A Novel Oxidation–Reduction Route for the Morphology-Controlled Synthesis of Manganese Oxide Nanocoating as Highly Effective Material for Pseudocapacitors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of the Nanolayers of Manganese Oxide via SILD Synthesis
2.3. Characterization
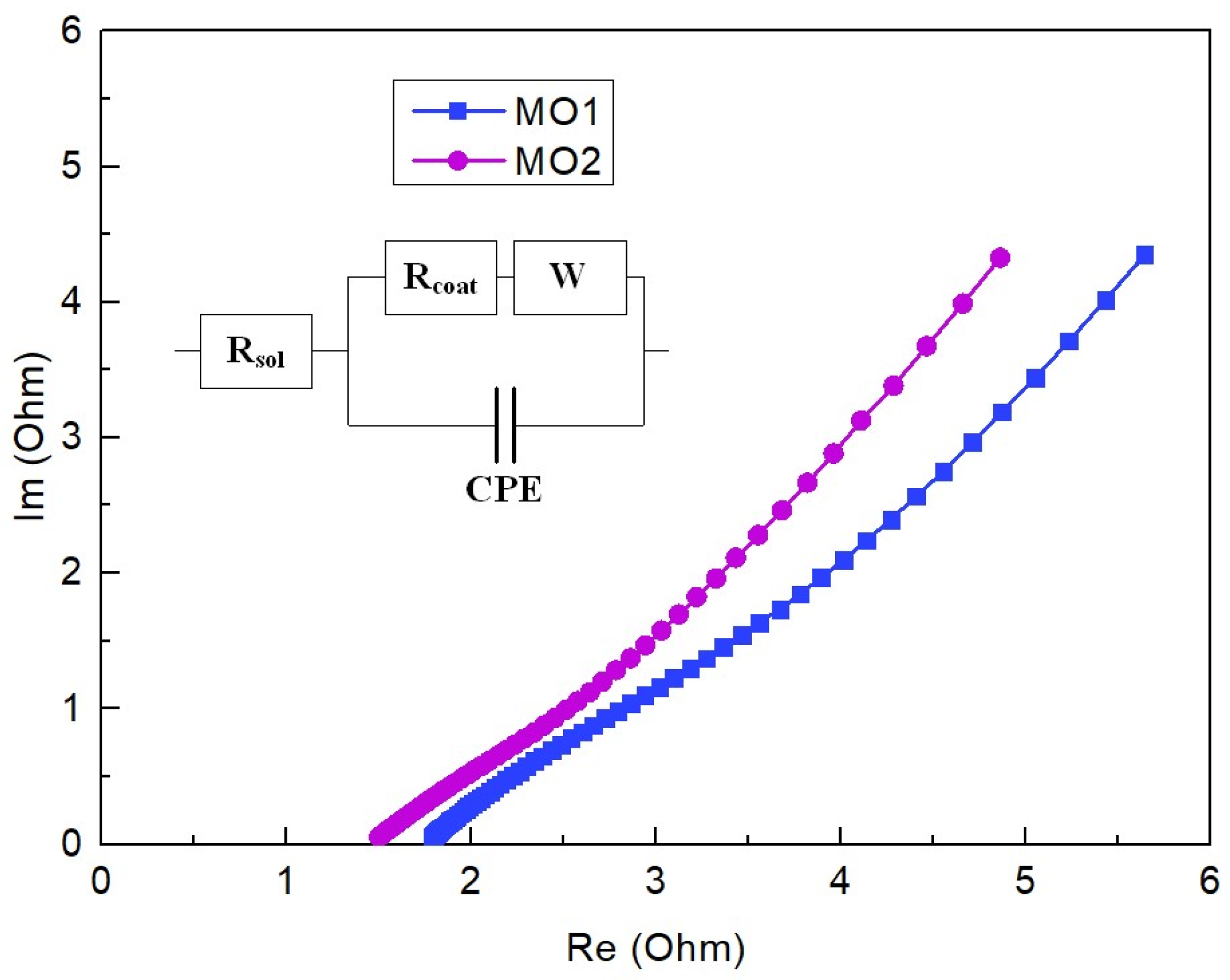
2.4. Electrochemical Measurement
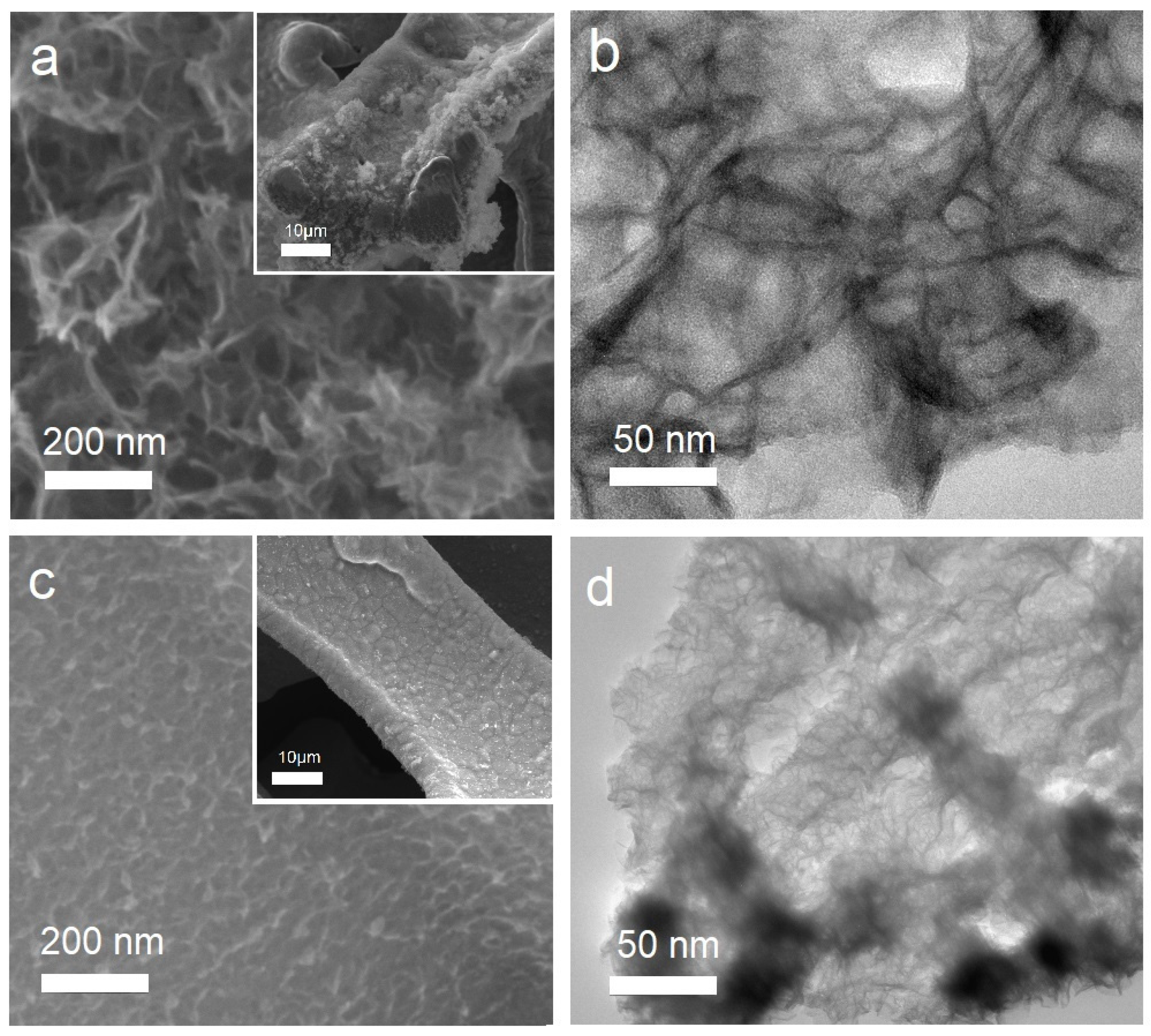
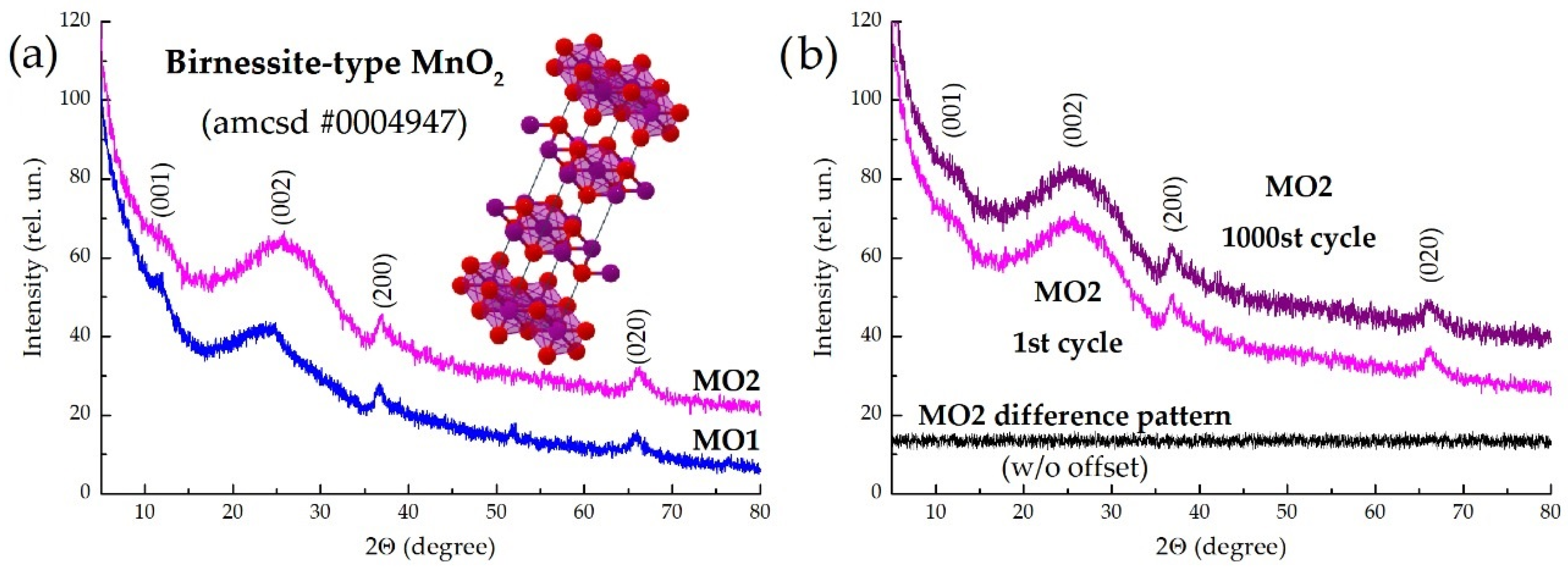
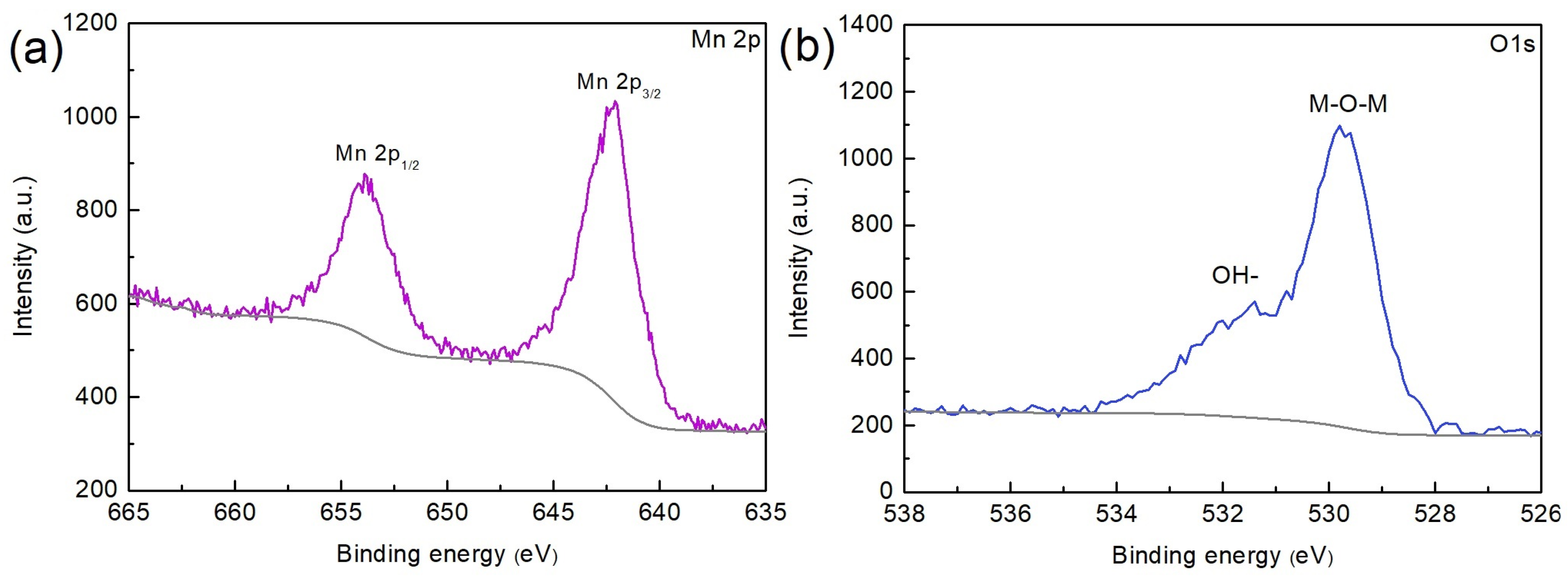
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, C.; Li, F.; Ma, L.-P.; Cheng, H.-M. Advanced Materials for Energy Storage. Adv. Mater. 2010, 22, E28–E62. [Google Scholar] [CrossRef]
- Zhai, Y.; Dou, Y.; Zhao, D.; Fulvio, P.F.; Mayes, R.; Dai, S. Carbon materials for chemical capacitive energy storage. Adv. Mater. 2011, 23, 4828–4850. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Li, F.; Cheng, H.-M. Progress in flexible lithium batteries and future prospects. Energy Environ. Sci. 2014, 7, 1307–1338. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Choi, K.-H.; Choi, W.-S.; Kwon, Y.H.; Jung, H.-R.; Shin, H.-C.; Kim, J.Y. Progress in flexible energy storage and conversion systems, with a focus on cable-type lithium-ion batteries. Energy Environ. Sci. 2013, 6, 2414. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, L.; Zhang, J. A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 2012, 41, 797–828. