Bioactivity and Mechanical Properties of Hydroxyapatite on Ti6Al4V and Si(100) Surfaces by Pulsed Laser Deposition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Details
2.1.1. Substrate Preparation
2.1.2. Coating Process
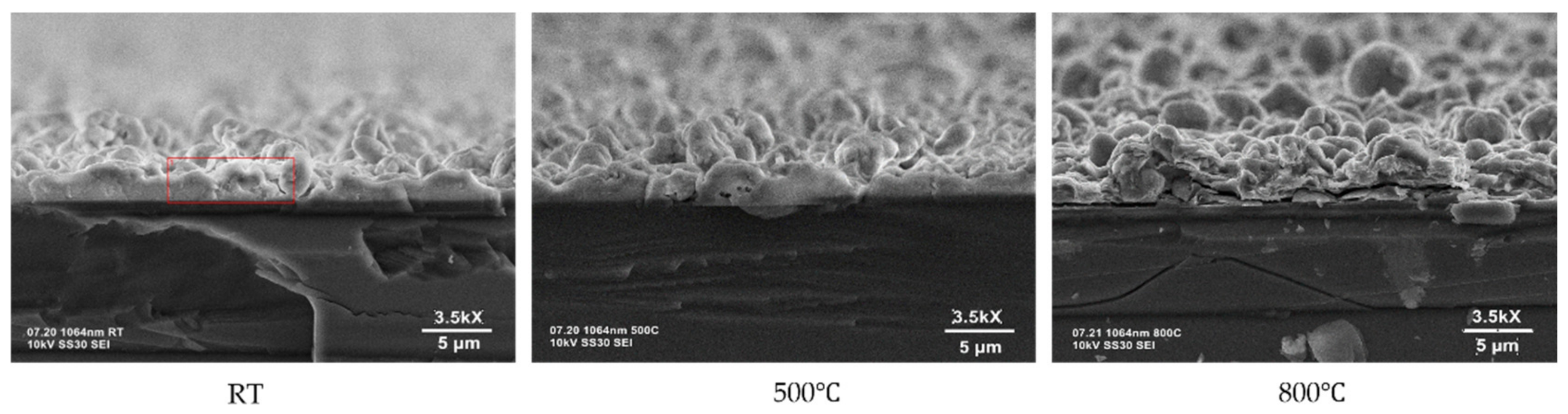
2.2. Surface Characterization: SEM, AFM, AmScope
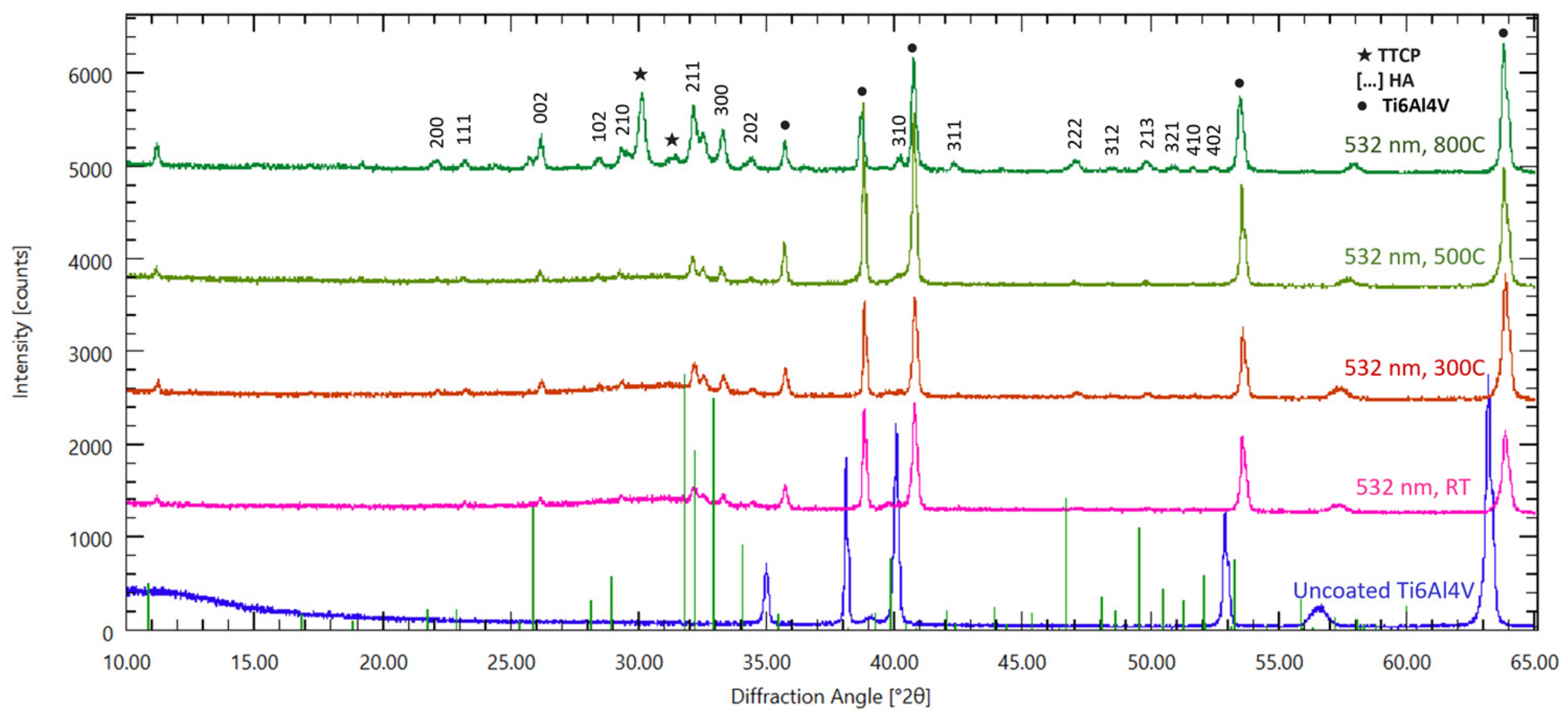
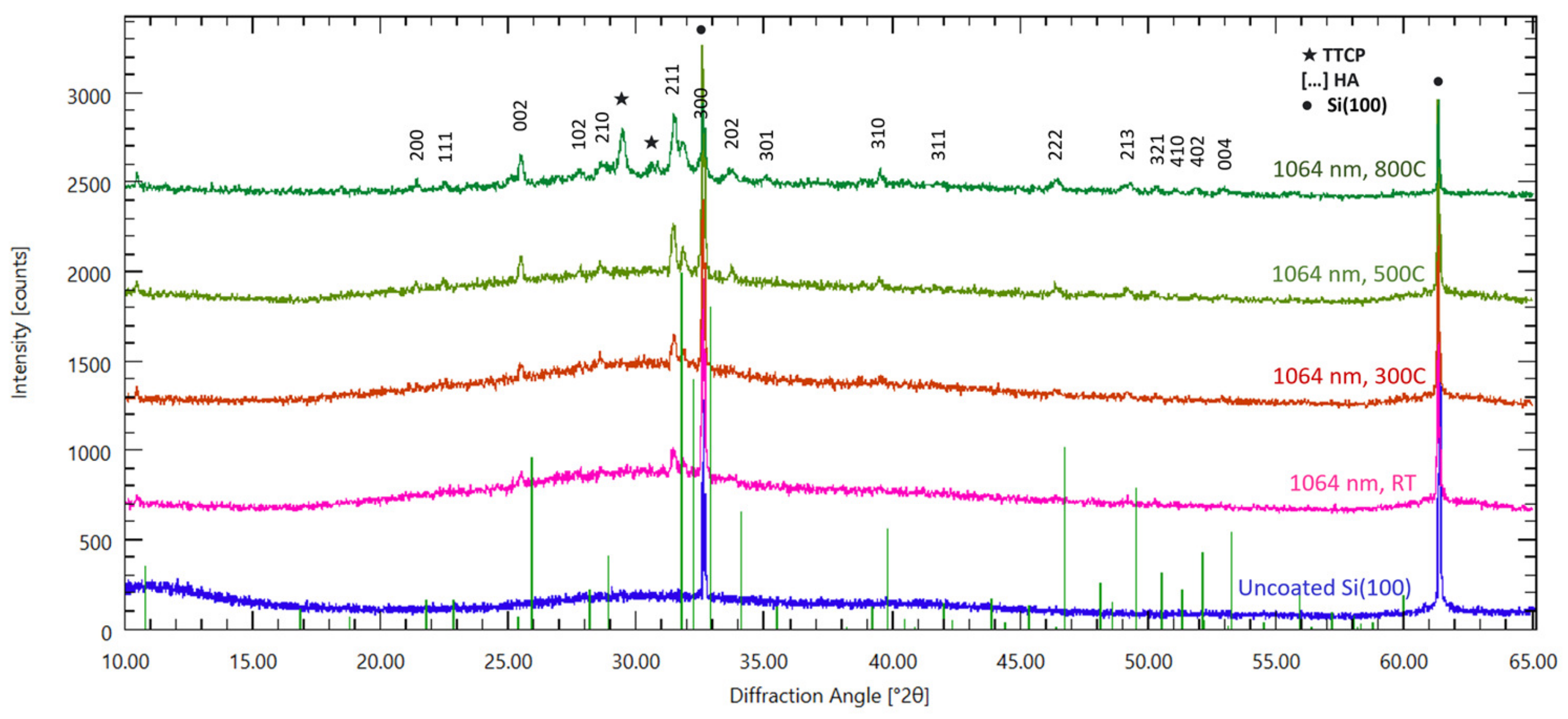
2.3. Structural Analysis: XRD, FTIR
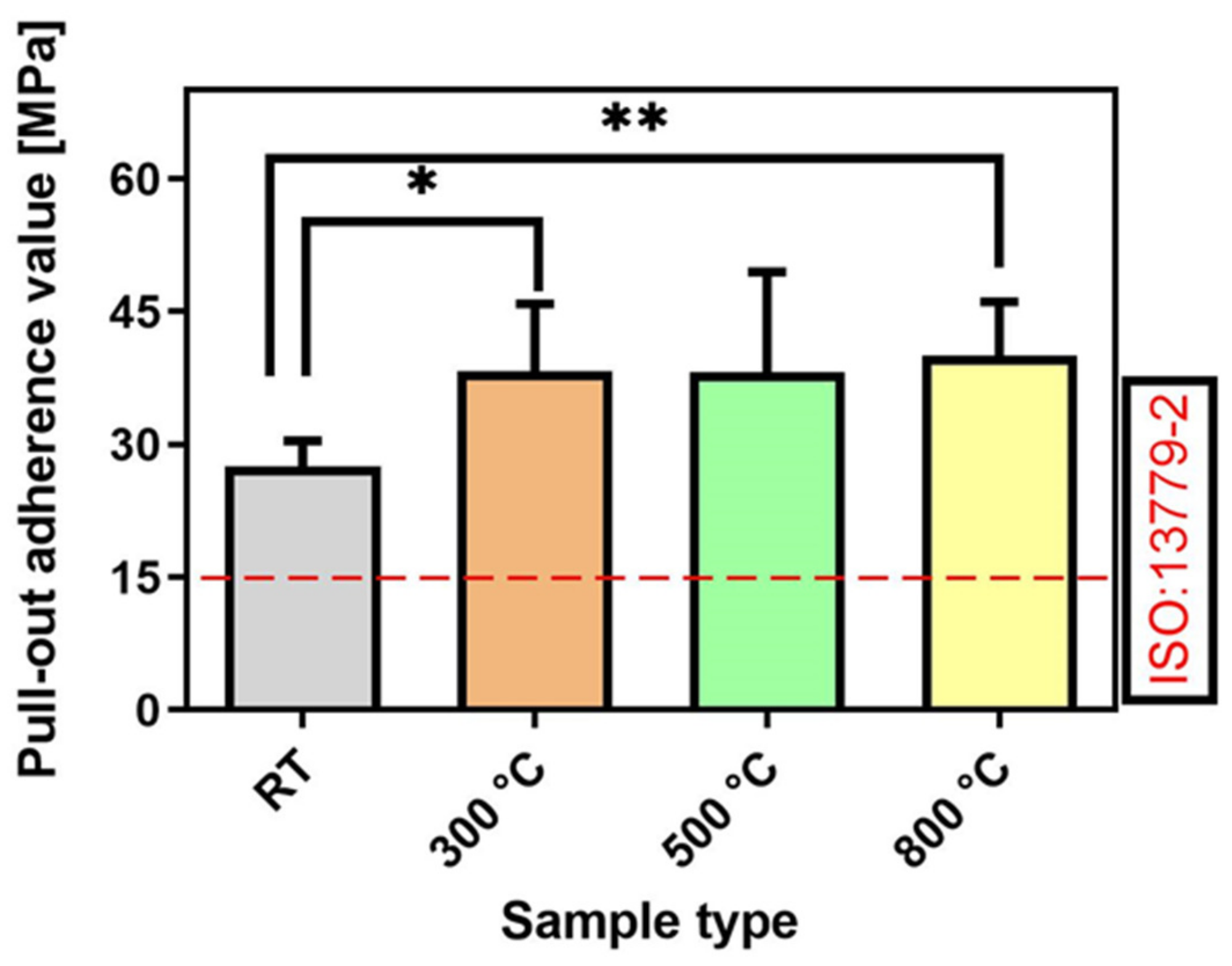
2.4. Mechanical Analysis and Adherence Strength: Pull-Out Tests
2.5. Biological Assessment
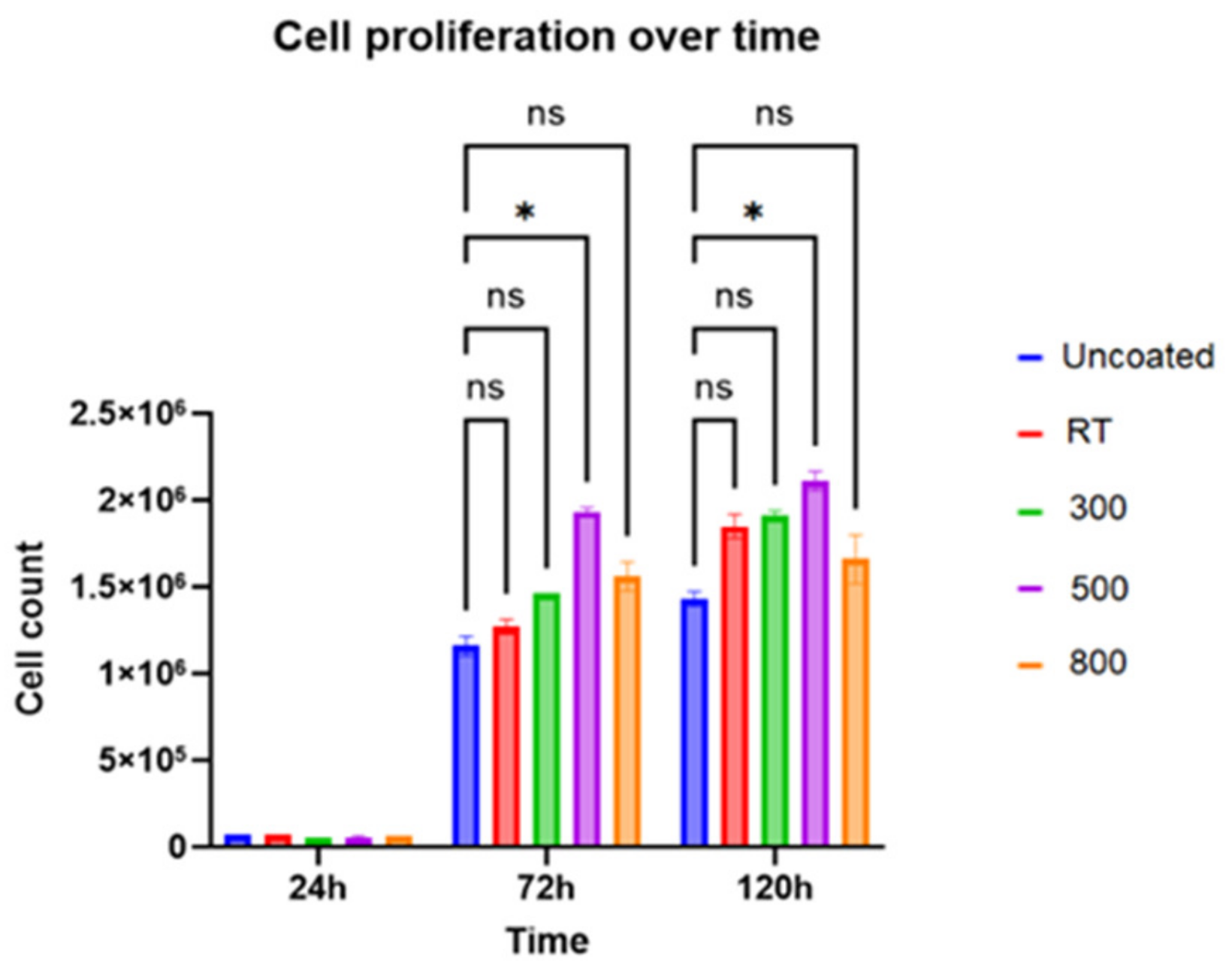
2.5.1. Cell Growth Experiment
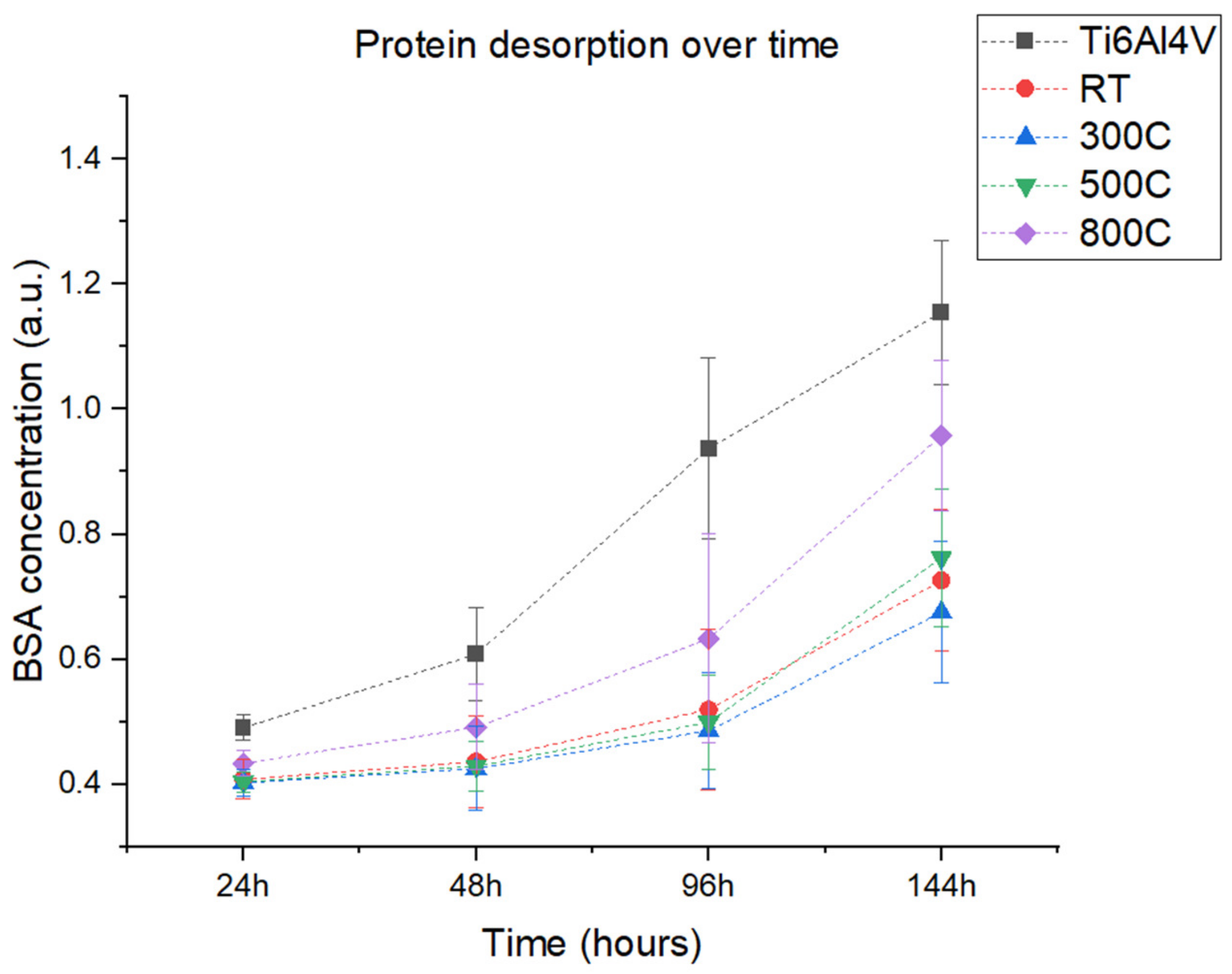
2.5.2. Protein Adsorption
3. Results and Discussion
Coating Adherence
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Breme, H.; Biehl, V.; Reger, N.; Gawalt, E. Chapter 1a Metallic Biomaterials: Introduction. In Handbook of Biomaterial Properties; Springer: Berlin/Heidelberg, Germany, 2016; pp. 151–158. [Google Scholar]
