Crosslinked Polymer Coatings of Poly (Acrylic Acid-co-acrylamide)/Polyethyleneimine (P(AA-co-AAm)/PEI) on Titanium Alloy with Excellent Lubrication Performance for Artificial Joints
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Reagents
2.2. Preparation of Hydrogel-Coated Ti6Al4V
2.3. Structural Characterization
2.4. Equilibrium Water Content Measurement
2.5. Porosity Measurement
2.6. Water Contact Angle Measurements
2.7. Thermal Characterization
2.8. Measurement of Tribological Properties
3. Results and Discussion
3.1. Preparation of P(AA-co-AAm)/PEI Coating on Ti6Al4V
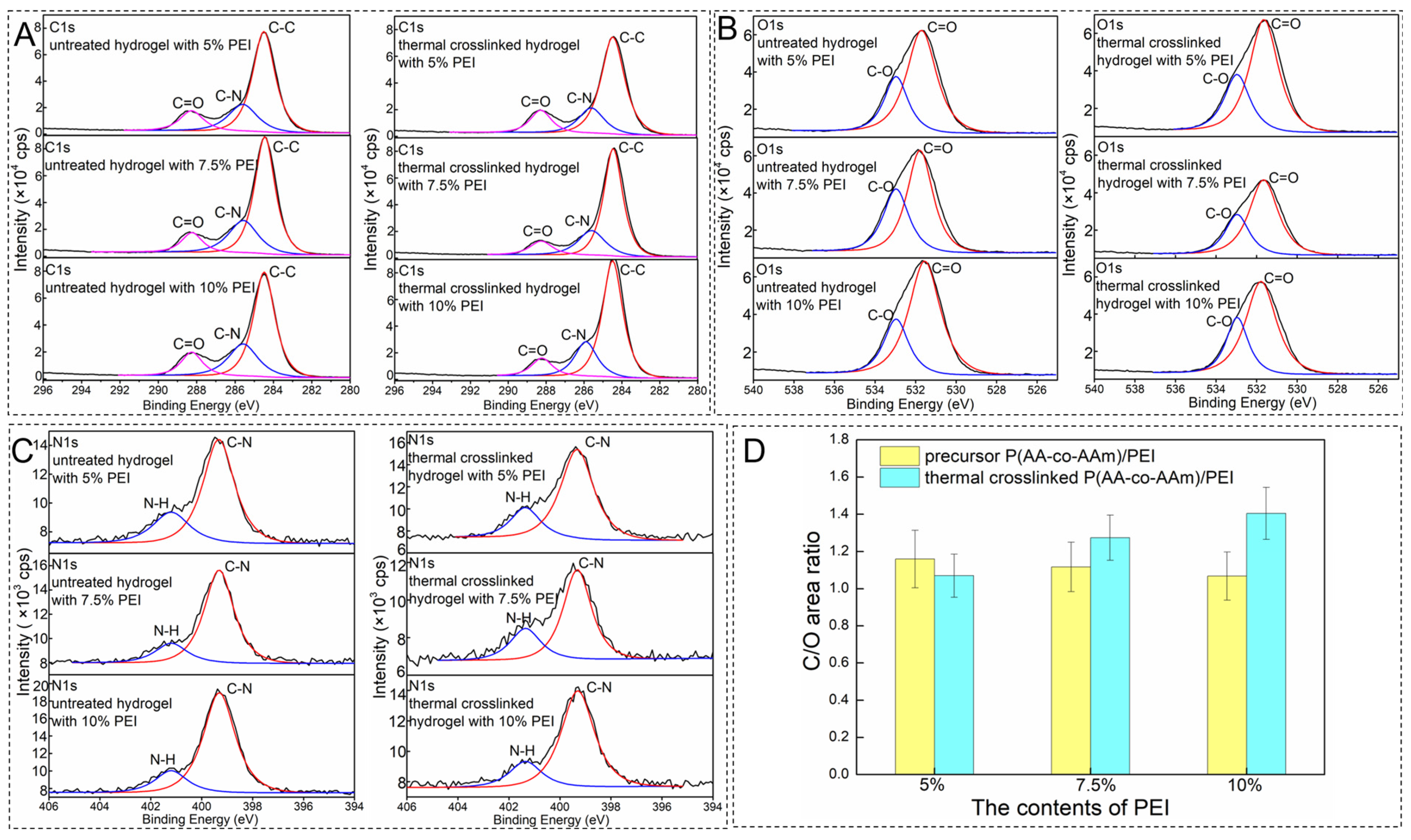
3.2. Chemical Composition and Surface Morphology
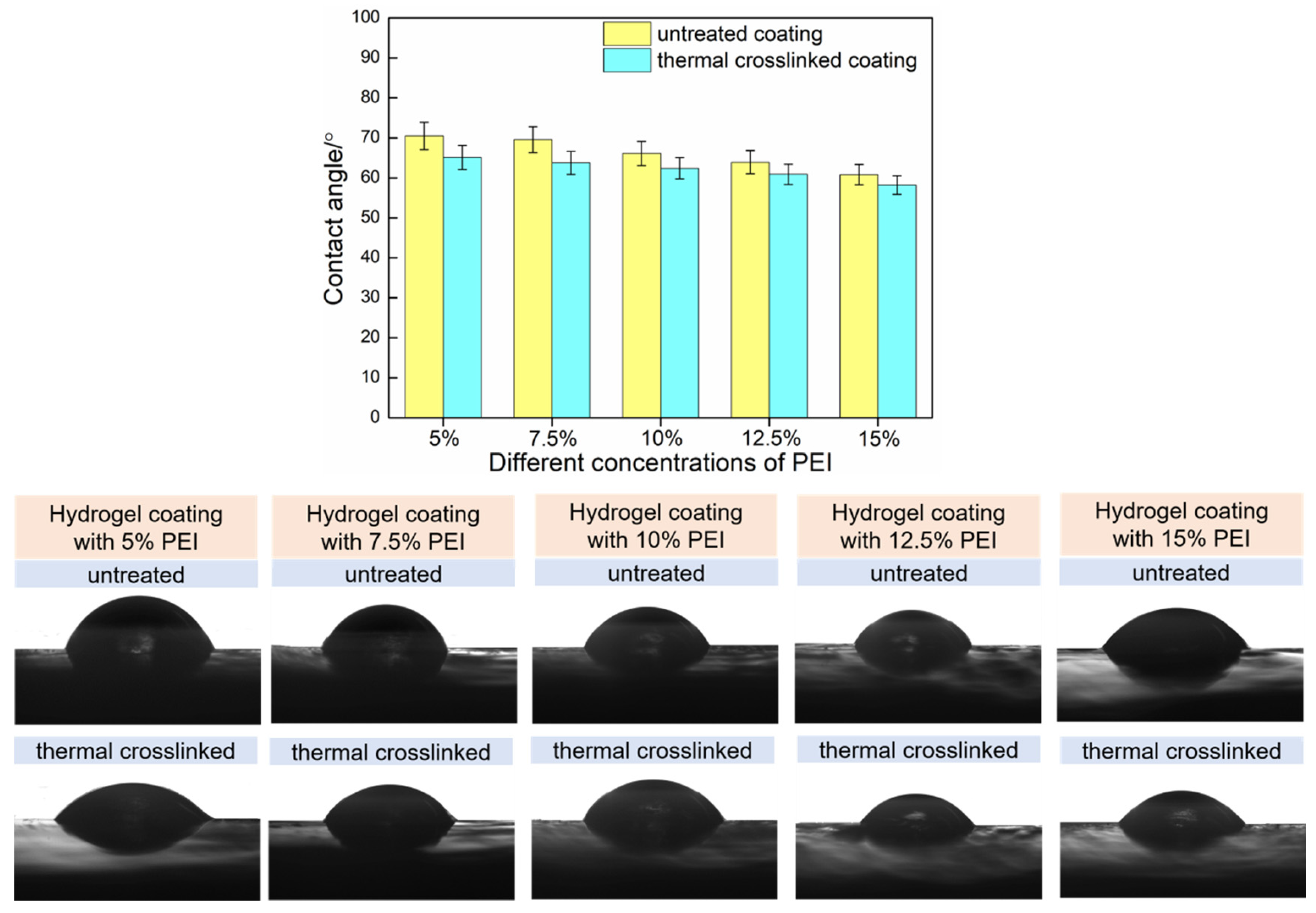
3.3. Surface Wettability
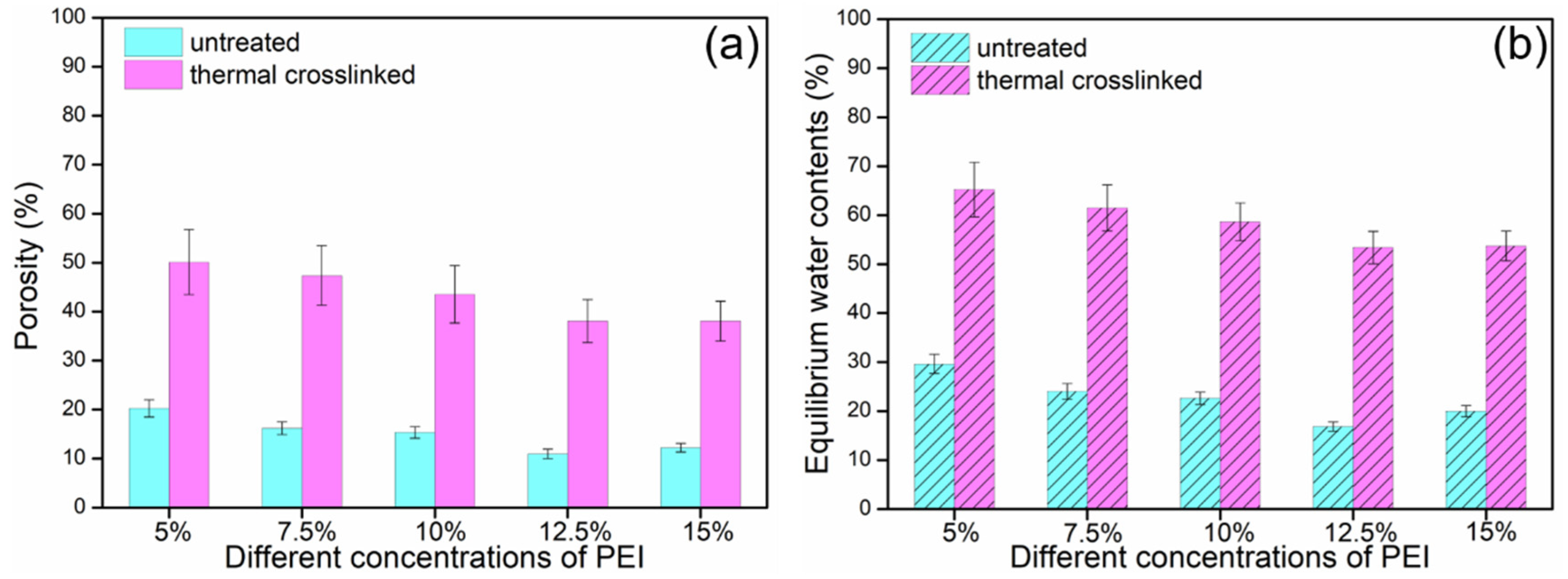
3.4. Porosity and Equilibrium Water Contents
3.5. Thermal Properties
3.6. Evaluation of the Lubrication Properties
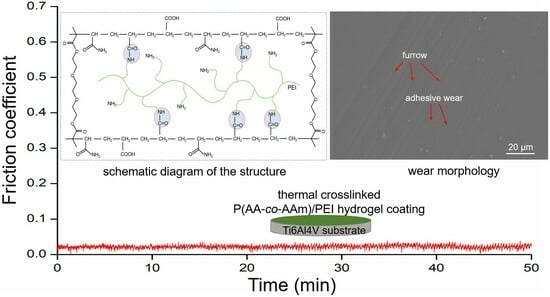
3.7. Assessment of the Wear Resistance
3.8. The Lubrication Mechanism of the Composite Hydrogel Coating
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cilingir, A.C. Effect of Rotational and Sliding Motions on Friction and Degeneration of Articular Cartilage under Dry and Wet Friction. J. Bionic Eng. 2015, 12, 464–472. [Google Scholar] [CrossRef]
- Jin, Z.; Dowson, D. Bio-friction. Friction 2013, 1, 100–113. [Google Scholar] [CrossRef]
- Forster, H.; Fisher, J. The Influence of Loading Time and Lubricant on the Friction of Articular Cartilage. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 1996, 210, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Macirowski, T.; Tepic, S.; Mann, R.W. Cartilage Stresses in the Human Hip Joint. J. Biomech. Eng. 1994, 116, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Briggs, A.M.; Cross, M.J.; Hoy, D.G.; Sànchez-Riera, L.; Blyth, F.M.; Woolf, A.D.; March, L. Musculoskeletal Health Conditions Represent a Global Threat to Healthy Aging: A Report for the 2015 World Health Organization World Report on Ageing and Health. Gerontol. 2016, 56, S243–S255. [Google Scholar] [CrossRef]
- Bloyce, A.; Qi, P.Y.; Dong, H.; Bell, T. Surface modification of titanium alloys for combined improvements in corrosion and wear resistance. Surf. Coat. Technol. 1998, 107, 125–132. [Google Scholar] [CrossRef]
- Pratap, T.; Patra, K. Mechanical micro-texturing of Ti-6Al-4V surfaces for improved wettability and bio-tribological performances. Surf. Coat. Technol. 2018, 349, 71–81. [Google Scholar] [CrossRef]
- Sawano, H.; Warisawa, S.i.; Ishihara, S. Study on long life of artificial joints by investigating optimal sliding surface geometry for improvement in wear resistance. Precis. Eng. 2009, 33, 492–498. [Google Scholar] [CrossRef]
- Luo, Y.; Chen, W.; Tian, M.; Teng, S. Thermal oxidation of Ti6Al4V alloy and its biotribological properties under serum lubrication. Tribol. Int. 2015, 89, 67–71. [Google Scholar] [CrossRef]
