Gas Sensitivity of IBSD Deposited TiO2 Thin Films
Abstract
:1. Introduction
2. Experimental Methods
3. Results and Discussion
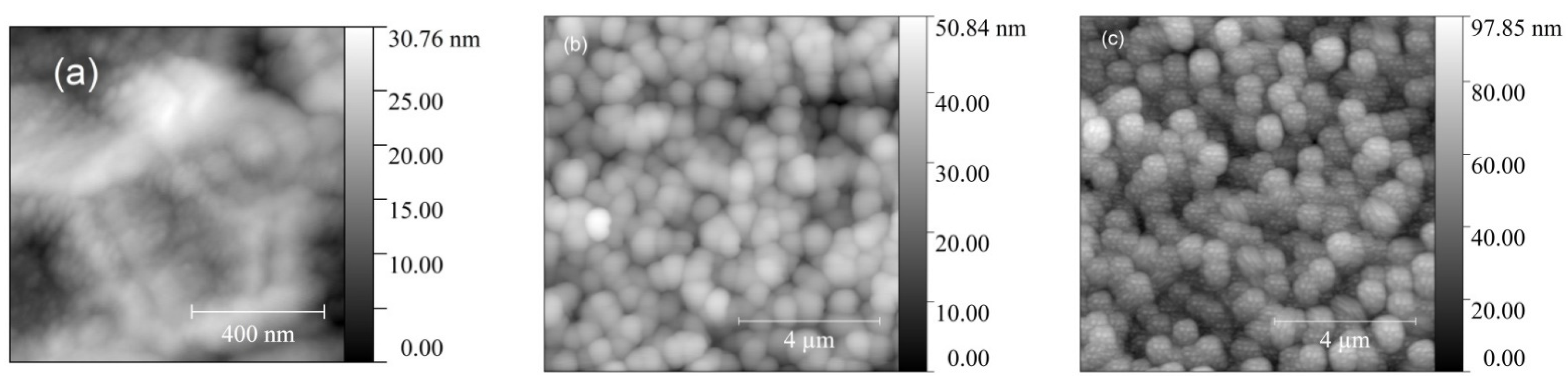
3.1. Structural Properties of TiO2 Films
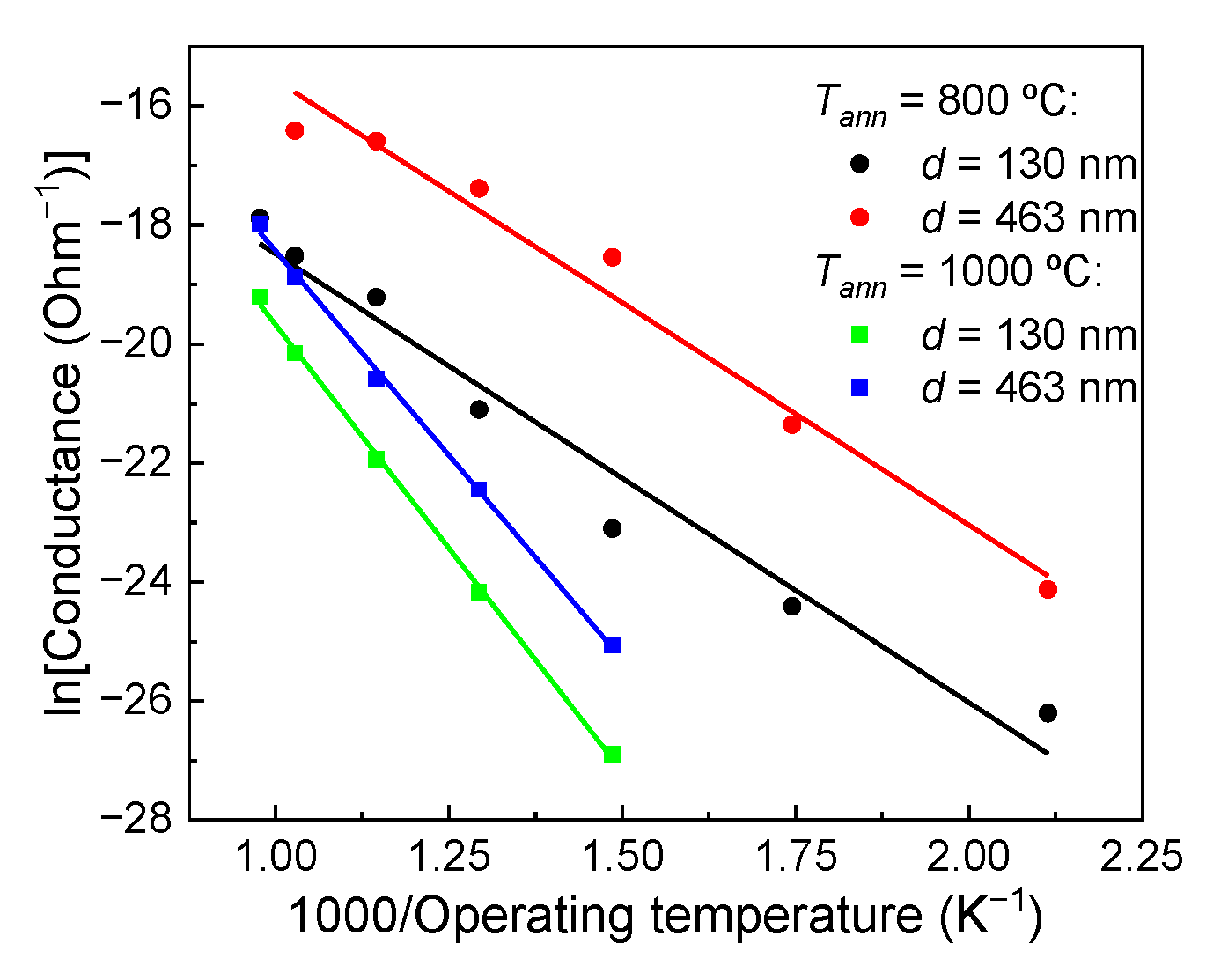
3.2. Electrically Conductive Properties of TiO2 Thin Films in Dry Pure Air
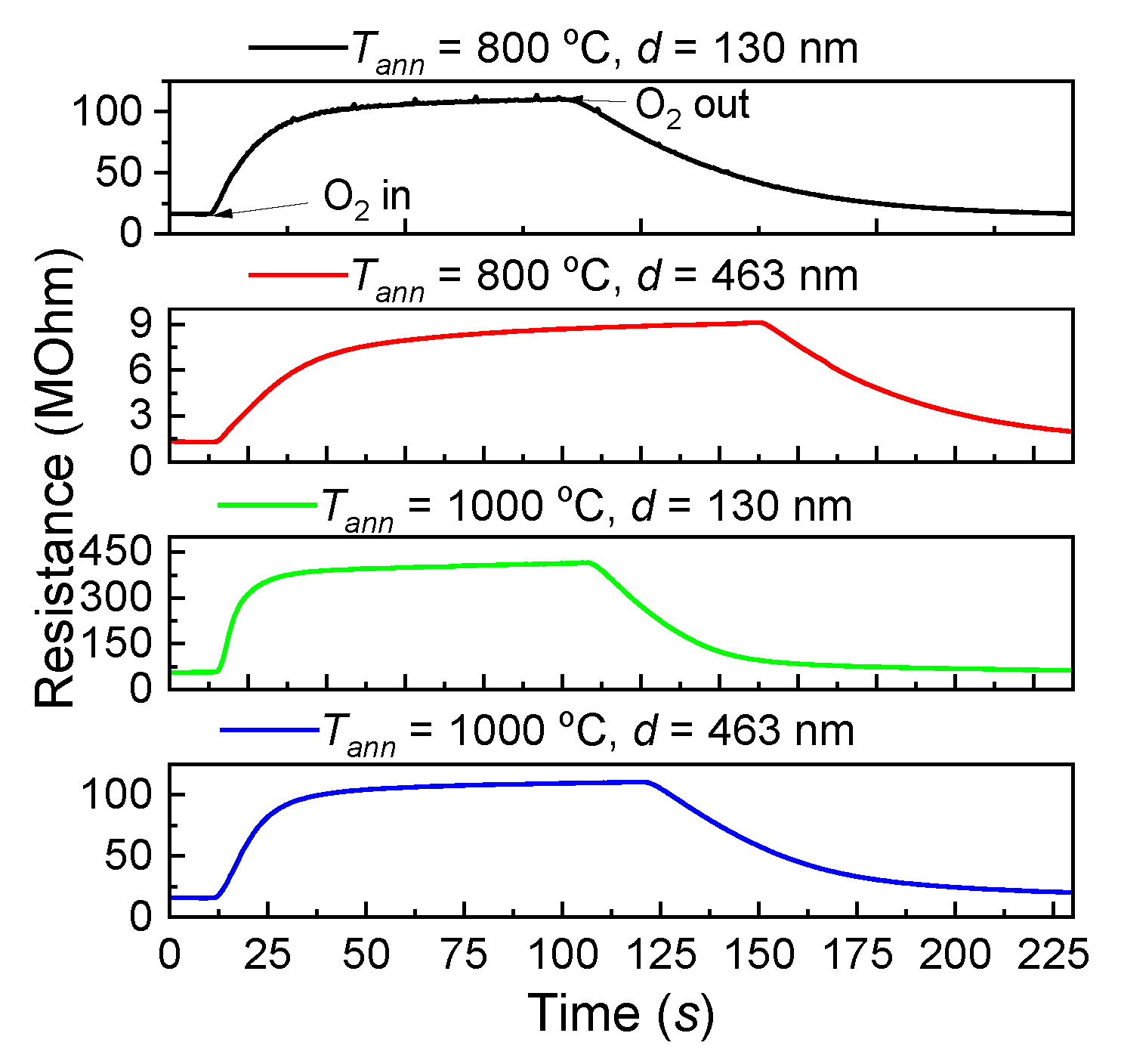
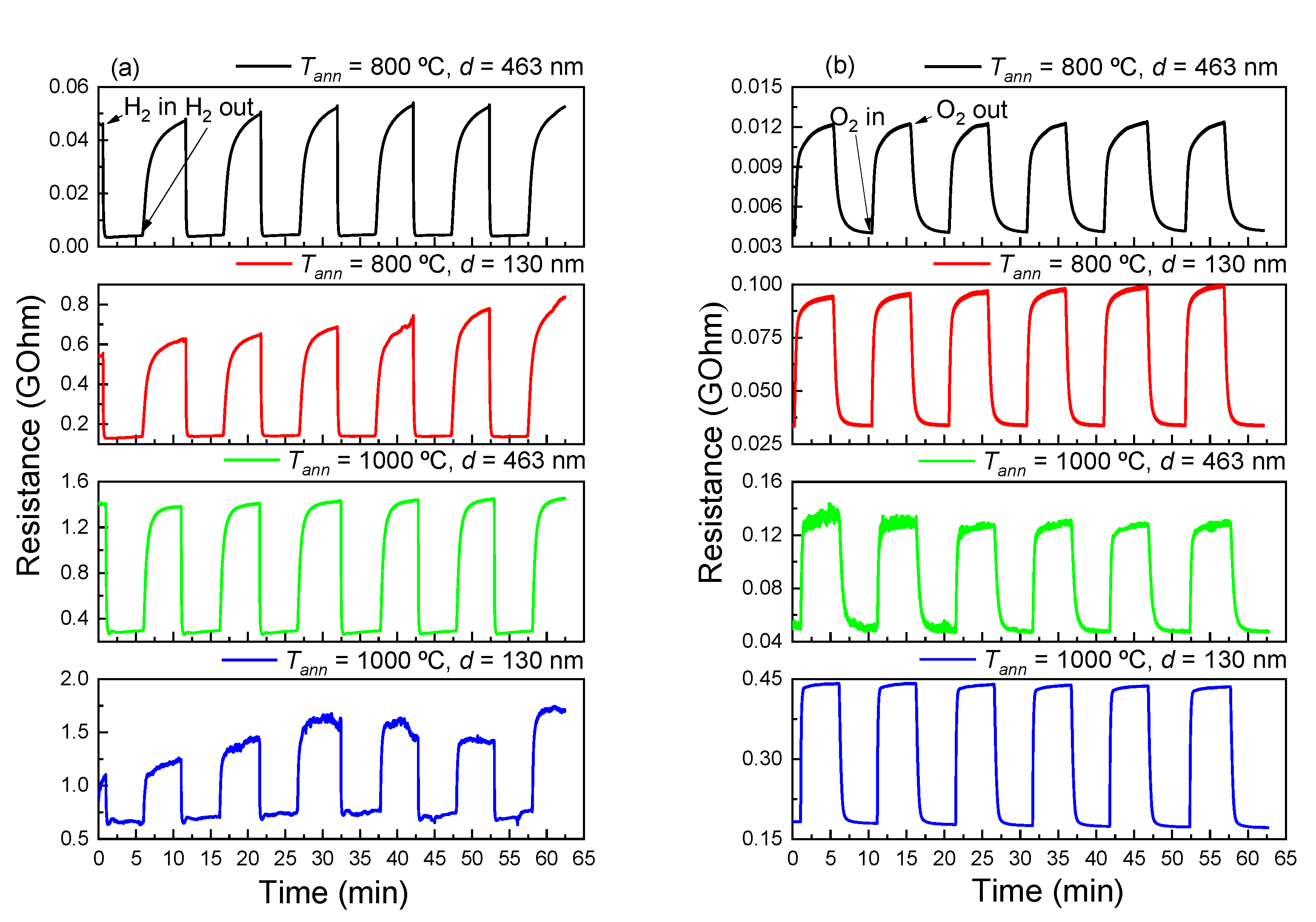
3.3. The Effect of Oxygen on the Electrically Conductive Properties of TiO2 Thin Films
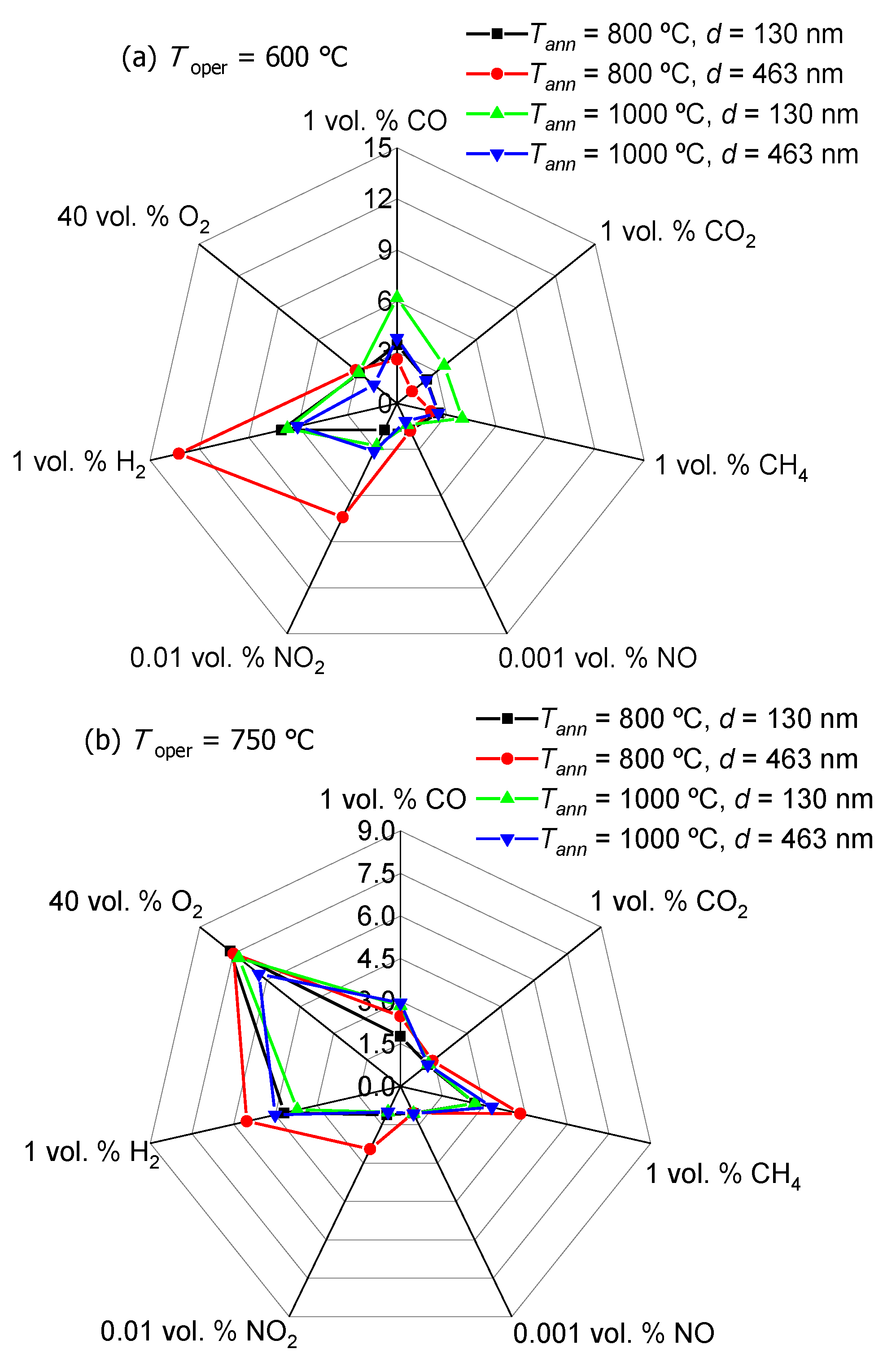
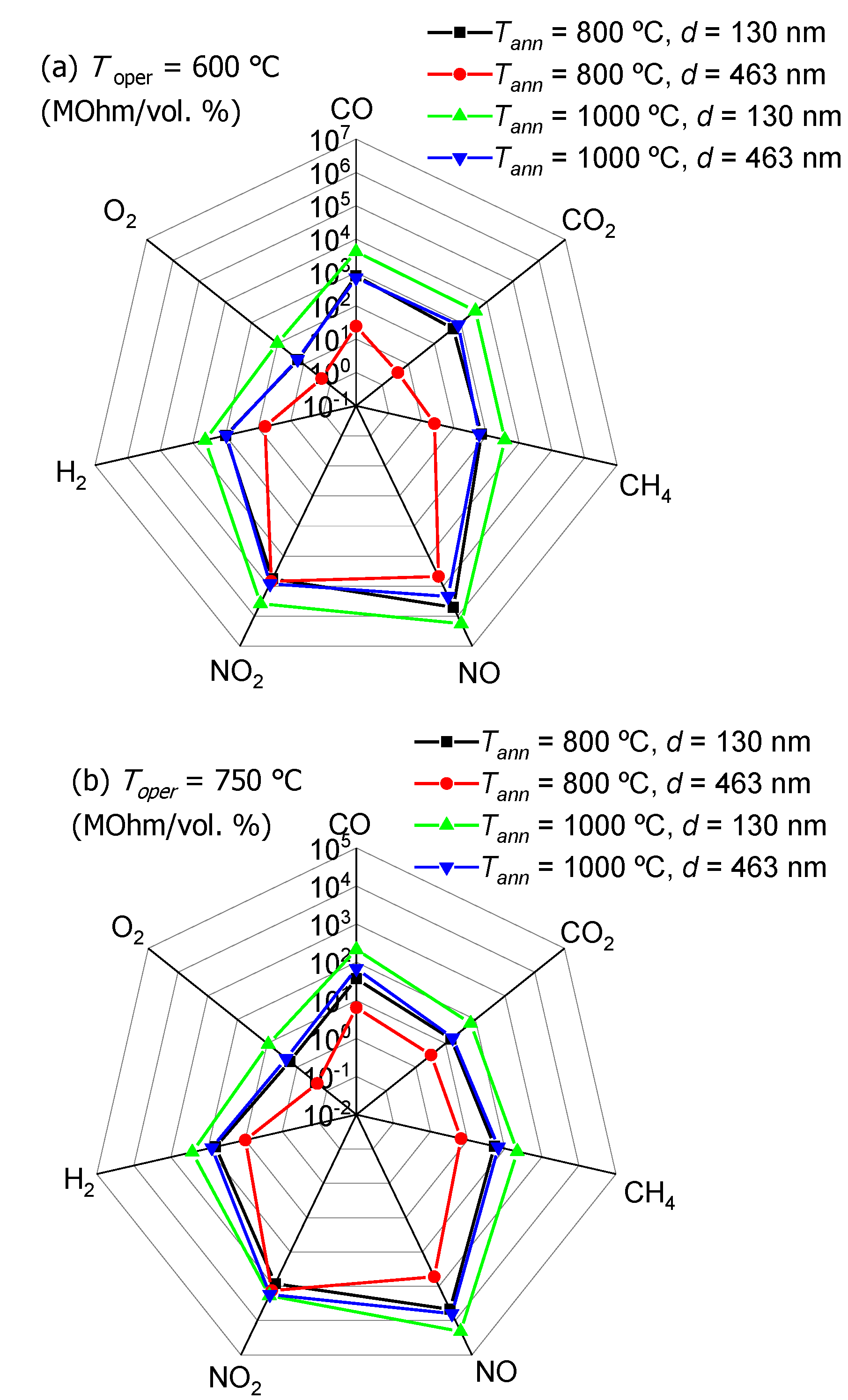
3.4. Sensitivity of TiO2 Thin Films to Reducing and Oxidizing Gases
β = (Rairst − Rgst)/ng for reducing gases,
β = (Rgst − Rairst)/ng for oxidizing gases.
3.5. Sensory Effect
CO + O−(c) → CO2 + e,
CH4 + 4O−(c) → CO2 + 2H2O + 4e.
NO2 + O−(c) → NO3−,
NO + Sa + e− → NO−,
NO + O−(c) → NO2−,
CO2 + Sa + e− → CO2−.
H + O2−(c) → OH−(c) + e;
OH−(c) → OH + e.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.; Wu, T.; Zhou, Y.; Meng, C.; Zhu, W.; Liu, L. TiO2-based nanoheterostructures for promoting gas sensitivity performance: Designs, developments, and prospects. Sensors 2017, 17, 1971. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Cui, X.; Lai, T.; Ren, J.; Yang, Z.; Xiao, M.; Wang, B.; Xiao, X.; Wang, Y. Gas sensors based on TiO2 nanostructured materials for the detection of hazardous gases: A review. Nano Mater. Sci. 2021, 3, 390–403. [Google Scholar] [CrossRef]
- Rzaij, J.; Abass, A. Review on: TiO2 thin film as a metal oxide gas sensor. J. Chem. Rev. 2020, 2, 114–121. [Google Scholar] [CrossRef] [Green Version]
- Larsson, H.; Yakimova, R.; Zolnai, Z.; Ivanov, I.; Monemar, B.; Gogova, D. Fast growth of high quality GaN. Phys. Status Solidi a 2003, 20, 13–17. [Google Scholar] [CrossRef]
- Almaev, A.; Nikolaev, V.; Butenko, P.; Stepanov, S.; Pechnikov, A.; Yakovlev, N.; Sinyugin, I.; Shapenkov, S.; Scheglov, M. Gas sensors based on pseudohexagonal phase of gallium oxide. Phys. Status Solidi b 2021, 259, 2100306. [Google Scholar] [CrossRef]
- Kachel, K.; Korytov, M.; Gogova, D.; Galazka, Z.; Albrecht, M.; Zwierz, R.; Siche, D.; Golka, S.; Kwasniewski, A.; Schmidbauer, M.; et al. A new approach to free-standing GaN using β-Ga2O3 as a substrate. CrystEngComm 2012, 14, 8536–8540. [Google Scholar] [CrossRef]
- Almaev, A.V.; Yakovlev, N.N.; Chernikov, E.V.; Tolbanov, O.P. Selective sensors of nitrogen dioxide based on thin tungsten oxide films under optical irradiation. Tech. Phys. Lett. 2019, 45, 1016–1019. [Google Scholar] [CrossRef]
