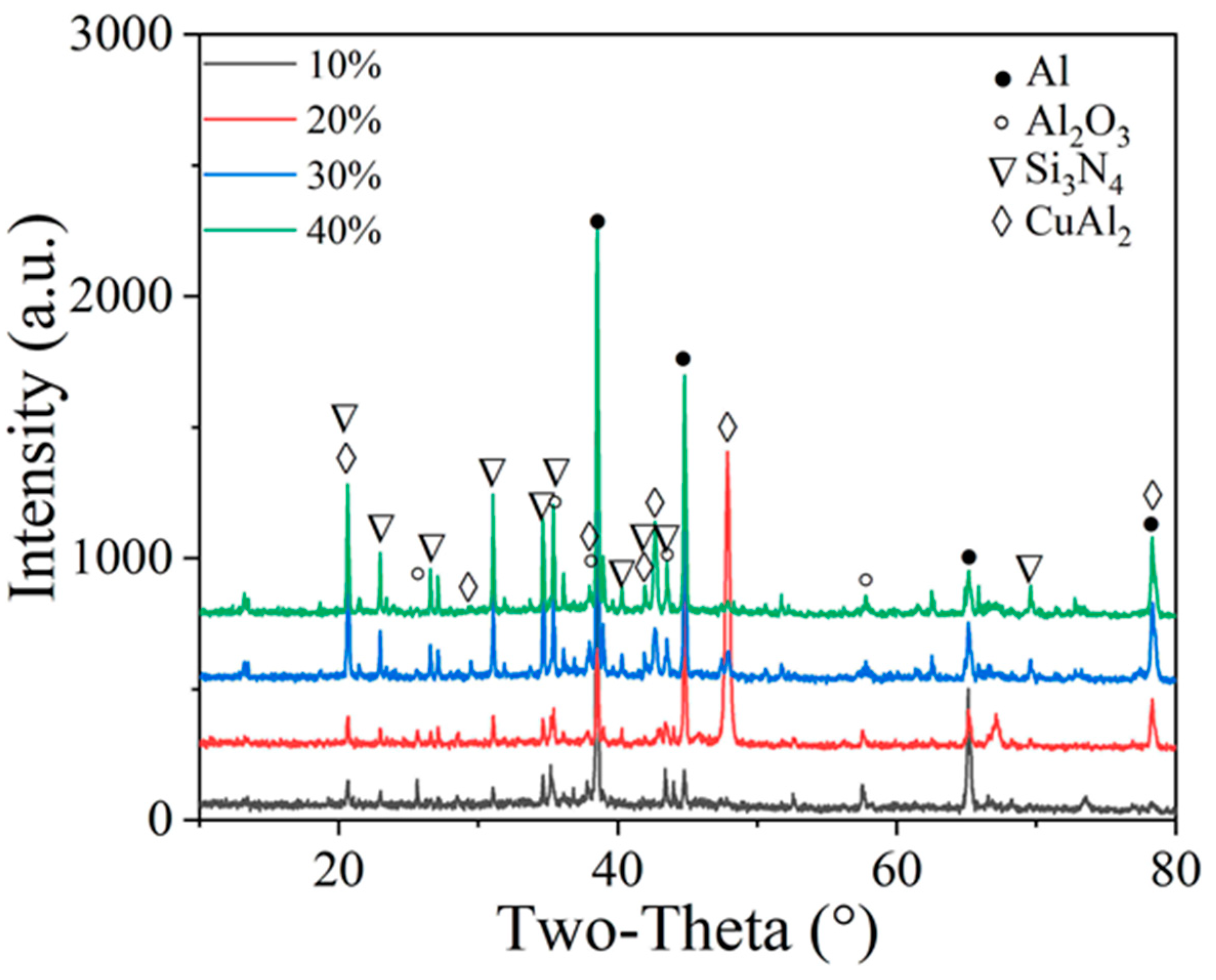
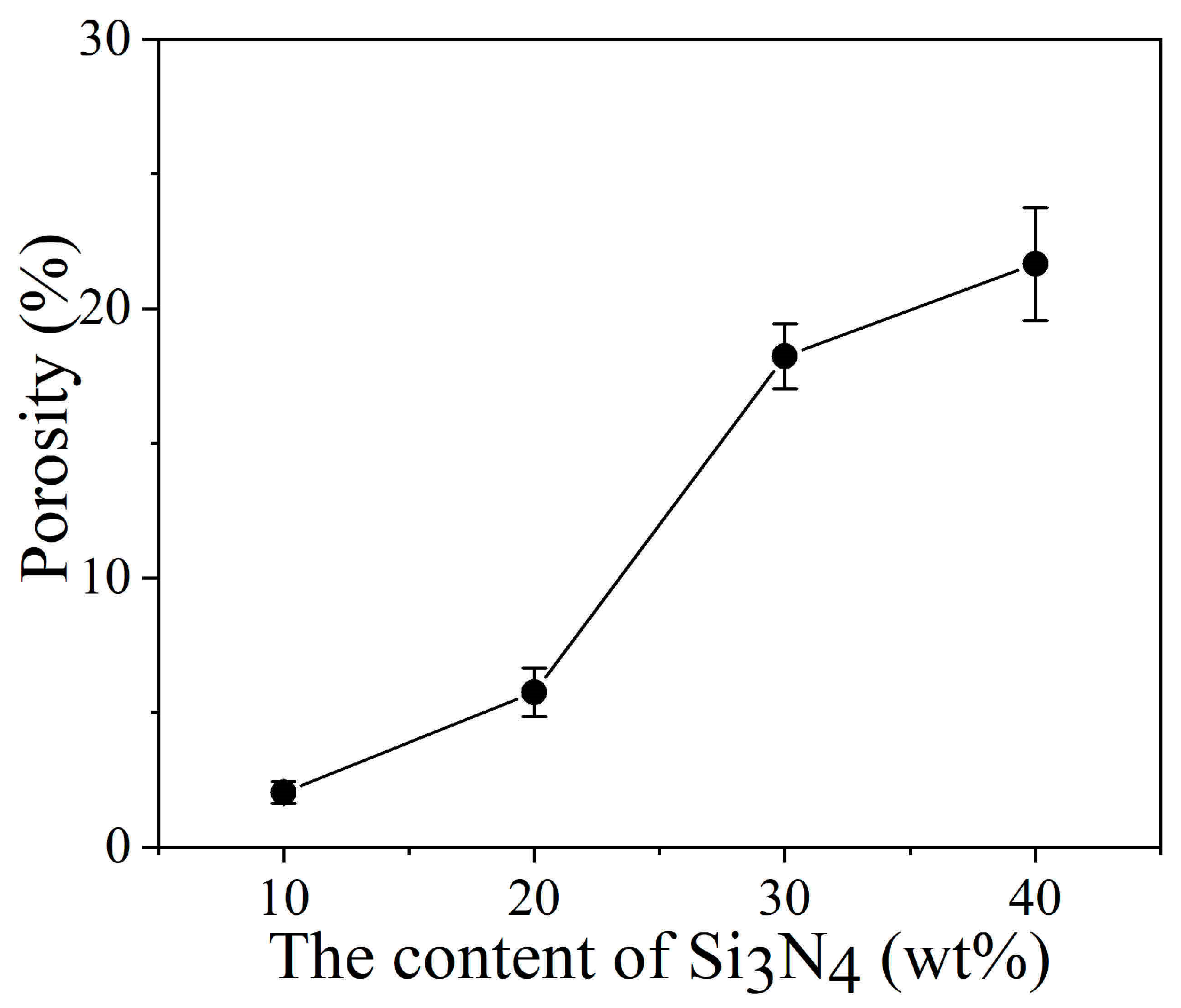
3.1. Effect of Particle Content on the Structure, Morphology, Porosity and Microhardness
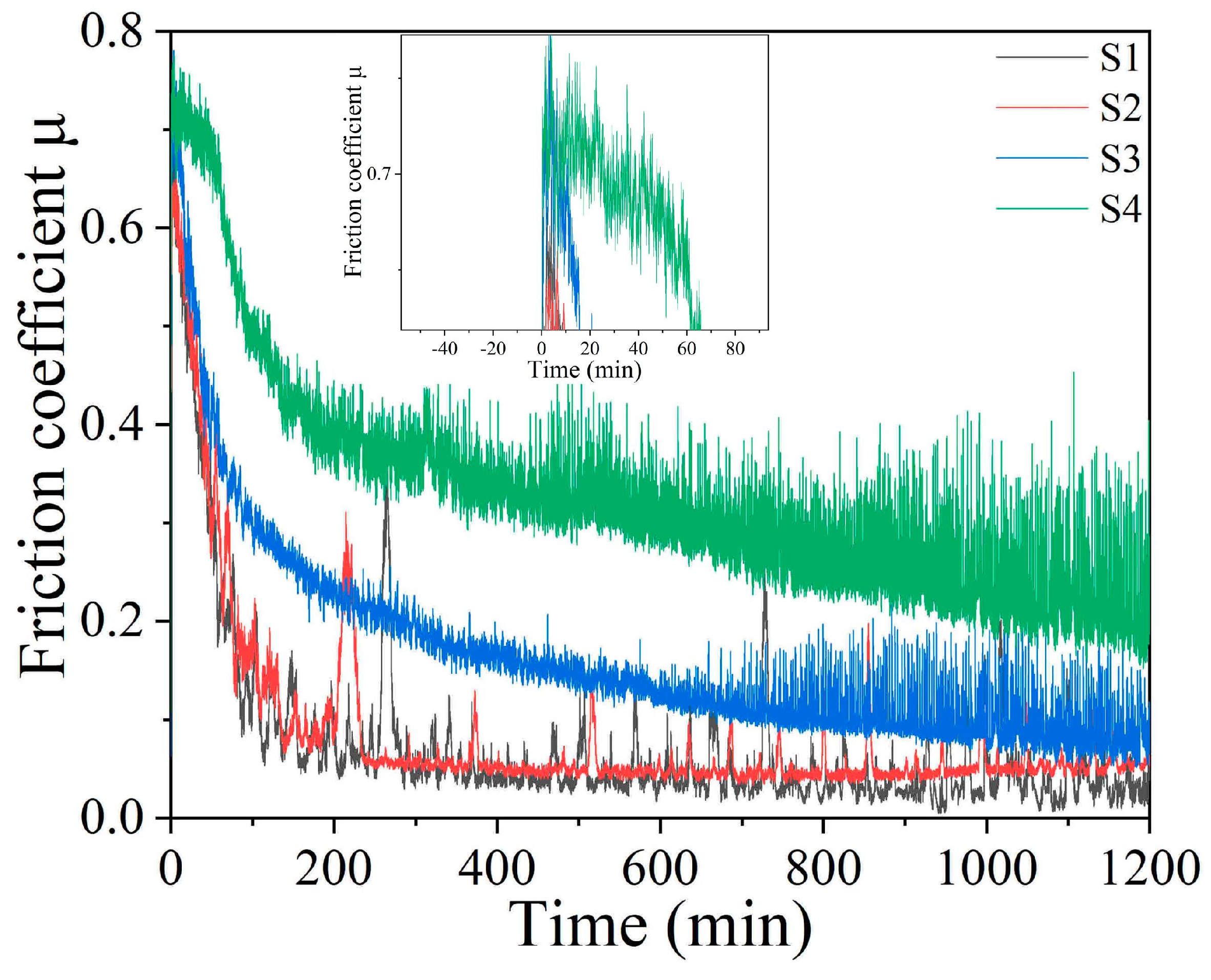
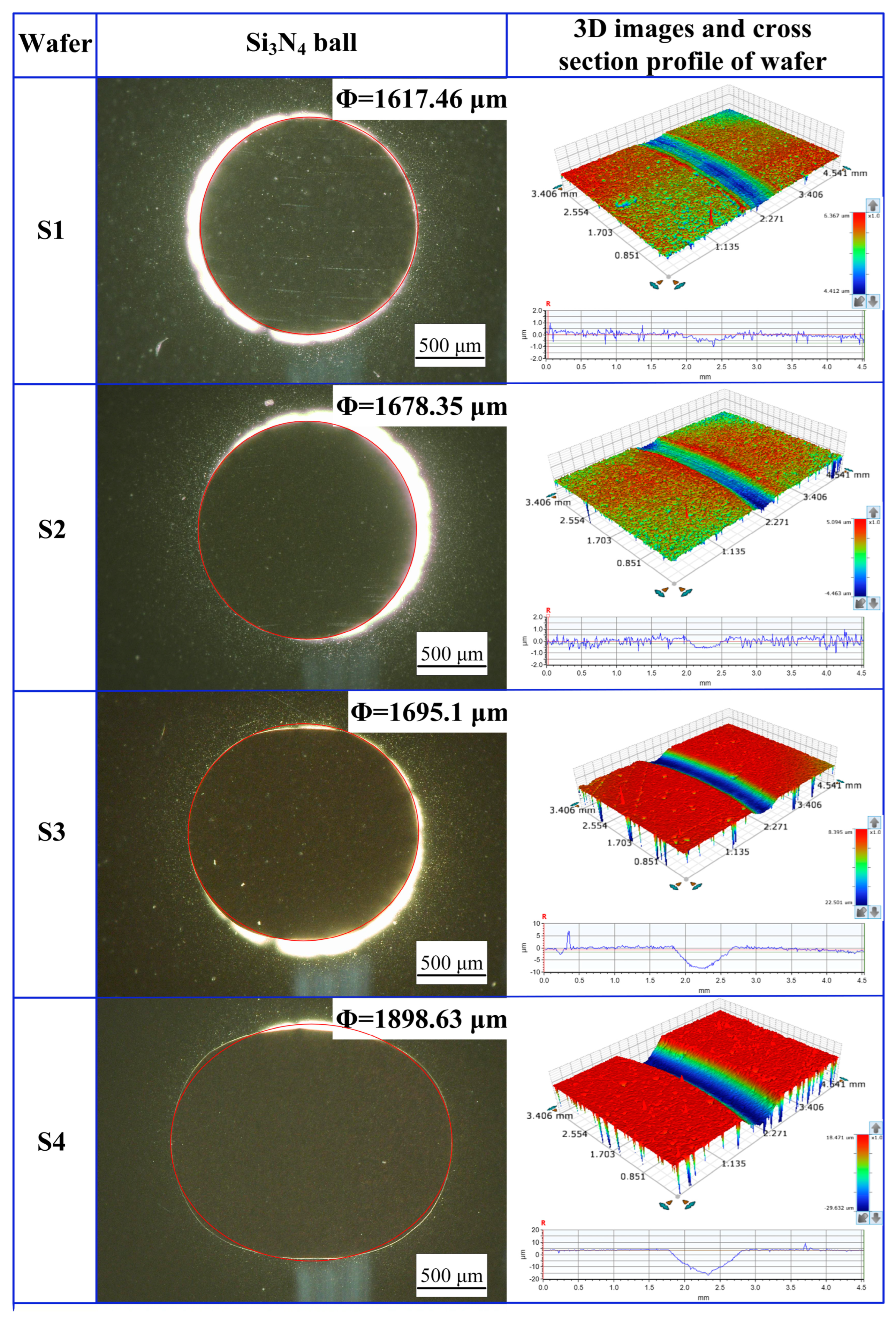
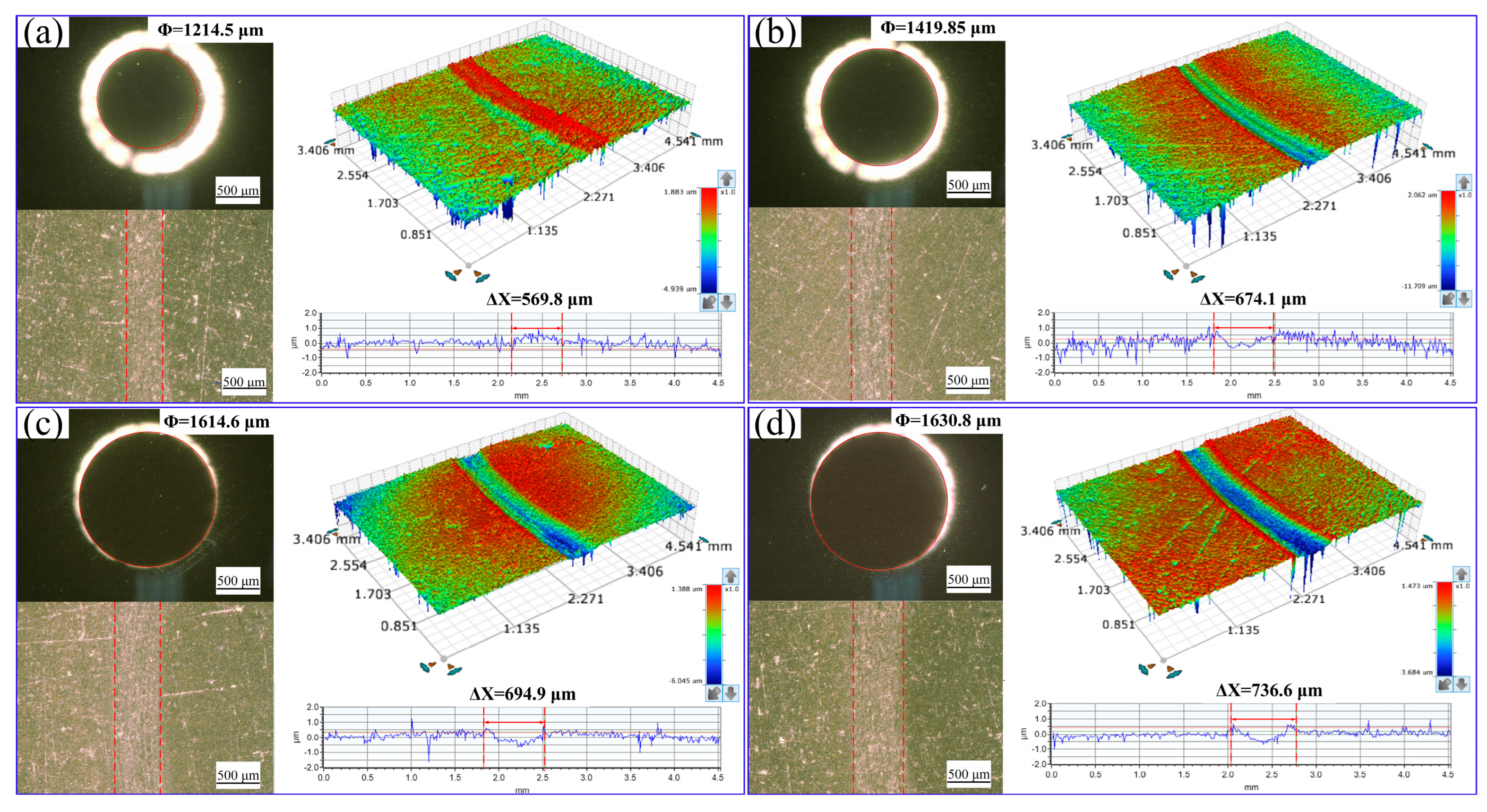
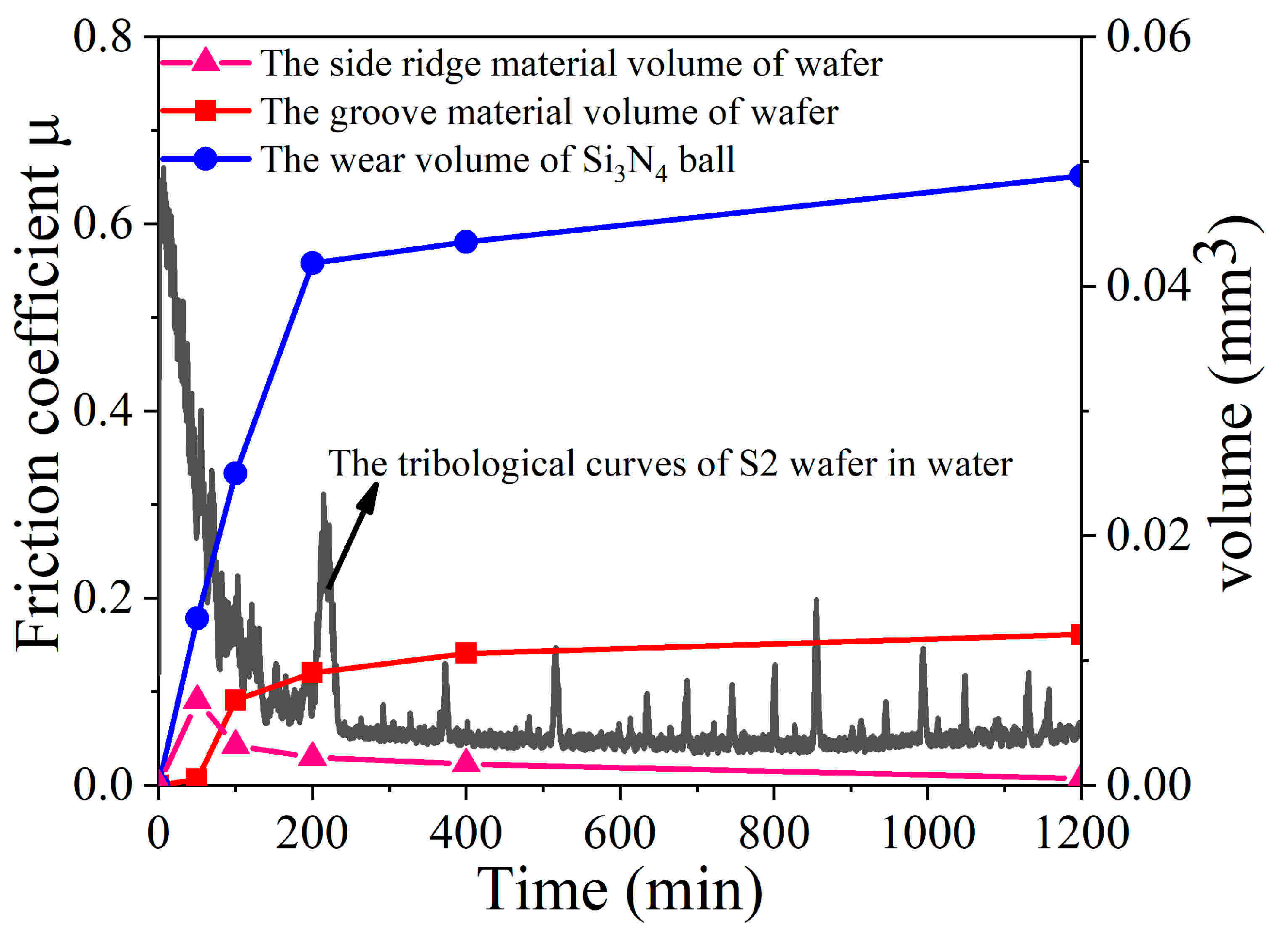
3.2. Water Lubrication Performance
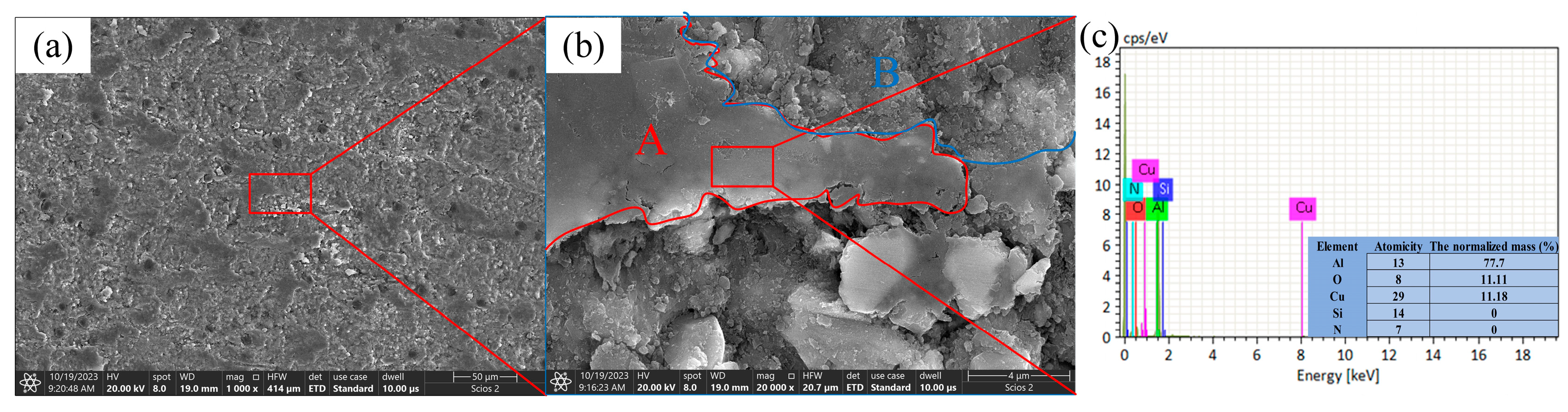
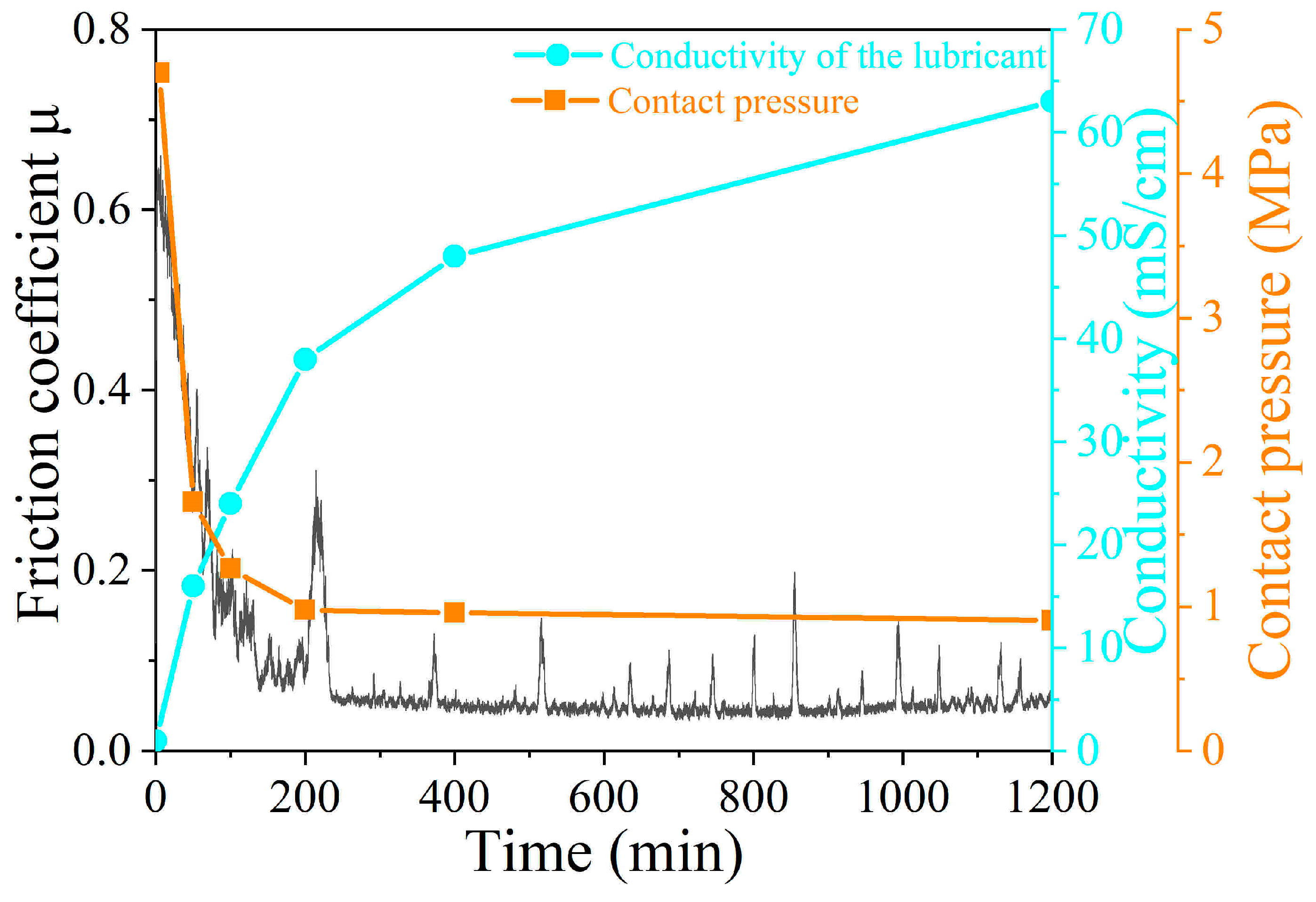
3.3. Water Lubrication Mechanism for the Low Friction Coefficient
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ramos-Masana, A.; Colominas, C. Evaluation of DC-MS and HiPIMS TiB2 and TaN coatings as diffusion barriers against molten aluminum: An insight into the wetting mechanism. Surf. Coat. Technol. 2019, 375, 171–181. [Google Scholar] [CrossRef]