- Chatterji, U.; Ashworth, M.J.; Lewis, P.L.; Dobson, P.J. Effect of total hip arthroplasty on recreational and sporting activity. ANZ J. Surg. 2004, 74, 446–449. [Google Scholar] [CrossRef]
- Flugsrud, G.B.; Nordsletten, L.; Espehaug, B.; Havelin, L.I.; Meyer, H.E. The effect of middle-age body weight and physical activity on the risk of early revision hip arthroplasty: A cohort study of 1535 individuals. Acta Orthop. 2007, 78, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Bao, Y.; Liu, Z.; Zheng, Q.; Dong, Y.; Song, R.; Yang, B. Temperature-sensitive tribological performance of titanium alloy lubricated with PNIPAM microgels. Appl. Surf. Sci. 2022, 572, 151392. [Google Scholar] [CrossRef]
- Mashtalyar, D.V.; Pleshkova, A.I.; Piatkova, M.A.; Nadaraia, K.V.; Imshinetskiy, I.M.; Belov, E.A.; Suchkov, S.N.; Sinebryukhov, S.L.; Gnedenkov, S.V. PTFE-Containing Coating Obtained on Ti by Spraying and PEO Pretreatment. Coatings 2023, 13, 1249. [Google Scholar] [CrossRef]
- Träger, D.; Słota, D.; Niziołek, K.; Florkiewicz, W.; Sobczak-Kupiec, A. Hybrid Polymer–Inorganic Coatings Enriched with Carbon Nanotubes on Ti-6Al-4V Alloy for Biomedical Applications. Coatings 2023, 13, 1813. [Google Scholar]
- Zhao, Y.; Chen, X.; Li, S.; Zeng, R.; Zhang, F.; Wang, Z.; Guan, S. Corrosion resistance and drug release profile of gentamicin-loaded polyelectrolyte multilayers on magnesium alloys: Effects of heat treatment. J. Colloid Interface Sci. 2019, 547, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, Y.; Li, S.; Lin, Y.-C.; Li, V.L.; Chen, Y.-H.; Lin, C.; Keerthi, M.; Shih, S.-J.; Chung, R.-J. Polyelectrolyte multilayer coatings for short/long-term release of antibacterial agents. Surf. Coat. Technol. 2020, 393, 125696. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, Z.; Li, T.; Liu, Z.; Sun, X.; Wang, W.; Chen, L.; He, C. Enhanced tissue infiltration and bone regeneration through spatiotemporal delivery of bioactive factors from polyelectrolytes modified biomimetic scaffold. Mater. Today Bio 2023, 20, 100681. [Google Scholar] [CrossRef]
- Deng, Y.; Xu, Y.; Ni, X.; LiYu, W.; Wang, Y.; Yang, Z. Mechanical and Tribological Behavior of Branched Polyethyleneimine/Polyacrylic Acid Coatings on Ti6Al4V Substrate. J. Mater. Eng. Perform. 2023, 32, 6123–6132. [Google Scholar] [CrossRef]
- Deng, Y.; Sun, J.; Ni, X.; Yu, B. Tribological properties of hierarchical structure artificial joints with poly acrylic acid (AA)—Poly acrylamide (AAm) hydrogel and Ti6Al4V substrate. J. Polym. Res. 2020, 27, 157. [Google Scholar] [CrossRef]
- Deng, Y.; Xiong, D. Fabrication and properties of UHMWPE grafted with acrylamide polymer brushes. J. Polym. Res. 2015, 22, 195. [Google Scholar] [CrossRef]