- Zhang, D.; Chen, Q.; Shi, C.; Chen, M.; Ma, K.; Wan, J.; Liu, R. Dealing with the Foreign-Body Response to Implanted Biomaterials: Strategies and Applications of New Materials. Adv. Funct. Mater. 2020, 31, 2007226. [Google Scholar] [CrossRef]
- Espallargas, N.; Torres, C.; Muñoz, A. A metal ion release study of CoCrMo exposed to corrosion and tribocorrosion conditions in simulated body fluids. Wear 2015, 332–333, 669–678. [Google Scholar] [CrossRef]
- Manivasagam, G.; Dhinasekaran, D.; Rajamanickam, A. Biomedical Implants: Corrosion and its Prevention—A Review. Recent Patents Corros. Sci. 2010, 2, 40–54. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, W.; Li, Y.; Li, W.; Tan, L.; Yang, K. Biofunctional magnesium coating of implant materials by physical vapour deposition. Biomater. Transl. 2021, 2, 248–256. [Google Scholar] [PubMed]
- Harun, W.S.; Asri, R.I.; Sulong, A.B.; Ghani, S.A.; Ghazalli, Z. Hydroxyapatite-Based Coating on Biomedical Implant. Hydroxyapatite Adv. Compos. Nanomater. Biomed. Appl. Its Technol. Facet. 2018, 6988. [Google Scholar] [CrossRef]