- Ramanavicius, S.; Tereshchenko, A.; Karpicz, R.; Ratautaite, V.; Bubniene, U.; Maneikis, A.; Jagminas, A.; Ramanavicius, A. TiO2-x/TiO2-structure based ‘self-heated’ sensor for the determination of some reducing gases. Sensors 2020, 20, 74. [Google Scholar] [CrossRef] [Green Version]
- Zakrzewska, K.; Radecka, M. TiO2-based nanomaterials for gas sensing—influence of anatase and rutile contributions. Nanoscale Res. Lett. 2017, 12, 89. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Jun, Y.X.; Haidry, A.A.; Plecenik, T.; Juan, X.L.; Chao, S.L.; Fatima, Q. Resistive-type hydrogen gas sensor based on TiO2: A review. Int. J. Hydrogen Energy 2018, 43, 21114–21132. [Google Scholar] [CrossRef]
- Nunes, D.; Fortunato, E.; Martins, R. Flexible nanostructured TiO2-based gas and UV sensors: A review. Discov. Mater. 2022, 2, 2. [Google Scholar] [CrossRef]
- Li, T.; Rui, Y.; Zhang, X.; Shi, J.; Wang, X.; Wang, Y.; Yang, J.; Zhang, Q. Anatase TiO2 nanorod arrays as high-performance electron transport layers for perovskite solar cells. J. Alloys Compd. 2020, 849, 156629. [Google Scholar] [CrossRef]
- Illarionov, G.A.; Morozova, S.M.; Chrishtop, V.V.; Einarsrud, M.A.; Morozov, M.I. Memristive TiO2: Synthesis, technologies, and applications. Front. Chem. 2020, 8, 724. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Heo, Y.U.; Nattestad, A.; Sun, Z.; Wang, L.; Kim, J.H.; Dou, Z.X. 3D hierarchical rutile TiO2 and metal-free organic sensitizer producing dye-sensitized solar cells 8.6 % conversion efficiency. Sci. Rep. 2014, 4, 5769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, X.; Wu, M.; Ao, Z.; Lai, B.; Zhou, Y.; An, T.; Wang, S. Metal–organic frameworks derived C/TiO2 for visible light photocatalysis: Simple synthesis and contribution of carbon species. J. Hazard. Mater. 2021, 403, 124048. [Google Scholar] [CrossRef]
- Ratan, S.; Kumar, C.; Kumar, A.; Kumar, D.J.; Kumar, A.M.; Kumar, R.U.; Pratap, A.S.; Jit, S. Room temperature high hydrogen gas response in Pd/TiO2/Si/Al capacitive sensor. Micro Nano Lett. 2020, 15, 632–635. [Google Scholar] [CrossRef]
- Dwivedi, D.; Dwivedi, R.; Srivastava, S.K. The effect of hydrogen-induced interface traps on a titanium dioxide-based palladium gate MOS capacitor (Pd-MOSC): A conductance study. Microelectron. J. 1998, 29, 445–450. [Google Scholar] [CrossRef]
- Mardare, D.; Cornei, N.; Mita, C.; Florea, D.; Stancu, A.; Tiron, V.; Manole, A.; Adomnitei, C. Low temperature TiO2 based gas sensors for CO2. Ceram. Int. 2016, 42, 7353–7359. [Google Scholar] [CrossRef]