- Xiong, D.; Deng, Y.; Wang, N.; Yang, Y. Influence of surface PMPC brushes on tribological and biocompatibility properties of UHMWPE. Appl. Surf. Sci. 2014, 298, 56–61. [Google Scholar] [CrossRef]
- Hafezi, M.; Qin, L.; Mahmoodi, P.; Yang, H.; Dong, G. Releasable agarose-hyaluronan hydrogel with anti-friction performance and enhanced stability for artificial joint applications. Tribol. Int. 2021, 153, 106622. [Google Scholar] [CrossRef]
- Feng, S.; Liu, Y.; Li, J.; Wen, S. Superlubricity Achieved with Zwitterionic Brushes in Diverse Conditions Induced by Shear Actions. Macromolecules 2021, 54, 5719–5727. [Google Scholar] [CrossRef]
- Vilela, C.A.; Correia, C.; da Silva Morais, A.; Santos, T.C.; Gertrudes, A.C.; Moreira, E.S.; Frias, A.M.; Learmonth, D.A.; Oliveira, P.; Oliveira, J.M.; et al. In vitro and in vivo performance of methacrylated gellan gum hydrogel formulations for cartilage repair*. J. Biomed. Mater. Res. Part A 2018, 106, 1987–1996. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.; Xia, H.; Jia, L.; Zhao, J.; Zhao, D.; Yan, X.; Zhang, Y.; Tang, S.; Zhou, G.; Zhu, L.; et al. Ultrafast, tough, and adhesive hydrogel based on hybrid photocrosslinking for articular cartilage repair in water-filled arthroscopy. Sci. Adv. 2021, 7, eabg0628. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zhao, J.; Koshut, W.J.; Watt, J.; Riboh, J.C.; Gall, K.; Wiley, B.J. A Synthetic Hydrogel Composite with the Mechanical Behavior and Durability of Cartilage. Adv. Funct. Mater. 2020, 30, 2003451. [Google Scholar] [CrossRef]
- Wei, Q.; Liu, H.; Zhao, X.; Zhao, W.; Xu, R.; Ma, S.; Zhou, F. Bio-inspired hydrogel-polymer brush bi-layered coating dramatically boosting the lubrication and wear-resistance. Tribol. Int. 2023, 177, 108000. [Google Scholar] [CrossRef]
- Gong, J.P.; Katsuyama, Y.; Kurokawa, T.; Osada, Y. Double-Network Hydrogels with Extremely High Mechanical Strength. Adv. Mater. 2003, 15, 1155–1158. [Google Scholar] [CrossRef]
- Pathak, A.K.; Singh, V.K. A wide range and highly sensitive optical fiber pH sensor using polyacrylamide hydrogel. Opt. Fiber Technol. 2017, 39, 43–48. [Google Scholar] [CrossRef]
- Jing, Z.; Xu, A.; Liang, Y.-Q.; Zhang, Z.; Yu, C.; Hong, P.; Li, Y. Biodegradable Poly(acrylic acid-co-acrylamide)/Poly(vinyl alcohol) Double Network Hydrogels with Tunable Mechanics and High Self-healing Performance. Polymers 2019, 11, 952. [Google Scholar] [CrossRef]
- Muniz, E.C.; Geuskens, G. Compressive Elastic Modulus of Polyacrylamide Hydrogels and Semi-IPNs with Poly(N-isopropylacrylamide). Macromolecules 2001, 34, 4480–4484. [Google Scholar] [CrossRef]
- Means, A.K.; Shrode, C.S.; Whitney, L.V.; Ehrhardt, D.A.; Grunlan, M.A. Double Network Hydrogels that Mimic the Modulus, Strength, and Lubricity of Cartilage. Biomacromolecules 2019, 20, 2034–2042. [Google Scholar] [CrossRef] [PubMed]