- Asri, R.I.M.; Harun, W.S.W.; Samykano, M.; Lah, A.N.C.; Ghani, S.A.C.; Tarlochan, F.; Raza, M.R. Corrosion and surface modification on biocompatible metals: A review. Mater. Sci. Eng. C 2017, 77, 1261–1274. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, W.; Li, X.; Zhang, X.; Wang, C.; Meng, X.; Pei, Y.; Fan, X.; Lan, P.; Wang, C.; et al. Improving Osteointegration and Osteogenesis of Three-Dimensional Porous Ti6Al4V Scaffolds by Polydopamine-Assisted Biomimetic Hydroxyapatite Coating. ACS Appl. Mater. Interfaces 2015, 7, 5715–5724. [Google Scholar] [CrossRef]
- Li, Y.; Jahr, H.; Zhou, J.; Zadpoor, A.A. Additively manufactured biodegradable porous metals. Acta Biomater. 2020, 115, 29–50. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, J.; Ong, M.T.; Yung, P.S.; Wang, J.; Qin, L. Update on the research and development of magnesium-based biodegradable implants and their clinical translation in orthopaedics. Biomater. Transl. 2021, 2, 188–196. [Google Scholar]
- Khuzhakulov, Z.; Kylychbekov, S.; Allamyradov, Y.; Majidov, I.; Ben Yosef, J.; Er, A.Y.; Kitchens, C.; Banga, S.; Badarudeen, S.; Er, A.O. Formation of picosecond laser-induced periodic surface structures on steel for knee arthroplasty prosthetics. Front. Met. Alloy. 2023, 1, 1090104. [Google Scholar] [CrossRef]
- Papynov, E.K.; Shichalin, O.O.; Belov, A.A.; Buravlev, I.Y.; Mayorov, V.Y.; Fedorets, A.N.; Buravleva, A.A.; Lembikov, A.O.; Gritsuk, D.V.; Kapustina, O.V.; et al. CaSiO3-HAp Metal-Reinforced Biocomposite Ceramics for Bone Tissue Engineering. J. Funct. Biomater. 2023, 14, 259. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Zhong, J.; Hou, R.; Hu, X.; Chen, Y.; Weng, H.; Zhang, Z.; Liu, B.; Yang, S.; Peng, Z. Polymer bilayer-Micro arc oxidation surface coating on pure magnesium for bone implantation. J. Orthop. Transl. 