- Rydosz, A.; Brudnik, A.; Staszek, K. Metal oxide thin films prepared by magnetron sputtering technology for volatile organic compound detection in the microwave frequency range. Materials 2019, 12, 877. [Google Scholar] [CrossRef] [Green Version]
- Moumen, A.; Kumarage, G.C.W.; Comini, E. P-type metal oxide semiconductor thin films: Synthesis and chemical sensor applications. Sensors 2022, 22, 1359. [Google Scholar] [CrossRef]
- Nafarizal, N. Precise control of metal oxide thin films deposition in magnetron sputtering plasmas for high performance sensing devices fabrication. Procedia Chem. 2016, 20, 93–97. [Google Scholar] [CrossRef]
- Chaluvadi, S.K.; Mondal, D.; Bigi, C.; Knez, D.; Rajak, P.; Ciancio, R.; Fujii, J.; Panaccione, G.; Vobornik, I.; Rossi, G.; et al. Pulsed laser deposition of oxide and metallic thin films by means of Nd:YAG laser source operating at its 1st harmonics: Recent approaches and advances. J. Phys. Mater. 2021, 4, 032001. [Google Scholar] [CrossRef]
- Filipescu, M.; Papavlu, A.P.; Dinescu, M. Functional metal oxide thin films grown by pulsed laser deposition. In Crystalline and Non-Crystalline Solids; Mandracci, P., Ed.; IntechOpen: London, UK, 2016. [Google Scholar] [CrossRef] [Green Version]
- Huotari, J.; Kekkonen, V.; Puustinen, J.; Liimatainen, J.; Lappalainen, J. Pulsed laser deposition for improved metal-oxide gas sensing layers. Procedia Eng. 2016, 168, 1066–1069. [Google Scholar] [CrossRef]
- Huotari, J.; Lappalainen, J.; Puustinen, J.; Baur, T.; Alepee, C.; Haapalainen, T.; Komulainen, S.; Pylvanainen, J.; Lloyd, A.S. Pulsed laser deposition of metal oxide nanoparticles, agglomerates, and nanotrees for chemical sensors. Procedia Eng. 2015, 120, 1158–1161. [Google Scholar] [CrossRef] [Green Version]
- Hamid, N.; Suhaimi, S.; Othman, M.Z.; Ismail, W.Z.W. A Review on thermal evaporation method to synthesis zinc oxide as photocatalytic material. Nano Hybrids Compos. 2021, 31, 55–63. [Google Scholar] [CrossRef]
- Mukherjee, S. Thin film deposition from dual ion beam sputtering system. CSI Trans. ICT 2019, 7, 99–104. [Google Scholar] [CrossRef]
- Bundesmann, C.; Neumann, H. Tutorial: The systematics of ion beam sputtering for deposition of thin films with tailored properties. J. Appl. Phys. 2018, 124, 231102. [Google Scholar] [CrossRef]
- Bundesmann, C.; Amelal, T. Secondary particle properties for the ion beam sputtering of TiO2 in a reactive oxygen atmosphere. Appl. Surf. Sci. 2019, 485, 391–401. [Google Scholar] [CrossRef]
- Bundesmann, C.; Lautenschläger, T.; Spemann, D.; Finzel, A.; Thelander, E.; Mensing, M.; Frost, F. Systematic investigation of the properties of TiO2 films grown by reactive ion beam sputter deposition. Appl. Surf. Sci. 2017, 421, 331–340. [Google Scholar] [CrossRef]