- Pagounis, E.; Talvitie, M.; Lindroos, V.K. Influence of the metal/ceramic interface on the microstructure and mechanical properties of HIPed iron-based composites. Compos. Sci. Technol. 1996, 56, 1329–1337. [Google Scholar] [CrossRef]
- Ru, J.; He, H.; Wang, X.; Wei, S. Preparation and characterization of Ni-Cu dual coated ZTA particles by ionic liquid-assisted electroless plating as reinforcement of metalbased composites. Surf. Coat. Tech. 2020, 387, 125476. [Google Scholar] [CrossRef]
- Loto, C.A. Electroless nickel plating–A review. Silicon-Neth. 2016, 8, 177–186. [Google Scholar] [CrossRef]
- Ghosh, S. Electroless copper deposition: A critical review. Thin Solid. Film. 2019, 669, 641–658. [Google Scholar] [CrossRef]
- Kumar, K.; Bansal, V.; Sharma, S.; Kimothi, S.; Sharma, A. Microhardness and wear resistance of alkaline electroless Ni-P/Ni-P-ZnO nanocomposite platings. Mater. Today Proc. 2023, 80, 1219–1224. [Google Scholar] [CrossRef]
- Wang, S.; Sun, Y.; Li, G. Study on cobalt coating on ZTA particles by electroless plating and impact-abrasive wear behavior of ZTAp reinforced iron matrix composite. Wear 2022, 510–511, 204489. [Google Scholar] [CrossRef]
- Li, G.; Huang, X.; Guo, J. Fabrication of Ni-coated Al2O3 powders by the heterogeneous precipitation method. Mater. Res. Bull. 2001, 36, 1307–1315. [Google Scholar] [CrossRef]
- Dios, M.; Gonzalez, Z.; Gordo, E.; Ferrari, B. Chemical precipitation of nickel nanoparticles on Ti(C,N) suspensions focused on cermet processing. Int. J. Refract. Met. Hard Mater. 2017, 63, 2–8. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, W. A facile fabrication of Ag/SiC composite coating with high mechanical properties and corrosion resistance by electroless plating. Mater. Today Commun. 2023, 36, 106737. [Google Scholar] [CrossRef]
- Nguyen, D.H.; Kim, K.M.; Nguyen Vo, T.T.; Shim, G.H.; Kim, J.H.; Ahn, H.S. Improvement of thermal-hydraulic performance of plate heat exchanger by electroless nickel, copper and silver plating. Case Stud. Therm. Eng. 2021, 23, 100797. [Google Scholar] [CrossRef]