2023, 40, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Chen, C.; Zhang, S.; Ren, L.; Zhao, Y.; Guo, W. Mediation of mechanically adapted TiCu/TiCuN/CFR-PEEK implants in vascular regeneration to promote bone repair in vitro and in vivo. J. Orthop. Transl. 2022, 33, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Fiume, E.; Magnaterra, G.; Rahdar, A.; Verné, E.; Baino, F. Hydroxyapatite for Biomedical Applications: A Short Overview. Ceramics 2021, 4, 542–563. [Google Scholar] [CrossRef]
- Pajor, K.; Pajchel, L.; Kolmas, J. Hydroxyapatite and Fluorapatite in Conservative Dentistry and Oral Implantology—A Review. Materials 2019, 12, 2683. [Google Scholar] [CrossRef] [PubMed]
- Ielo, I.; Calabrese, G.; De Luca, G.; Conoci, S. Recent Advances in Hydroxyapatite-Based Biocomposites for Bone Tissue Regeneration in Orthopedics. Int. J. Mol. Sci. 2022, 23, 9721. [Google Scholar] [CrossRef]
- Medical Implants Market Size to Surpass US$ 145.6 Bn by 2030. Available online: https://www.precedenceresearch.com/press-release/medical-implants-market (accessed on 28 August 2023).