- Shahzamani, M.; Taheri, S.; Roghanizad, A.; Naseri, N.; Dinari, M. Preparation and characterization of hydrogel nanocomposite based on nanocellulose and acrylic acid in the presence of urea. Int. J. Biol. Macromol. 2020, 147, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Cozens, E.J.; Roohpour, N.; Gautrot, J.E. Comparative adhesion of chemically and physically crosslinked poly(acrylic acid)-based hydrogels to soft tissues. Eur. Polym. J. 2021, 146, 110250. [Google Scholar] [CrossRef]
- Erceg, T.; Cakić, S.; Cvetinov, M.; Dapčević-Hadnađev, T.; Budinski-Simendić, J.; Ristić, I. The properties of conventionally and microwave synthesized poly(acrylamide-co-acrylic acid) hydrogels. Polym. Bull. 2020, 77, 2089–2110. [Google Scholar] [CrossRef]
- Kim, D.; Park, K. Swelling and mechanical properties of superporous hydrogels of poly(acrylamide-co-acrylic acid)/polyethylenimine interpenetrating polymer networks. Polymer 2004, 45, 189–196. [Google Scholar] [CrossRef]
- Caruso, F.; Lichtenfeld, H.; Donath, E.; Möhwald, H. Investigation of Electrostatic Interactions in Polyelectrolyte Multilayer Films: Binding of Anionic Fluorescent Probes to Layers Assembled onto Colloids. Macromolecules 1999, 32, 2317–2328. [Google Scholar] [CrossRef]
- Zhu, X.; Jańczewski, D.; Lee, S.S.C.; Teo, S.L.-M.; Vancso, G.J. Cross-Linked Polyelectrolyte Multilayers for Marine Antifouling Applications. ACS Appl. Mater. Interfaces 2013, 5, 5961–5968. [Google Scholar] [CrossRef]
- Zhang, S.-w.; Feng, D.; Wang, D. Tribological behaviors of composite molecular deposition films. Tribol. Int. 2005, 38, 959–965. [Google Scholar] [CrossRef]
- Král, M.; Dendisová, M.; Matějka, P.; Svoboda, J.; Pop-Georgievski, O. Infrared imaging of surface confluent polydopamine (PDA) films at the nanoscale. Colloids Surf. B Biointerfaces 2023, 221, 112954. [Google Scholar] [CrossRef]
- Mondal, A.; Devine, R.; Estes, L.; Manuel, J.; Singha, P.; Mancha, J.; Palmer, M.; Handa, H. Highly hydrophobic polytetrafluoroethylene particle immobilization via polydopamine anchor layer on nitric oxide releasing polymer for biomedical applications. J. Colloid Interface Sci. 2021, 585, 716–728. [Google Scholar] [CrossRef] [PubMed]
- Tran, V.T.; Mredha, M.T.I.; Jeon, I. High-water-content hydrogels exhibiting superior stiffness, strength, and toughness. Extrem. Mech. Lett. 2020, 37, 100691. [Google Scholar] [CrossRef]
- Santos, L.; Ferraz, M.P.; Shirosaki, Y.; Lopes, M.A.; Fernandes, M.H.; Osaka, A.; Santos, J.D. Degradation Studies and Biological Behavior on an Artificial Cornea Material. Investig. Ophthalmol. Vis. Sci. 2011, 52, 4274–4281. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Zhang, Y.; Cui, Z.; Du, Y.; Shi, Z. New strategy for design and fabrication of polymer hydrogel with tunable porosity as artificial corneal skirt. Mater. Sci. Eng. C 2017, 70, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Xu, N.; Tian, K.; Qing, T.; Hao, Y.; Liang, P.; Li, M. Nitrilotriacetic acid modified magnetic Prussian blue for efficient removal of cadmium from wastewater. Appl. Surf. Sci. 2022, 600, 154102. [Google Scholar] [CrossRef]