- Bundesmann, C.; Lautenschläger, T.; Spemann, D.; Thelander, E. Reactive Ar ion beam sputter deposition of TiO2 films: Influence of process parameters on film properties. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2017, 395, 17–23. [Google Scholar] [CrossRef]
- Amelal, T.; Pietzonka, L.; Rohkamm, E.; Bundesmann, C. Properties of secondary particles for the reactive ion beam sputtering of Ti and TiO2 using oxygen ions. J. Vac. Sci. Technol. A 2020, 38, 033403. [Google Scholar] [CrossRef]
- Jung, H.; Min, H.; Hwang, J.; Kim, J.; Choe, Y.; Lee, H.; Lee, W. Selective detection of sub-1-ppb level isoprene using Pd-coated In2O3 thin film integrated in portable gas chromatography. Appl. Surf. Sci. 2022, 586, 152827. [Google Scholar] [CrossRef]
- Kalanov, D.; Unutulmazsoy, Y.; Spemann, D.; Bauer, J.; Anders, A.; Bundesmann, C. Properties of gallium oxide thin films grown by ion beam sputter deposition at room temperature. J. Vac. Sci. Technol. A 2022, 40, 033409. [Google Scholar] [CrossRef]
- Choe, Y. New gas sensing mechanism for SnO2 thin-film gas sensors fabricated by using dual ion beam sputtering. Sens. Actuators B Chem. 2001, 77, 200–208. [Google Scholar] [CrossRef]
- Min, B.K.; Choi, S.D. C4H10 sensing characteristics of ion beam sputtered SnO2 sensors. Sens. Actuators B Chem. 2005, 108, 125–129. [Google Scholar] [CrossRef]
- Min, B.K.; Choi, S.D. SnO2 thin film gas sensor fabricated by ion beam deposition. Sens. Actuators B Chem. 2004, 98, 239–246. [Google Scholar] [CrossRef]
- Prasad, A.K.; Kubinski, D.J.; Gouma, P.I. Comparison of sol–gel and ion beam deposited MoO3 thin film gas sensors for selective ammonia detection. Sens. Actuators B Chem. 2003, 93, 25–30. [Google Scholar] [CrossRef]
- Simion, C.E.; Schipani, F.; Papadogianni, A.; Stanoiu, A.; Budde, M.; Oprea, A.; Weimar, U.; Bierwagen, O.; Barsan, N. Conductance model for single-crystalline/compact metal oxide gas-sensing layers in the nondegenerate limit: Example of epitaxial SnO2 (101). ACS Sens. 2019, 4, 2420–2428. [Google Scholar] [CrossRef]
- Liu, Y.; Parisi, J.; Sun, X.; Lei, Y. Solid-state gas sensors for high temperature applications—A review. J. Mater. Chem. A 2014, 2, 9919. [Google Scholar] [CrossRef]
- Golubović, A.; Nikolić, S.; Djurić, S.; Valčić, A. The growth of sapphire single crystals. J. Serb. Chem. Soc. 2001, 66, 411–418. [Google Scholar] [CrossRef]
- Almaev, A.V.; Kushnarev, B.O.; Chernikov, E.V.; Novikov, V.A.; Korusenko, P.M.; Nesov, S.N. Structural, electrical and gas-sensitive properties of Cr2O3 thin films. Superlattices Microstruct. 2021, 151, 106835. [Google Scholar] [CrossRef]