- Kim, H.; Camata, R.P.; Vohra, Y.K.; Lacefield, W.R. Control of phase composition in hydroxyapatite/tetracalcium phosphate biphasic thin coatings for biomedical applications. J. Mater. Sci. Mater. Med. 2005, 16, 961–966. [Google Scholar] [CrossRef]
- Klein, C.P.; de Blieck-Hogemrst, J.; Wolket, J.; de Groot, K. Studies of the solubility of different calcium phosphate ceramic particles in vitro. Biomaterials 1990, 11, 509–512. [Google Scholar] [CrossRef]
- Sobczak-Kupiec, A.; Drabczyk, A.; Florkiewicz, W.; Głąb, M.; Kudłacik-Kramarczyk, S.; Słota, D.; Tomala, A.; Tyliszczak, B. Review of the Applications of Biomedical Compositions Containing Hydroxyapatite and Collagen Modified by Bioactive Components. Materials 2021, 14, 2096. [Google Scholar] [CrossRef]
- Duta, L.; Stan, G.E.; Popescu-Pelin, G.; Zgura, I.; Anastasescu, M.; Oktar, F.N. Influence of Post-Deposition Thermal Treatments on the Morpho-Structural, and Bonding Strength Characteristics of Lithium-Doped Biological-Derived Hydroxyapatite Coatings. Coatings 2022, 12, 1883. [Google Scholar] [CrossRef]
- Mihailescu, N.; Stan, G.; Duta, L.; Chifiriuc, M.C.; Bleotu, C.; Sopronyi, M.; Luculescu, C.; Oktar, F.; Mihailescu, I. Structural, compositional, mechanical characterization and biological assessment of bovine-derived hydroxyapatite coatings reinforced with MgF 2 or MgO for implants functionalization. Mater. Sci. Eng. C 2016, 59, 863–874. [Google Scholar] [CrossRef] [PubMed]
- Duta, L.; Oktar, F.; Stan, G.; Popescu-Pelin, G.; Serban, N.; Luculescu, C.; Mihailescu, I. Novel doped hydroxyapatite thin films obtained by pulsed laser deposition. Appl. Surf. Sci. 2013, 265, 41–49. [Google Scholar] [CrossRef]
- Er, A.O.; Ren, W.; Elsayed-Ali, H.E. Low temperature epitaxial growth of Ge quantum dot on Si(100)-(2 × 1) by femtosecond laser excitation. Appl. Phys. Lett. 2011, 98, 013108. [Google Scholar] [CrossRef]
- Farha, A.H.; Er, A.O.; Ufuktepe, Y.; Myneni, G.; Elsayed-Ali, H.E. Effects of substrate temperature on properties of NbNx films grown on Nb by pulsed laser deposition. Appl. Surf. Sci. 2011, 258, 1613–1618. [Google Scholar] [CrossRef]
- Er, A.O.; Elsayed-Ali, H.E. Excitation-Induced Germanium Quantum Dot Formation on Si(100)−(2 × 1). J. Appl. Phys. 2010, 108, 034303–034310. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, J.; Tian, Y. A Review on Macroscopic and Microstructural Features of Metallic Coating Created by Pulsed Laser Material Deposition. Micromachines 2022, 13, 659. [Google Scholar] [CrossRef] [PubMed]
- Er, A.O.; Elsayed-Ali, H.E. Femtosecond pulsed laser deposition of Ge quantum dot growth on Si (100)−(2 × 1). Appl. Surf. Sci. 2011, 257, 8078–8084. [Google Scholar] [CrossRef]
- Er, A.O.; Elsayed-Ali, H.E. Electronically enhanced surface diffusion during Ge growth on Si(100). J. Appl. Phys. 2011, 109, 084320–084326. [Google Scholar]
- Koren, G.; Gupta, A.; Baseman, R.J.; Lutwyche, M.I.; Laibowitz, R.B. Laser wavelength dependent properties of YBa2Cu3O7-d thin films deposited by laser ablation. Appl. Phys. Lett. 1989, 55, 2450–2452. [Google Scholar] [CrossRef]
- György, E.; Mihailescu, I.N.; Kompitsasa, M.; Giannoudakosa, A. Particulates generation and solutions for their elimination in pulsed laser deposition. J. Optoelectron. Adv. Mater. 2004, 6, 39–46. [Google Scholar]
- Mihailescu, I.N.; Teodorescu, V.S.; Gyorgy, E.; Luches, A.; Perrone, A.; Martino, M. About the nature of particulates covering the surface of thin films obtained by reactive pulsed laser deposition. J. Phys. D Appl. Phys. 1998, 31, 2236–2240. [Google Scholar] [CrossRef]
- Hontsu, S. Preparation of Biomaterial Thin Films by Pulsed-Laser Deposition Technique. Rev. Laser Eng. 2000, 28, 407–412. [Google Scholar] [CrossRef]
- Oladijo, S.; Akinlabi, E.; Mwema, F.; Stamboulis, A. An Overview of Sputtering Hydroxyapatite for Biomedical Application. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2021. [Google Scholar]
- Sun, L.; Berndt, C.C.; Gross, K.A.; Kucuk, A. Material fundamentals and clinical performance of plasma-sprayed hydroxyapatite coatings: A review. J. Biomed. Mater. Res. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2001, 58, 570–592. [Google Scholar] [CrossRef] [PubMed]
- Cheang, P.; Khor, K. Addressing processing problems associated with plasma spraying of hydroxyapatite coatings. Biomaterials 1996, 17, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Massaro, C.; Baker, M.A.; Cosentino, F.; Ramires, P.A.; Klose, S.; Milella, E. Surface and biological evaluation of hydroxyapatite-based coatings on titanium deposited by different techniques. J. Biomed. Mater. Res. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2001, 58, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Cotell, C.M.; Chrisey, D.B.; Grabowski, K.S.; Sprague, J.A.; Gossett, C.R. Pulsed laser deposition of hydroxylapatite thin films on Ti-6Al-4V. J. Appl. Biomater. 1992, 3, 87–93. [Google Scholar] [CrossRef]