- Ji, J.; Ke, Y.; Pei, Y.; Zhang, G. Effects of highly exfoliated montmorillonite layers on the properties of clay reinforced terpolymer nanocomposite plugging microspheres. J. Appl. Polym. Sci. 2017, 134, 44894. [Google Scholar] [CrossRef]
- Li, K.; Zhang, C.; Du, Z.; Li, H.; Zou, W. Preparation of humidity-responsive antistatic carbon nanotube/PEI nanocomposites. Synth. Met. 2012, 162, 2010–2015. [Google Scholar] [CrossRef]
- Liu, Y.; Fu, R.; Sun, Y.; Zhou, X.; Baig, S.A.; Xu, X. Multifunctional nanocomposites Fe3O4@SiO2-EDTA for Pb(II) and Cu(II) removal from aqueous solutions. Appl. Surf. Sci. 2016, 369, 267–276. [Google Scholar] [CrossRef]
- Sehaqui, H.; de Larraya, U.P.; Liu, P.; Pfenninger, N.; Mathew, A.P.; Zimmermann, T.; Tingaut, P. Enhancing adsorption of heavy metal ions onto biobased nanofibers from waste pulp residues for application in wastewater treatment. Cellulose 2014, 21, 2831–2844. [Google Scholar] [CrossRef]
- Lee, H.; Sun, J.-Y. Strong and Facile Adhesives Based on Phase Transitional Poly(acrylic acid)/Poly(ethylenimine) Complexes. ACS Appl. Eng. Mater. 2023, 1, 229–240. [Google Scholar] [CrossRef]
- Tang, S.; Lin, L.; Wang, X.; Sun, X.; Yu, A. Adsorption of fulvic acid onto polyamide 6 microplastics: Influencing factors, kinetics modeling, site energy distribution and interaction mechanisms. Chemosphere 2021, 272, 129638. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Jiang, Z.; Fu, X.; Ni, F.; Shen, F.; Zhang, S.; Cheng, Z.; Lei, Y.; Zhang, Y.; He, Y. Unveiling interactions of norfloxacin with microplastic in surface water by 2D FTIR correlation spectroscopy and X-ray photoelectron spectroscopy analyses. Ecotoxicol. Environ. Saf. 2023, 251, 114521. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Zhang, L.; Luo, X.; Su, T.; Zhang, Y.; Qin, Z.; Ji, H. PEI modified magnetic porous cassava residue microspheres for adsorbing Cd(II) from aqueous solution. Eur. Polym. J. 2021, 159, 110741. [Google Scholar] [CrossRef]
- Yu, D.; Wang, Y.; Wu, M.; Zhang, L.; Wang, L.; Ni, H. Surface functionalization of cellulose with hyperbranched polyamide for efficient adsorption of organic dyes and heavy metals. J. Clean. Prod. 2019, 232, 774–783. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, G.; Weng, H.; Wang, L.; Chen, J.; Cheng, S.; Zhang, P.; Wang, M.; Ge, X.; Chen, H.; et al. Carbon-doped boron nitride nanosheets with adjustable band structure for efficient photocatalytic U(VI) reduction under visible light. Chem. Eng. J. 2021, 410, 128280. [Google Scholar] [CrossRef]