- Qing, H.; Lian, G. A simple route for the synthesis of rutile TiO2 nanorods. Chem. Lett. 2003, 32, 638–639. [Google Scholar] [CrossRef]
- Wang, D.; Choi, D.; Yang, Z.; Viswanathan, V.V.; Nie, Z.; Wang, C.; Song, Y.; Zhang, J.-G.; Liu, J. Synthesis and Li-ion insertion properties of highly crystalline mesoporous rutile TiO2. Chem. Mater. 2008, 20, 3435–3442. [Google Scholar] [CrossRef]
- Abazović, N.D.; Čomor, M.I.; Dramićanin, M.D.; Jovanović, D.J.; Ahrenkiel, S.P.; Nedeljković, J.M. Photoluminescence of anatase and rutile TiO2 particles. J. Phys. Chem. B 2006, 110, 25366–25370. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Jin, P.; Kaneko, K.; Terai, A.; Nabatova-Gabain, N.; Tanemura, S. Preparation and characterization of polycrystalline anatase and rutile TiO2 thin films by rf magnetron sputtering. Appl. Surf. Sci. 2003, 212–213, 255–263. [Google Scholar] [CrossRef]
- Haidry, A.A.; Schlosser, P.; Durina, P.; Mikula, M.; Tomasek, M.; Plecenik, T.; Roch, T.; Pidik, A.; Stefecka, M.; Noskovic, J.; et al. Hydrogen gas sensors based on nanocrystalline TiO2 thin films. Cent. Eur. J. Phys. 2011, 9, 1351. [Google Scholar] [CrossRef]
- Nadzirah, S.; Hashim, U.; Kashif, M.; Shamsuddin, S.A. Stable electrical, morphological and optical properties of titanium dioxide nanoparticles affected by annealing temperature. Microsyst. Technol. 2017, 23, 1743–1750. [Google Scholar] [CrossRef]
- Soussi, A.; Ait Hssi, A.; Boujnah, M.; Boulkadat, L.; Abouabassi, K.; Asbayou, A.; Elfanaoui, A.; Markazi, R.; Ihlal, A.; Bouabid, K. Electronic and optical properties of TiO2 thin films: Combined experimental and theoretical study. J. Electron. Mater. 2021, 50, 4497–4510. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Brinzari, V.; Golovanov, V.; Blinov, Y. Kinetics of gas response to reducing gases of SnO2 films, deposited by spray pyrolysis. Sens. Actuators B Chem. 2004, 98, 41–45. [Google Scholar] [CrossRef]
- Weibel, A.; Bouchet, R.; Knauth, P. Electrical properties and defect chemistry of anatase (TiO2). Solid State Ion. 2006, 177, 229–236. [Google Scholar] [CrossRef] [Green Version]
- Nowotny, M.K.; Bak, T.; Nowotny, J. Electrical properties and defect chemistry of TiO2 single crystal. I. Electrical conductivity. J. Phys. Chem. B 2006, 110, 16270–16282. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Wu, C.; Tan, Y.; Chi, B.; Pu, J.; Jian, L. Oxygen sensing performance of Nb-doped TiO2 thin film with porous structure. J. Alloy. Compd. 2014, 585, 729–733. [Google Scholar] [CrossRef]