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Deng, Y.; Hu, W.; Qiao, J.; Zhang, L.; Zhang, J. A review of electrolyte materials and compositions for electrochemical supercapacitors. Chem. Soc. Rev. 2015, 44, 7484–7539. [Google Scholar] [CrossRef] [PubMed]
- Bhojane, P. Recent advances and fundamentals of Pseudocapacitors: Materials, mechanism, and its understanding. J. Energy Storage 2022, 45, 103654. [Google Scholar] [CrossRef]
- Abdah, M.A.A.M.; Azman, N.H.N.; Kulandaivalu, S.; Sulaiman, Y. Review of the use of transition-metal-oxide and conducting polymer-based fibers for high-performance supercapacitors. Mater. Design 2020, 186, 108199. [Google Scholar] [CrossRef]
- Palem, R.; Ramesh, S.; Bathula, C.; Kakani, V.; Saratale, G.; Yadav, H.; Kim, J.-H.; Kim, H.; Lee, S.-H. Enhanced supercapacitive behavior by CuO@MnO2/carboxymethyl cellulose composites. Ceram. Int. 2021, 47, 26738–26747. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, X.; Han, Y.; Li, T. Flexible supercapacitor: Overview and outlooks. J. Energy Storage 2021, 42, 103053. [Google Scholar] [CrossRef]
- Zan, G.; Wu, T.; Zhu, F.; He, P.; Cheng, Y.; Chai, S.; Wang, Y.; Huang, X.; Zhang, W.; Wan, Y.; et al. A biomimetic conductive super-foldable material. Matter 2021, 4, 3232–3247. [Google Scholar] [CrossRef]