- Zhang, T.; Xu, Y.; Li, H.; Zhang, B. Interfacial adhesion between carbon fibers and nylon 6: Effect of fiber surface chemistry and grafting of nano-SiO2. Compos. Part A Appl. Sci. Manuf. 2019, 121, 157–168. [Google Scholar] [CrossRef]
- Li, L.; Huang, S.; Wen, T.; Ma, R.; Yin, L.; Li, J.; Chen, Z.; Hayat, T.; Hu, B.; Wang, X. Fabrication of carboxyl and amino functionalized carbonaceous microspheres and their enhanced adsorption behaviors of U(VI). J. Colloid Interface Sci. 2019, 543, 225–236. [Google Scholar] [CrossRef]
- Yang, B.; Niu, K.; Haag, F.; Cao, N.; Zhang, J.; Zhang, H.; Li, Q.; Allegretti, F.; Björk, J.; Barth, J.V.; et al. Abiotic Formation of an Amide Bond via Surface-Supported Direct Carboxyl–Amine Coupling. Angew. Chem. Int. Ed. 2022, 61, e202113590. [Google Scholar] [CrossRef]
- Wang, Y.; Gu, Z.; Yang, J.; Liao, J.; Yang, Y.; Liu, N.; Tang, J. Amidoxime-grafted multiwalled carbon nanotubes by plasma techniques for efficient removal of uranium(VI). Appl. Surf. Sci. 2014, 320, 10–20. [Google Scholar] [CrossRef]
- Ma, L.; Wu, G.; Zhao, M.; Li, X.; Han, P.; Song, G. Modification of carbon fibers surfaces with polyetheramines: The role of interphase microstructure on adhesion properties of CF/epoxy composites. Polym. Compos. 2018, 39, E2346–E2355. [Google Scholar] [CrossRef]
- Pan, M.; Cruz, G.M.; Grazon, C.; Kechkeche, D.; Renault, L.H.; Clavier, G.; Méallet-Renault, R. Luminescence-Sensitive Surfaces Bearing Ratiometric Nanoparticles for Bacteria Growth Detection. ACS Appl. Polym. Mater. 2022, 4, 5482–5492. [Google Scholar] [CrossRef]
- Zhai, S.; Li, M.; Wang, D.; Ju, X.; Fu, S. Cyano and acylamino group modification for tannery sludge bio-char: Enhancement of adsorption universality for dye pollutants. J. Environ. Chem. Eng. 2021, 9, 104939. [Google Scholar] [CrossRef]
- Wang, Z.; Dong, Y.; Yang, J.-C.; Wang, X.-J.; Zhang, M.-l.; Zhang, G.; Long, S.-R.; Liu, S.; Yang, J. Improved interfacial shear strength in carbon fiber enhanced semi-aromatic polyamide 6T composite via in-situ polymerization on fiber surface. Compos. Sci. Technol. 2022, 223, 109401. [Google Scholar] [CrossRef]
- Wu, Y.; Han, C.; Yang, J.; Jia, S.; Wang, S. Polypropylene films modified by air plasma and feather keratin graft. Surf. Coat. Technol. 2011, 206, 506–510. [Google Scholar] [CrossRef]
- Sun, D.; Wang, J.; Wang, H.; Yan, Z.; Ma, W.; Ma, X.; Wang, Y.; Ma, Z.; Jin, X.; Sun, S.; et al. Influence of crosslink density on the interfacial bonding performance for epoxy resin towards load-induced calcium silicate hydrate gel. J. Non-Cryst. Solids 2023, 619, 122553. [Google Scholar] [CrossRef]
- Souza Almeida, F.; Guedes Silva, K.C.; Matias Navarrete de Toledo, A.; Kawazoe Sato, A.C. Modulating porosity and mechanical properties of pectin hydrogels by starch addition. J. Food Sci. Technol. 2021, 58, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Mohammadian, M.; Madadlou, A. Cold-set hydrogels made of whey protein nanofibrils with different divalent cations. Int. J. Biol. Macromol. 2016, 89, 499–506. [Google Scholar] [CrossRef]