- Li, M.; Chen, Y. An investigation of response time of TiO2 thin-film oxygen sensors. Sens. Actuators B Chem. 1996, 32, 83–85. [Google Scholar] [CrossRef]
- Kirner, U.; Schierbaum, K.D.; Göpel, W.; Leibold, B.; Nicoloso, N.; Weppner, W.; Fischer, D.; Chu, W.F. Low and high temperature TiO2 oxygen sensors. Sens. Actuators B Chem. 1990, 1, 103–107. [Google Scholar] [CrossRef]
- Bartic, M.; Toyoda, Y.; Baban, C.; Ogita, M. Oxygen sensitivity in gallium oxide thin films and single crystals at high temperatures. Jpn. J. Appl. Phys. 2006, 45, 5186. [Google Scholar] [CrossRef]
- Maksimova, N.K.; Almaev, A.V.; Sevastyanov, E.Y.; Potekaev, A.I.; Chernikov, E.V.; Sergeychenko, N.V.; Korusenko, P.M.; Nesov, S.N. Effect of additives Ag and rare-earth elements Y and Sc on the properties of hydrogen sensors based on thin SnO2 films during long-term testing. Coatings 2019, 9, 423. [Google Scholar] [CrossRef] [Green Version]
- Lu, C.; Huang, Y.; Huang, J.; Chang, C.; Wu, S. A Macroporous TiO2 oxygen sensor fabricated using anodic aluminium oxide as an etching mask. Sensors 2010, 10, 670–683. [Google Scholar] [CrossRef]
- Wang, Y.; Lai, X.; Liu, B.; Chen, Y.; Lu, Y.; Wang, F.; Zhang, L. UV-induced desorption of oxygen at the TiO2 surface for highly sensitive room temperature O2 sensing. J. Alloy. Compd. 2019, 793, 583–589. [Google Scholar] [CrossRef]
- Haidry, A.A.; Xie, L.; Wang, Z.; Zavabeti, A.; Li, Z.; Plecenik, T.; Gregor, M.; Roch, T.; Plecenik, A. Remarkable improvement in hydrogen sensing characteristics with Pt/TiO2 interface control. ACS Sens. 2019, 4, 2997–3006. [Google Scholar] [CrossRef]
- Kumar, M.; Singh Bhati, V.; Kumar, M. Effect of Schottky barrier height on hydrogen gas sensitivity of metal/TiO2 nanoplates. Int. J. Hydrog. Energy 2017, 42, 22082–22089. [Google Scholar] [CrossRef]
- Arifin, P.; Mustajab, M.A.; Haryono, S.; Adhika, D.R.; Nugraha, A.A. MOCVD growth and characterization of TiO2 thin films for hydrogen gas sensor application. Mater. Res. Express 2019, 6, 076313. [Google Scholar] [CrossRef]
- Lu, C.; Chen, Z. High-temperature resistive hydrogen sensor based on thin nanoporous rutile TiO2 film on anodic aluminum oxide. Sens. Actuators B Chem. 2009, 140, 109–115. [Google Scholar] [CrossRef]
- Samransuksamer, B.; Jutarosaga, T.; Horprathum, M.; Wisitsoraat, A.; Eiamchai, P.; Limwichean, S.; Patthanasettakul, V.; Chananonnawathorn, C.; Chindaudom, P. Highly sensitive H2 sensors based on Pd- and PdO-decorated TiO2 thin films at low-temperature operation. Key Eng. Mater. 2016, 675–676, 277–280. [Google Scholar] [CrossRef]