- Zan, G.; Wu, T.; Dong, W.; Zhou, J.; Tu, T.; Xu, R.; Chen, Y.; Wang, Y.; Wu, Q. Two-Level Biomimetic Designs Enable Intelligent Stress Dispersion for Super-Foldable C/NiS Nanofiber Free-Standing Electrode. Adv. Fiber Mater. 2022, 4, 1117–1190. [Google Scholar] [CrossRef]
- Julien, C.M.; Mauger, A. Nanostructured MnO2 as Electrode Materials for Energy Storage. Nanomaterials 2017, 7, 396. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-Y.; Han, L.-Q.; Sun, S.; Wang, C.-Y.; Chen, M.-M. MnO2/C composite electrodes free of conductive enhancer for supercapacitors. J. Alloys Compd. 2015, 653, 539–545. [Google Scholar] [CrossRef]
- Huang, M.; Li, F.; Dong, F.; Zhang, Y.X.; Zhang, L.L. MnO2-based nanostructures for high-performance supercapacitors. J. Mater. Chem. A 2015, 3, 21380–23423. [Google Scholar] [CrossRef]
- Yin, B.; Zhang, S.; Jiang, H.; Qu, F.; Wu, X. Phase-controlled synthesis of polymorphic MnO2 structures for electrochemical energy storage. J. Mater. Chem. 2015, 3, 5722–5729. [Google Scholar] [CrossRef]
- Sun, P.; Yi, H.; Peng, T.; Jing, Y.; Wang, R.; Wang, H.; Wang, X. Ultrathin MnO2 nanoflakes deposited on carbon nanotube networks for symmetrical supercapacitors with enhanced performance. J. Power Sources 2017, 341, 27–35. [Google Scholar] [CrossRef]
- Tolstoy, V.P. Successive ionic layer deposition. An application in nanotechnology. Russ. Chem. Rev. 2006, 75, 161. [Google Scholar] [CrossRef]