- Ming, H.; Yunlong, Z.; Lili, T.; Lin, S.; Jing, G.; Peiling, D. Surface modifying of SiC particles and performance analysis of SiCp/Cu composites. Appl. Surf. Sci. 2015, 332, 720–725. [Google Scholar] [CrossRef]
- Wang, H.; Jia, J.; Song, H.; Hu, X.; Sun, H.; Yang, D. The preparation of Cu-coated Al2O3 composite powders by electroless plating. Ceram. Int. 2011, 37, 2181–2184. [Google Scholar] [CrossRef]
- Beigi Khosroshahi, N.; Taherzadeh Mousavian, R.; Azari Khosroshahi, R.; Brabazon, D. Mechanical properties of rolled A356 based composites reinforced by Cu-coated bimodal ceramic particles. Mater. Des. 2015, 83, 678–688. [Google Scholar] [CrossRef]
- León, C.A.; Drew, R.A.L. The influence of nickel coating on the wettability of aluminum on ceramics. Compos. Part. A Appl. Sci. Manuf. 2002, 33, 1429–1432. [Google Scholar] [CrossRef]
- Kretz, F.; Gácsi, Z.; Kovács, J.; Pieczonka, T. The electroless deposition of nickel on SiC particles for aluminum matrix composites. Surf. Coat. Technol. 2004, 180–181, 575–579. [Google Scholar] [CrossRef]
- Lü, P.; Wang, X.; Dong, C.; Peng, C.; Wang, R. Preparation and characterization of different surface modified SiCp reinforced Al-matrix composites. J. Cent. South. Univ. 2020, 27, 2567–2577. [Google Scholar] [CrossRef]
- Kang, J.Y.; Heo, Y.U.; Kim, H.; Suh, D.W.; Son, D.; Lee, D.H.; Lee, T.H. Effect of copper addition on the characteristics of high-carbon and high-chromium steels. Mater. Sci. Eng. A 2014, 614, 36–44. [Google Scholar] [CrossRef]
- Li, T.Z.; Li, L.; Lu, H.; Parent, L.; Tian, H.; Chung, R.J.; Li, D.Y. Effect of trace Ni on the resistance of high-Cr cast iron to slurry erosion. Wear 2019, 426–427, 605–611. [Google Scholar] [CrossRef]