- Xie, T.; Sullivan, N.; Steffens, K.; Wen, B.; Liu, G.; Debnath, R.; Davydov, A.; Gomez, R.; Motayed, A. UV-assisted room-temperature chemiresistive NO2 sensor based on TiO2 thin film. J. Alloy. Compd. 2015, 653, 255–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boyadjiev, S.; Georgieva, V.; Vergov, L.; Baji, Z.; Gáber, F.; Szilágyi, I.M. Gas sensing properties of very thin TiO2 films prepared by atomic layer deposition (ALD). J. Phys. Conf. Ser. 2014, 559, 012013. [Google Scholar] [CrossRef]
- Rumyantseva, M.N.; Makeeva, E.A.; Gaskov, A.M. Influence of the microstructure of semiconductor sensor materials on oxygen chemisorption on their surface. Russ. J. Gen. Chem. 2008, 78, 2556–2565. [Google Scholar] [CrossRef]
- Almaev, A.V.; Chernikov, E.V.; Novikov, V.V.; Kushnarev, B.O.; Yakovlev, N.N.; Chuprakova, E.V.; Oleinik, V.L.; Lozinskaya, A.D.; Gogova, D.S. Impact of Cr2O3 additives on the gas-sensitive properties of β-Ga2O3 thin films to oxygen, hydrogen, carbon monoxide, and toluene vapors. J. Vac. Sci. Technol. A 2021, 39, 023405. [Google Scholar] [CrossRef]
- Saruhan, B.; Fomekong, L.R.; Nahirniak, S. Review: Influences of semiconductor metal oxide properties on gas sensing characteristics. Front. Sens. 2021, 2, 657931. [Google Scholar] [CrossRef]
- Badalyan, S.M.; Rumyantseva, M.N.; Smirnov, V.V.; Alikhanyan, A.S.; Gaskov, A.M. Effect of Au and NiO catalysts on the NO2 sensing properties of nanocrystalline SnO2. Inorg. Mater. 2010, 46, 232–236. [Google Scholar] [CrossRef]
- Gautam, Y.K.; Sharma, K.; Tyagi, S.; Ambedkar, A.K.; Chaudhary, M. Nanostructured metal oxide semiconductor-based sensors for greenhouse gas detection: Progress and challenges. R. Soc. Open Sci. 2021, 8, 201324. [Google Scholar] [CrossRef]
- Jiménez-Cadena, G.; Riu, J.; Rius, F.X. Gas sensors based on nanostructured materials. Analyst 2007, 132, 1083–1099. [Google Scholar] [CrossRef]
- Yamazoe, N.; Fuchigami, J.; Kishikawa, M.; Seiyama, T. Interactions of tin oxide surface with O2, H2O and H2. Surf. Sci. 1979, 86, 335–344. [Google Scholar] [CrossRef]
- Yamazoe, N.; Sakai, G.; Shimanoe, K. Oxide semiconductor gas sensors. Catal. Surv. Asia 2003, 7, 63–75. [Google Scholar] [CrossRef]
- Gaman, V.I.; Almaev, A.V. Dependences of characteristics of sensors based on tin dioxide on the hydrogen concentration and humidity of gas mixture. Russ. Phys. J. 2017, 60, 90–100. [Google Scholar] [CrossRef]



| MOS | d (nm) | Gas | ng (vol. %) | TMAX (°C) | S | Ref. |
|---|---|---|---|---|---|---|
| In2O3 | 40 | Isoprene | 25 × 10−4 | 432 | 1.02 | [33] |
| Au/In2O3 | 364 | 2.82 | ||||