- Ratnayake, S.P.; Ren, J.; Colusso, E.; Guglielmi, M.; Martucci, A.; Della Gaspera, E. SILAR Deposition of Metal Oxide Nanostructured Films. Small 2021, 17, 2101666. [Google Scholar] [CrossRef]
- Kodintsev, I.A.; Martinson, K.D.; Lobinsky, A.A.; Popkov, V.I. Successive ionic layer deposition of co-doped Cu(OH)2 nanorods as electrode material for electrocatalytic reforming of ethanol. Nanosyst. Phys. Chem. Math. 2019, 10, 573–578. [Google Scholar] [CrossRef]
- Kodintsev, I.A.; Martinson, K.D.; Lobinsky, A.A.; Popkov, V.I. SILD synthesis of the efficient and stable electrocatalyst based on CoO-NiO solid solution toward hydrogen production. Nanosyst. Phys. Chem. Math. 2019, 10, 681–685. [Google Scholar] [CrossRef]
- Lobinsky, A.A.; Popkov, V.I. Direct SILD synthesis of efficient electroactive materials based on ultrathin nanosheets of amorphous CoCr-LDH. Mater. Lett. 2022, 322, 132472. [Google Scholar] [CrossRef]
- Lobinsky, A.A.; Kaneva, M.V. Synthesis Ni-doped CuO nanorods via successive ionic layer deposition method and their capacitive performance. Nanosyst. Phys. Chem. Math. 2020, 11, 608–614. [Google Scholar] [CrossRef]
- Lobinsky, A.A.; Tolstoy, V.P. Synthesis of 2D Zn–Co LDH nanosheets by a successive ionic layer deposition method as a material for electrodes of high-performance alkaline battery–supercapacitor hybrid devices. RSC Adv. 2018, 8, 29607–29612. [Google Scholar] [CrossRef]