- Almeida, A.P.C.; Saraiva, J.N.; Cavaco, G.; Portela, R.P.; Leal, C.R.; Sobral, R.G.; Almeida, P.L. Crosslinked bacterial cellulose hydrogels for biomedical applications. Eur. Polym. J. 2022, 177, 111438. [Google Scholar] [CrossRef]
- Deng, Y.; Xiong, D.; Wang, K. The mechanical properties of the ultra high molecular weight polyethylene grafted with 3-dimethy (3-(N-methacryamido) propyl) ammonium propane sulfonate. J. Mech. Behav. Biomed. Mater. 2014, 35, 18–26. [Google Scholar] [CrossRef]
- Huang, X.; Chrisman, J.D.; Zacharia, N.S. Omniphobic Slippery Coatings Based on Lubricant-Infused Porous Polyelectrolyte Multilayers. ACS Macro Lett. 2013, 2, 826–829. [Google Scholar] [CrossRef]
- Gu, Y.; Huang, X.; Wiener, C.G.; Vogt, B.D.; Zacharia, N.S. Large-Scale Solvent Driven Actuation of Polyelectrolyte Multilayers Based on Modulation of Dynamic Secondary Interactions. ACS Appl. Mater. Interfaces 2015, 7, 1848–1858. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Pettitt, M.E.; Wode, F.; Arpa Sancet, M.P.; Fu, J.; Ji, J.; Callow, M.E.; Callow, J.A.; Rosenhahn, A.; Grunze, M. Interaction of Zoospores of the Green Alga Ulva with Bioinspired Micro- and Nanostructured Surfaces Prepared by Polyelectrolyte Layer-by-Layer Self-Assembly. Adv. Funct. Mater. 2010, 20, 1984–1993. [Google Scholar] [CrossRef]
- Klein, J. Hydration lubrication. Friction 2013, 1, 1–23. [Google Scholar] [CrossRef]
- Sappidi, P.; Natarajan, U. Effect of salt valency and concentration on structure and thermodynamic behavior of anionic polyelectrolyte Na+-polyethacrylate aqueous solution. J. Mol. Model. 2016, 22, 274. [Google Scholar] [CrossRef] [PubMed]
- Schweins, R.; Hollmann, J.; Huber, K. Dilute solution behaviour of sodium polyacrylate chains in aqueous NaCl solutions. Polymer 2003, 44, 7131–7141. [Google Scholar] [CrossRef]
- Yu, J.; Jackson, N.E.; Xu, X.; Brettmann, B.K.; Ruths, M.; de Pablo, J.J.; Tirrell, M. Multivalent ions induce lateral structural inhomogeneities in polyelectrolyte brushes. Sci. Adv. 2017, 3, eaao1497. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, C.; Luo, J. Hydration Lubrication Applicable to Artificial Joints through Polyelectrolyte-Embedded Modification on UHMWPE. ACS Appl. Polym. Mater. 2022, 4, 7487–7497. [Google Scholar] [CrossRef]
- Chen, K.; Liu, S.; Wu, X.; Wang, F.; Chen, G.; Yang, X.; Xu, L.; Qi, J.; Luo, Y.; Zhang, D. Mussel-inspired construction of Ti6Al4V-hydrogel artificial cartilage material with high strength and low friction. Mater. Lett. 2020, 265, 127421. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, X.; Liu, S.; Chen, K.; Feng, C.; Li, X.; Qi, J.; Luo, Y.; Liu, H.; Zhang, D. Cartilage-bone inspired the construction of soft-hard composite material with excellent interfacial binding performance and low friction for artificial joints. Friction 2023, 11, 1177–1193. [Google Scholar] [CrossRef]