- Peng, Y.; Ma, C.; Dai, Q.; Huang, W.; Wang, X. Preparation and Water Lubrication Behaviors of Al-Cu Alloy-Based Si3N4 Composites. J. Mater. Eng. Perform. 2023, 1–11. [Google Scholar] [CrossRef]
- Ashkenazi, D. How aluminum changed the world: A metallurgical revolution through technological and cultural perspectives. Technol. Forecast. Soc. 2019, 143, 101–113. [Google Scholar] [CrossRef]
- Wang, S.; Xie, G.; Yang, J.; Liu, F.; Liu, X. Analysis of microstructure evolution and deformation mechanism of nano-oxides Al2O3 dispersion strengthened copper alloy during compression at room temperature. J. Alloy Compd. 2023, 949, 169837. [Google Scholar] [CrossRef]
- Chen, J.; Sun, T.; Su, J.; Li, J.; Zhou, P.; Peng, Y.; Zhu, Y. A novel agglomerated diamond abrasive with excellent micro-cutting and self-sharpening capabilities in fixed abrasive lapping processes. Wear 2021, 464–465, 203531. [Google Scholar] [CrossRef]
- Chen, J.; Zhu, N.; Niu, F.; Peng, Y.; Su, J.; Zhu, Y. Influence of agglomerated diamond abrasive wear on sapphire material removal behavior. Diam. Relat. Mater. 2020, 108, 107965. [Google Scholar] [CrossRef]
- Tomizawa, H.; Fischer, T.E. Friction and wear of silicon nitride and silicon Carbide in water: Hydrodynamic lubrication at low sliding speed obtained by tribochemical wear. ASLE Trans. 1987, 30, 41–46. [Google Scholar] [CrossRef]
- Xu, J.; Kato, K. Formation of tribochemical layer of ceramics sliding in water and its role for low friction. Wear 2000, 245, 61–75. [Google Scholar] [CrossRef]




| Sensitization | Activation | |||
|---|---|---|---|---|
| Reagent | SnCl2·2H2O | HCl | PdCl2 | HCl |
| Concentration | 16 g/L | 50 mL/L | 0.25 g/L | 10 mL/L |
| Condition | Constantly stirred for 30 min, cleaned 3 times in deionized water | Constantly stirred for 20 min | ||
| Reagent | Concentration | Condition |
|---|---|---|