- Baeri, P.; Torrisi, L.; Marino, N.; Foti, G. Ablation of hydroxyapatite by pulsed laser irradiation. Appl. Surf. Sci. 1992, 54, 210–214. [Google Scholar] [CrossRef]
- Dinda, G.; Shin, J.; Mazumder, J. Pulsed laser deposition of hydroxyapatite thin films on Ti–6Al–4V: Effect of heat treatment on structure and properties. Acta Biomater. 2009, 5, 1821–1830. [Google Scholar] [CrossRef]
- Blind, O.; Klein, L.H.; Dailey, B.; Jordan, L. Characterization of hydroxyapatite films obtained by pulsed-laser deposition on Ti and Ti-6AL-4v substrates. Dent. Mater. 2005, 21, 1017–1024. [Google Scholar] [CrossRef]
- Arias, J.L.; Mayor, B.; Pou, J.; Rehman, I.; Knowles, J.; Best, S.; Bonfield, W.; García, F.; León, B.; Pérez-Amor, M. Effect of heat treatment on pulsed laser deposited amorphous calcium phosphate coatings. J. Biomed. Mater. Res. 1998, 43, 69–76. [Google Scholar]
- Gomes, G.; Borghi, F.; Ospina, R.; López, E.; Borges, F.; Mello, A. Nd: YAG (532 nm) pulsed laser deposition produces crystalline hydroxyapatite thin coatings at room temperature. Surf. Coat. Technol. 2017, 329, 174–183. [Google Scholar] [CrossRef]
- Yuan, B.; Chen, H.; Zhao, R.; Deng, X.; Chen, G.; Yang, X.; Xiao, Z.; Aurora, A.; Iulia, B.A.; Zhang, K.; et al. Construction of a magnesium hydroxide/graphene oxide/hydroxyapatite composite coating on Mg–Ca–Zn–Ag alloy to inhibit bacterial infection and promote bone regeneration. Bioact. Mater. 2022, 18, 354–367. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo-Robatto, B.; López-Álvarez, M.; Azevedo, A.; Dorado, J.; Serra, J.; Azevedo, N.; González, P. Pulsed laser deposition of copper and zinc doped hydroxyapatite coatings for biomedical applications. Surf. Coat. Technol. 2018, 333, 168–177. [Google Scholar] [CrossRef]
- Ungureanu, E.; Dragomir, A.V.; Parau, A.C.; Mitran, V.; Cimpean, A.; Tarcolea, M.; Vranceanu, D.M.; Cotrut, C.M. In Vitro Evaluation of Ag- and Sr-Doped Hydroxyapatite Coatings for Medical Applications. Materials 2023, 16, 5428. [Google Scholar] [CrossRef] [PubMed]
- Prodan, A.M.; Iconaru, S.L.; Predoi, M.V.; Predoi, D.; Motelica-Heino, M.; Turculet, C.S.; Beuran, M. Silver-Doped Hydroxyapatite Thin Layers Obtained by Sol-Gel Spin Coating Procedure. Coatings 2020, 10, 14. [Google Scholar] [CrossRef]
- Cao, Z.; Li, L.; Yang, L.; Yao, L.; Wang, H.; Yu, X.; Shen, X.; Yao, L.; Wu, G. Osteoinduction Evaluation of Fluorinated Hydroxyapatite and Tantalum Composite Coatings on Magnesium Alloys. Front. Chem. 2021, 9, 727356. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, W.; Yue, C.; Zhang, T.; Li, P.; Xing, Z.; Chen, Y. A tough graphene nanosheet/hydroxyapatite composite with improved in vitro biocompatibility. Carbon 2013, 61, 105–115. [Google Scholar] [CrossRef]
- Cotrut, C.M.; Ungureanu, E.; Ionescu, I.C.; Zamfir, R.I.; Kiss, A.E.; Parau, A.C.; Vladescu, A.; Vranceanu, D.M.; Saceleanu, A. Influence of Magnesium Content on the Physico-Chemical Properties of Hydroxyapatite Electrochemically Deposited on a Nanostructured Titanium Surface. Coatings 2022, 12, 1097. [Google Scholar] [CrossRef]
- Nistor, L.; Ghica, C.; Teodorescu, V.; Nistor, S.; Dinescu, M.; Matei, D.; Frangis, N.; Vouroutzis, N.; Liutas, C. Deposition of hydroxyapatite thin films by Nd: YAG laser ablation: A microstructural study. Mater. Res. Bull. 2004, 39, 2089–2101. [Google Scholar] [CrossRef]
- Yashiro, H.; Kakehata, M. A High-Quality Hydroxyapatite Layer Coated by Droplets Eliminated the Pulsed-Laser Deposition Scheme. In Laser Applications in Microelectronic and Optoelectronic Manufacturing (LAMOM) XXVIII.; SPIE: Bellingham, WA, USA, 2023. [Google Scholar]
- Yashiro, H.; Kakehata, M. High crystalline hydroxyapatite coating by eclipse type pulsed-laser deposition for low annealing temperature. Appl. Phys. Lett. 2022, 120, 131602. [Google Scholar] [CrossRef]
- Lin, K.C.; Chiu, Y.H.; Lin, J.H.; Pai, W.W. A quantitative analysis of the shape transition of Ge islands on Si(100) with NC-AFM. Nanotechnology 2005, 16, S63–S67. [Google Scholar] [CrossRef]
- Popa, A.; Stan, G.; Enculescu, M.; Tanase, C.; Tulyaganov, D.; Ferreira, J. Superior biofunctionality of dental implant fixtures uniformly coated with durable bioglass films by magnetron sputtering. J. Mech. Behav. Biomed. Mater. 2015, 51, 313–327. [Google Scholar] [CrossRef] [PubMed]
- ISO 13779-2:2018; Implants for surgery—Hydroxyapatite—Part 2: Thermally Sprayed Coatings of Hydroxyapatite. Available online: https://www.iso.org/standard/64617.html (accessed on 19 September 2023).