- Cui, L.; Li, H.; Huang, J.; Xiong, D. Improved biotribological performance of Ti6Al4V alloy through the synergetic effects of porous TiO2 layer and zwitterionic hydrogel coating. Ceram. Int. 2021, 47, 34970–34978. [Google Scholar] [CrossRef]
- Wang, C.; Zhu, K.; Gao, Y.; Han, S.; Ju, J.; Ren, T.; Zhao, X. Multifunctional GO-based hydrogel coating on Ti-6Al-4 V Alloy with enhanced bioactivity, anticorrosion and tribological properties against cortical bone. Tribol. Int. 2023, 184, 108423. [Google Scholar] [CrossRef]
- Sadeghi, M.; Kharaziha, M.; Salimijazi, H.R. Double layer graphene oxide-PVP coatings on the textured Ti6Al4V for improvement of frictional and biological behavior. Surf. Coat. Technol. 2019, 374, 656–665. [Google Scholar] [CrossRef]





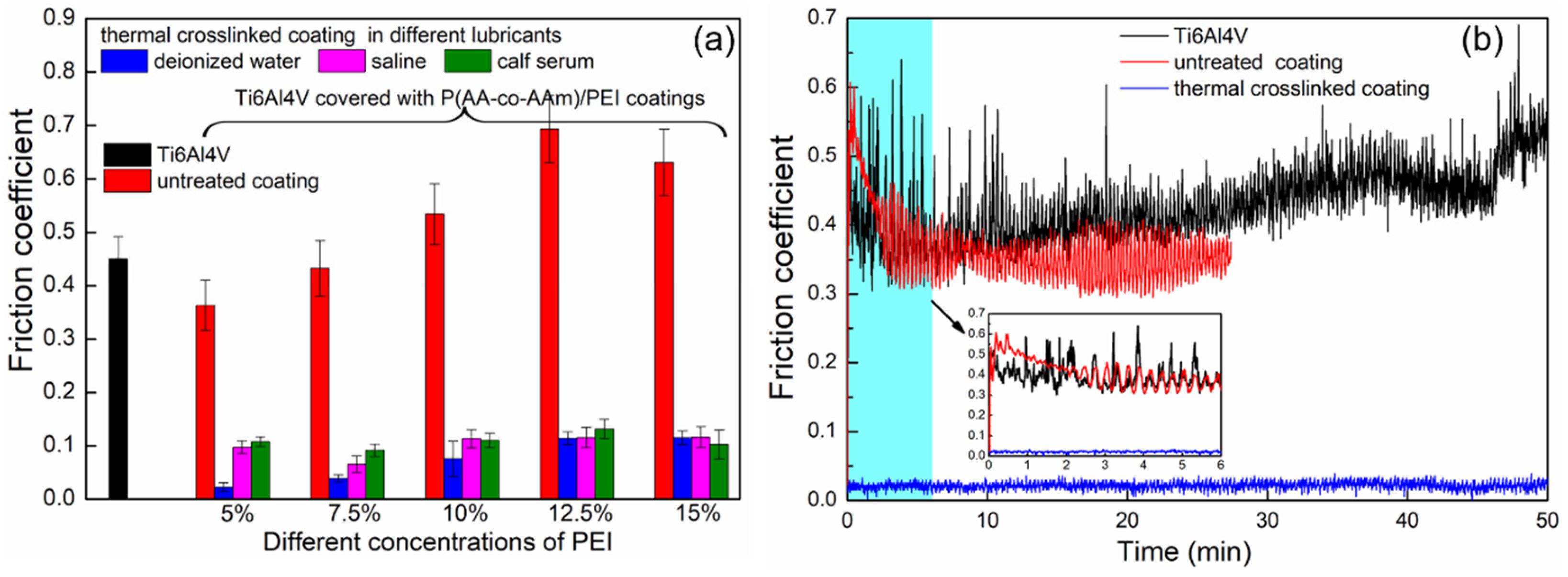


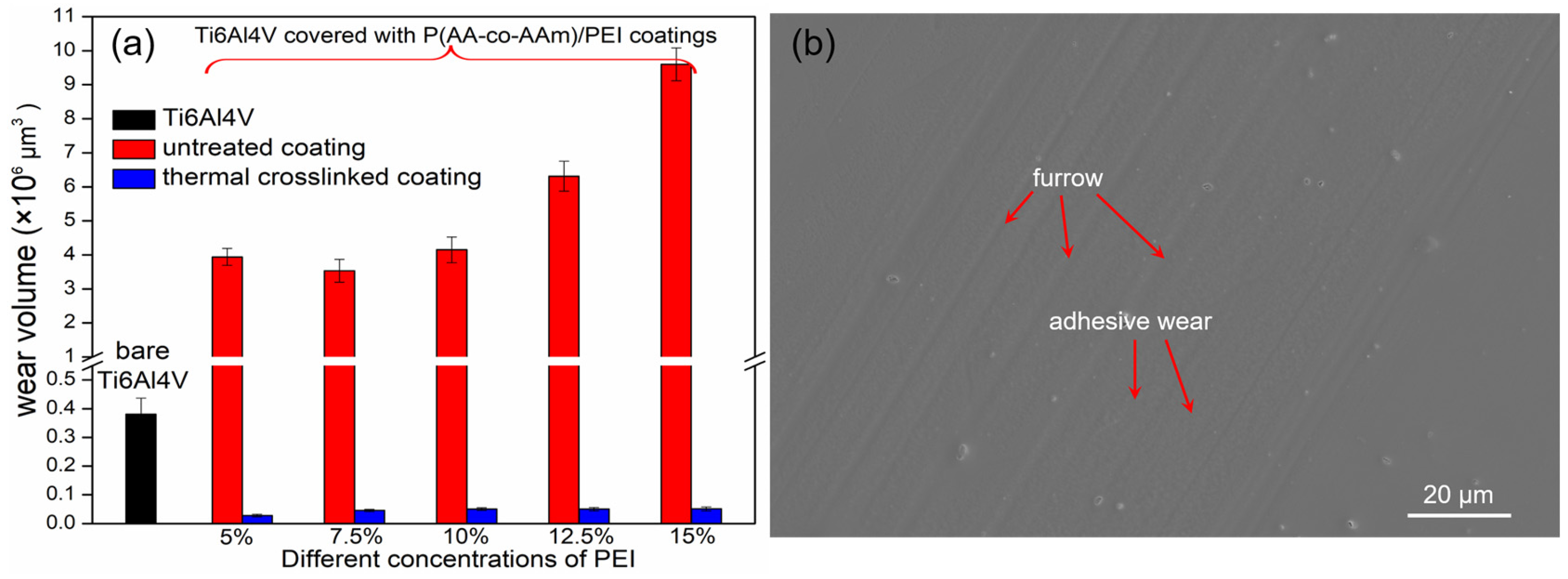
| Type of Hydrogel Coating | Titanium Pretreatment | Preparation Method of Hydrogel Coating | Friction Coefficient | References |
|---|---|---|---|---|
| PVA/HA/PAA | Treated by laser and modified by dopamine | Freeze–thaw and annealing | 0.08 | [78] |
| PVA/HA/PAA/PDA | Treated by laser and modified by dopamine | Freeze–thaw and annealing | 0.065 | [78,79] |
| PVA-PAM-PMPC | Micro-arc oxidation | UV irradiation and freeze–thaw | 0.052 | [80] |
| PVA/PAA/GO/PDA | Modified by 3-aminopropyltriethoxysilane (APTES) and dopamine | Freeze–thaw and annealing | 0.144 | [81] |
| PAA-PAM | Polish | UV irradiation | 0.085 | [19] |
| double-layer GO and PVP | Treated by laser | Electrophoretic deposition | <0.03 | [82] |
| P(AA-co-AAm)/PEI | Modified by dopamine | UV irradiation and annealing | 0.022 | this paper |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, Y.; Xu, Y.; Nie, L.; Huang, Y. Crosslinked Polymer Coatings of Poly (Acrylic Acid-co-acrylamide)/Polyethyleneimine (P(AA-co-AAm)/PEI) on Titanium Alloy with Excellent Lubrication Performance for Artificial Joints. Coatings 2024, 14, 28. https://doi.org/10.3390/coatings14010028
Deng Y, Xu Y, Nie L, Huang Y. Crosslinked Polymer Coatings of Poly (Acrylic Acid-co-acrylamide)/Polyethyleneimine (P(AA-co-AAm)/PEI) on Titanium Alloy with Excellent Lubrication Performance for Artificial Joints. Coatings. 2024; 14(1):28. https://doi.org/10.3390/coatings14010028
Chicago/Turabian StyleDeng, Yaling, Yu Xu, Lei Nie, and Yiyang Huang. 2024. "Crosslinked Polymer Coatings of Poly (Acrylic Acid-co-acrylamide)/Polyethyleneimine (P(AA-co-AAm)/PEI) on Titanium Alloy with Excellent Lubrication Performance for Artificial Joints" Coatings 14, no. 1: 28. https://doi.org/10.3390/coatings14010028