| NiSO4·6H2O | 25 g/L | pH = 5.5 Temperature: 85 °C Time: 2 h |
| NaH2PO2·H2O | 18 g/L | |
| CH3COOH | 20 mL/L | |
| CH3COONa | 15 g/L | |
| Thiourea | 0.25 mg/L | |
| NH3·H2O | / |
| Wafer Sample | S1 | S2 | S3 | S4 |
|---|---|---|---|---|
| Ni-coated Si3N4 mass percent (wt%) | 10 | 20 | 30 | 40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, Y.; Dai, Q.; Huang, W.; Wang, X. Water Lubrication of Al-Cu Composites Reinforced by Nickel-Coated Si3N4 Particles. Coatings 2024, 14, 225. https://doi.org/10.3390/coatings14020225
Peng Y, Dai Q, Huang W, Wang X. Water Lubrication of Al-Cu Composites Reinforced by Nickel-Coated Si3N4 Particles. Coatings. 2024; 14(2):225. https://doi.org/10.3390/coatings14020225
Chicago/Turabian StylePeng, Yanan, Qinqwen Dai, Wei Huang, and Xiaolei Wang. 2024. "Water Lubrication of Al-Cu Composites Reinforced by Nickel-Coated Si3N4 Particles" Coatings 14, no. 2: 225. https://doi.org/10.3390/coatings14020225