| Pt/In2O3 | 255 | 2.51 | ||||
| Pd/In2O3 | 196 | 6.31 | ||||
| SnO2 | 10 | H2 | 0.1 | 350 | 20 | [35] |
| 20 | 25 | |||||
| 50 | 60 | |||||
| 100 | 70 | |||||
| 200 | 30 | |||||
| 500 | 20 | |||||
| SnO2 | 50 | C4H10 | 0.5 | 400 | 4 | [36] |
| Pt/SnO2 | 4.5 | |||||
| SnO2:Ca | 2.5 | |||||
| Pt/SnO2:Ca | 2.3 | |||||
| SnO2 | 50 | CH4 | 0.5 | 400 | 1.75 | [37] |
| SnO2:Ca | 1.4 | |||||
| MoO3 | - | NH3 | 0.01 | 450 | 4 | [38] |
| d (nm) | Tann (℃) | Da (nm) | Dg (nm) |
|---|---|---|---|
| 130 | 0 | - | 10–30 |
| 800 | 320–550 | 100 | |
| 1000 | 500–700 | 125 | |
| 463 | 0 | - | 10–30 |
| 800 | 350–600 | 100–110 | |
| 1000 | 600–800 | 150 |
| Tann (℃) | d (nm) | tres (s) | trec (s) |
|---|---|---|---|
| 800 | 130 | 35.0 | 85.3 |
| 463 | 54.7 | 85.1 | |
| 1000 | 130 | 12.6 | 47.8 |
| 463 | 23.9 | 92.6 |
| Tann (°C) | d (nm) | l |
|---|---|---|
| 800 | 130 | 0.227 ± 0.004 |
| 463 | 0.235 ± 0.006 | |
| 1000 | 130 | 0.195 ± 0.003 |
| 463 | 0.212 ± 0.002 |
| Methods | d (nm) | ng (vol. %) | Toper (°C) | S | Ref. |
|---|---|---|---|---|---|
| O2 | |||||
| RFMS | 50 | 0.6 | 500 | 1.14 | [58] |
| DCMS | 60 | 10 | RT | 76 | [59] |
| IBSD | 130 | 40 | 750 | 7.64 | This work |
| H2 | |||||
| DCMS | 50 | 1 | 450 | ~104 | [47] |
| DCMS | 160 | 1 | 120 | 107 | [60] |
| RFMS | ~300 | 1 | 175 | 1.8 | [61] |
| MOCVD | 71 | 1 | 100 | 1.3 | [62] |
| EBE + oxidation | 25 | 0.05 | 500 | 91 | [63] |
| DCMS | 400 | 3 | 150 | 1.1 × 104 | [64] |
| IBSD | 463 | 1 | 600 | 13.25 | This work |
| NO2 | |||||
| RFMS | 10 | 0.05 | RT | 1.021 | [65] |
| ALD | 8 | 0.1 | RT | 7.5 × 10−7 * | [66] |
| IBSD | 463 | 0.01 | 600 | 7.41 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almaev, A.V.; Yakovlev, N.N.; Kushnarev, B.O.; Kopyev, V.V.; Novikov, V.A.; Zinoviev, M.M.; Yudin, N.N.; Podzivalov, S.N.; Erzakova, N.N.; Chikiryaka, A.V.; et al. Gas Sensitivity of IBSD Deposited TiO2 Thin Films. Coatings 2022, 12, 1565. https://doi.org/10.3390/coatings12101565
Almaev AV, Yakovlev NN, Kushnarev BO, Kopyev VV, Novikov VA, Zinoviev MM, Yudin NN, Podzivalov SN, Erzakova NN, Chikiryaka AV, et al. Gas Sensitivity of IBSD Deposited TiO2 Thin Films. Coatings. 2022; 12(10):1565. https://doi.org/10.3390/coatings12101565
Chicago/Turabian StyleAlmaev, Aleksei V., Nikita N. Yakovlev, Bogdan O. Kushnarev, Viktor V. Kopyev, Vadim A. Novikov, Mikhail M. Zinoviev, Nikolay N. Yudin, Sergey N. Podzivalov, Nadezhda N. Erzakova, Andrei V. Chikiryaka, and et al. 2022. "Gas Sensitivity of IBSD Deposited TiO2 Thin Films" Coatings 12, no. 10: 1565. https://doi.org/10.3390/coatings12101565