- ASTM D4541; Standard Test Method for Pull-Off Strength of Coatings Using Portable Adhesion Testers. ASTM: West Conshohocken, PA, USA, 2017.
- ISO 4624; Paints and varnishes—Pull-off test for adhesion. ISO: Geneva, Switzerland, 2016.
- Bäuerle, D. Laser Processing and Chemistry; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Chen, L.-C.; Chrisey, D.; Hubler, G. Pulsed Laser Deposition of Thin Films; Wiley: New York, NY, USA, 1994. [Google Scholar]
- Kautek, W.; Roas, B.; Schultz, L. Formation of Y-Ba-Cu-oxide thin films by pulsed laser deposition: A comparative study in the UV, visible and IR range. Thin Solid Film. 1990, 191, 317–334. [Google Scholar] [CrossRef]
- Liu, H.; Mao, X.; Yoo, J.; Russo, R. Early phase laser induced plasma diagnostics and mass removal during single-pulse laser ablation of silicon. Spectrochim. Acta Part B At. Spectrosc. 1999, 54, 1607–1624. [Google Scholar] [CrossRef]
- Ojeda-G-P, A.; Döbeli, M.; Lippert, T. Influence of Plume Properties on Thin Film Composition in Pulsed Laser Deposition. Adv. Mater. Interfaces 2018, 5, 1701062. [Google Scholar] [CrossRef]
- Agop, M.; Cimpoesu, N.; Gurlui, S.; Irimiciuc, S.A. Investigations of Transient Plasma Generated by Laser Ablation of Hydroxyapatite during the Pulsed Laser Deposition Process. Symmetry 2020, 12, 132. [Google Scholar] [CrossRef]
- Mayor, B.; Arias, J.; Chiussi, S.; Garcia, F.; Pou, J.; Fong, B.L.; Pérez-Amor, M. Calcium phosphate coatings grown at different substrate temperatures by pulsed ArF-laser deposition. Thin Solid Film. 1998, 317, 363–366. [Google Scholar] [CrossRef]
- Abdisatarov, B.; Ilhom, S.; Kholikov, K.; Loomis, D.; Dobrokhotov, V.; Khenner, M.; Er, A.O. Morphology and structure of Pb thin films grown on Si(111) by pulsed laser deposition. Appl. Phys. A 2020, 126, 1–7. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.J.; Martin, A.E., Jr.; Conrad, C.M. An Empirical Method for Estimating the Degree of Crystallinity of Native Cellulose Using the X-Ray Diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Murphy, B.; Baez, J.; Morris, M.A. Characterizing Hydroxyapatite Deposited from Solution onto Novel Substrates in Terms of Growth Mechanism and Physical Chemical Properties. Mater. Proc. 2023, 14, 34. [Google Scholar]
- Duta, L.; Mihailescu, N.; Popescu, A.C.; Luculescu, C.R.; Mihailescu, I.N.; Çetin, G.; Gunduz, O.; Oktar, F.N.; Popa, A.C.; Kuncser, A.; et al. Comparative physical, chemical and biological assessment of simple and titanium-doped ovine dentine-derived hydroxyapatite coatings fabricated by pulsed laser deposition. Appl. Surf. Sci. 2017, 413, 129–139. [Google Scholar] [CrossRef]
- Dong, X.; Wang, Q.; Wu, T.; Pan, H. Understanding Adsorption-Desorption Dynamics of BMP-2 on Hydroxyapatite (001) Surface. Biophys. J. 2007, 93, 750–759. [Google Scholar] [CrossRef]
- May, R.M.; Hoffman, M.G.; Sogo, M.J.; Parker, E.A.; O’toole, A.G.; Brennan, A.B.; Reddy, S.T. Micro-patterned surfaces reduce bacterial colonization and biofilm formation in vitro: Potential for enhancing endotracheal tube designs. Clin. Transl. Med. 2014, 3, 8. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kylychbekov, S.; Allamyradov, Y.; Khuzhakulov, Z.; Majidov, I.; Banga, S.; ben Yosef, J.; Duta, L.; Er, A.O. Bioactivity and Mechanical Properties of Hydroxyapatite on Ti6Al4V and Si(100) Surfaces by Pulsed Laser Deposition. Coatings 2023, 13, 1681. https://doi.org/10.3390/coatings13101681
Kylychbekov S, Allamyradov Y, Khuzhakulov Z, Majidov I, Banga S, ben Yosef J, Duta L, Er AO. Bioactivity and Mechanical Properties of Hydroxyapatite on Ti6Al4V and Si(100) Surfaces by Pulsed Laser Deposition. Coatings. 2023; 13(10):1681. https://doi.org/10.3390/coatings13101681
Chicago/Turabian StyleKylychbekov, Salizhan, Yaran Allamyradov, Zikrulloh Khuzhakulov, Inomjon Majidov, Simran Banga, Justice ben Yosef, Liviu Duta, and Ali Oguz Er. 2023. "Bioactivity and Mechanical Properties of Hydroxyapatite on Ti6Al4V and Si(100) Surfaces by Pulsed Laser Deposition" Coatings 13, no. 10: 1681. https://doi.org/10.3390/coatings13101681