- Soonmin, H. Recent advances in the growth and characterizations of SILAR-deposited thin films. Appl. Sci. 2022, 12, 8184. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, G.; Ouyang, N.; Qin, Z.; Lan, S.; Zhang, Q. The controlled synthesis of birnessite nanoflowers via H2O2 reducing KMnO4 for efficient adsorption and photooxidation activity. Front. Chem. 2021, 9, 699513. [Google Scholar] [CrossRef] [PubMed]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; St, R.; Smart, C. Resolving surface chemical states in XPS analysis of first-row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lobinsky, A.A.; Kodintzev, I.A.; Tenevich, M.I.; Popkov, V.I. A Novel Oxidation–Reduction Route for the Morphology-Controlled Synthesis of Manganese Oxide Nanocoating as Highly Effective Material for Pseudocapacitors. Coatings 2023, 13, 361. https://doi.org/10.3390/coatings13020361
Lobinsky AA, Kodintzev IA, Tenevich MI, Popkov VI. A Novel Oxidation–Reduction Route for the Morphology-Controlled Synthesis of Manganese Oxide Nanocoating as Highly Effective Material for Pseudocapacitors. Coatings. 2023; 13(2):361. https://doi.org/10.3390/coatings13020361
Chicago/Turabian StyleLobinsky, Artem A., Ilya A. Kodintzev, Maxim I. Tenevich, and Vadim I. Popkov. 2023. "A Novel Oxidation–Reduction Route for the Morphology-Controlled Synthesis of Manganese Oxide Nanocoating as Highly Effective Material for Pseudocapacitors" Coatings 13, no. 2: 361. https://doi.org/10.3390/coatings13020361




