High Temperature Corrosion Behaviour of Aluminide-Coated Cast Iron for an Exhaust Manifold Application
Abstract
:1. Introduction
2. Materials and Methods
3. Results
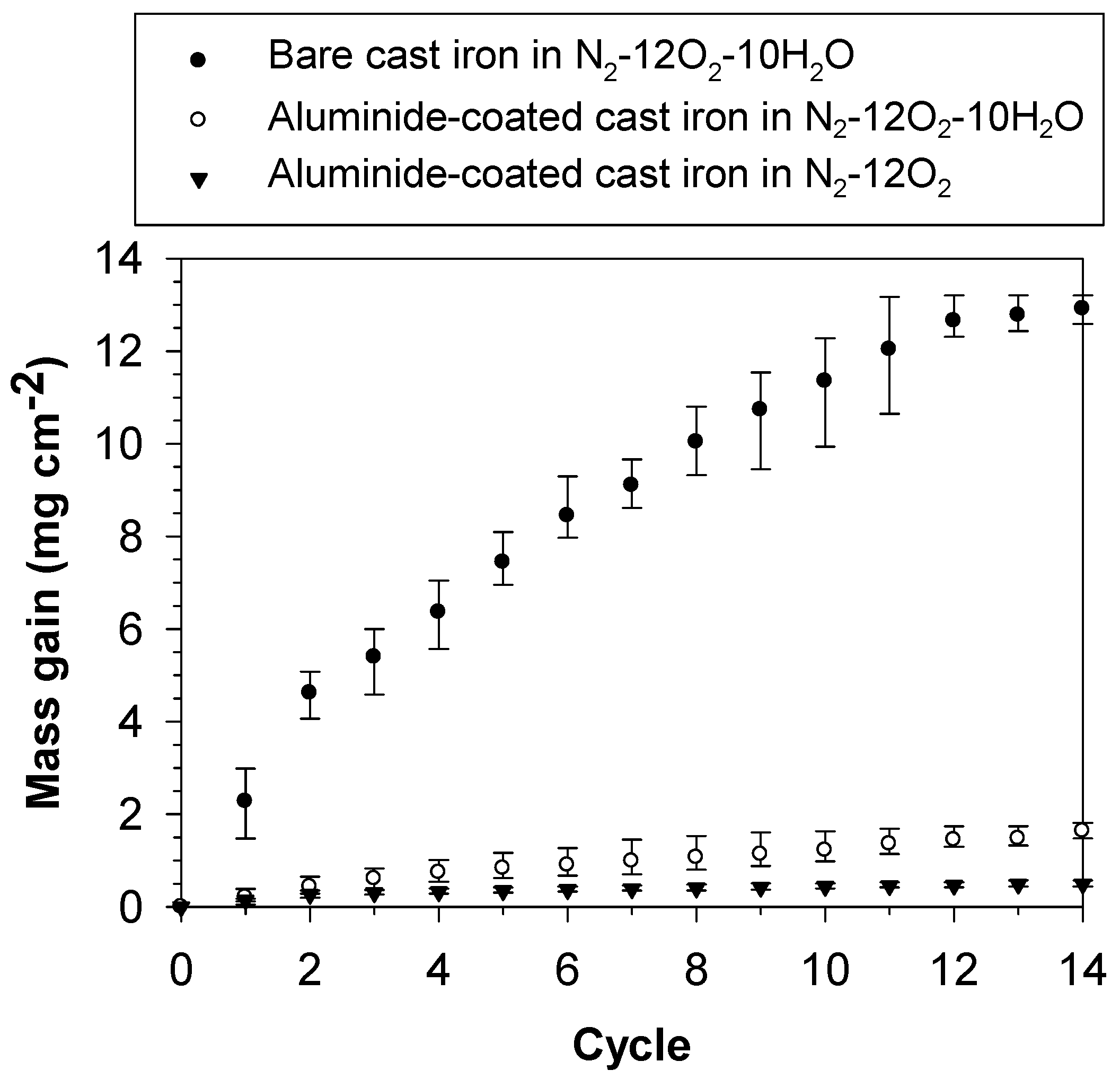
3.1. Mass Gain of the Bare and Aluminide-Coated Cast Irons after Oxidation
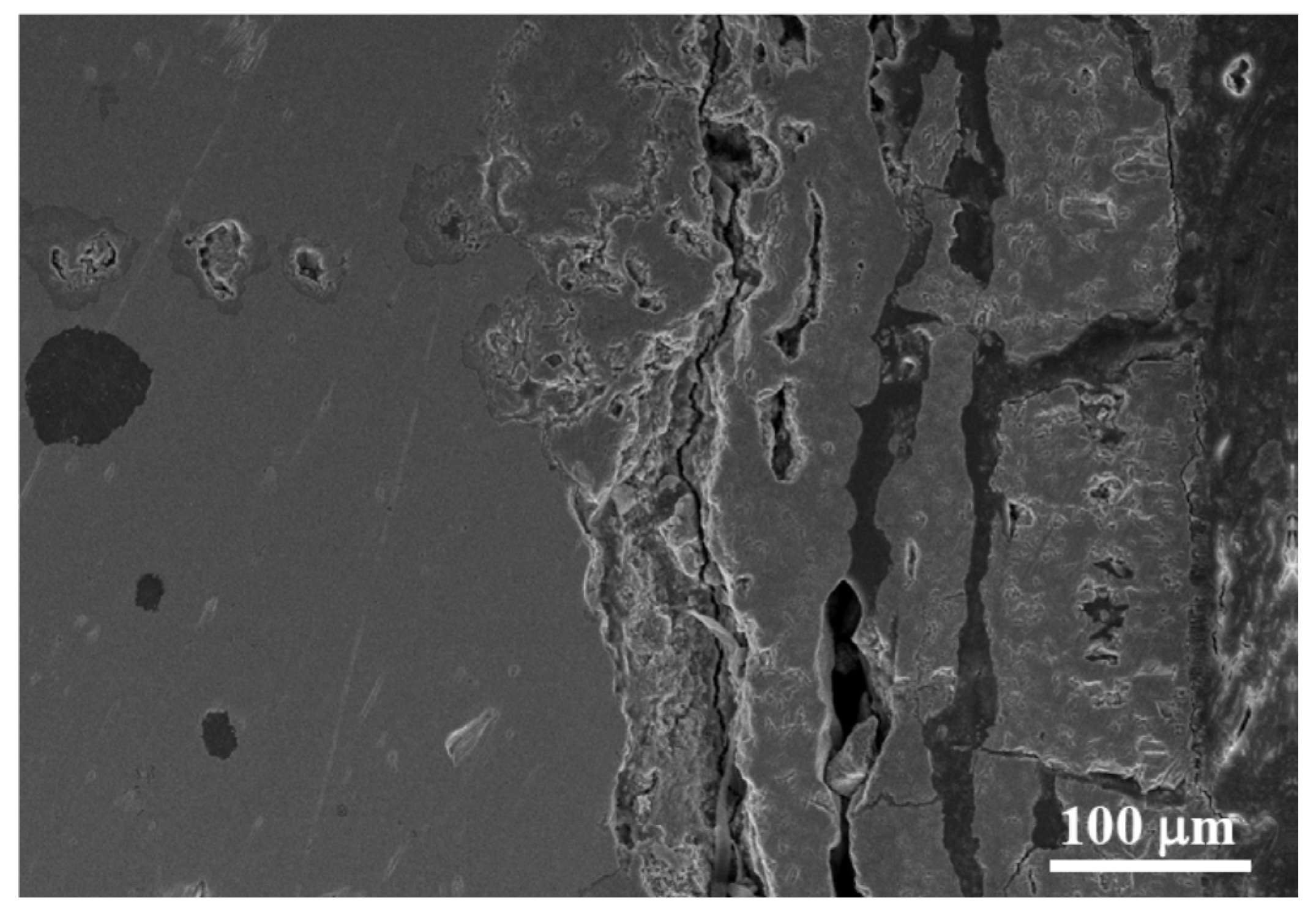
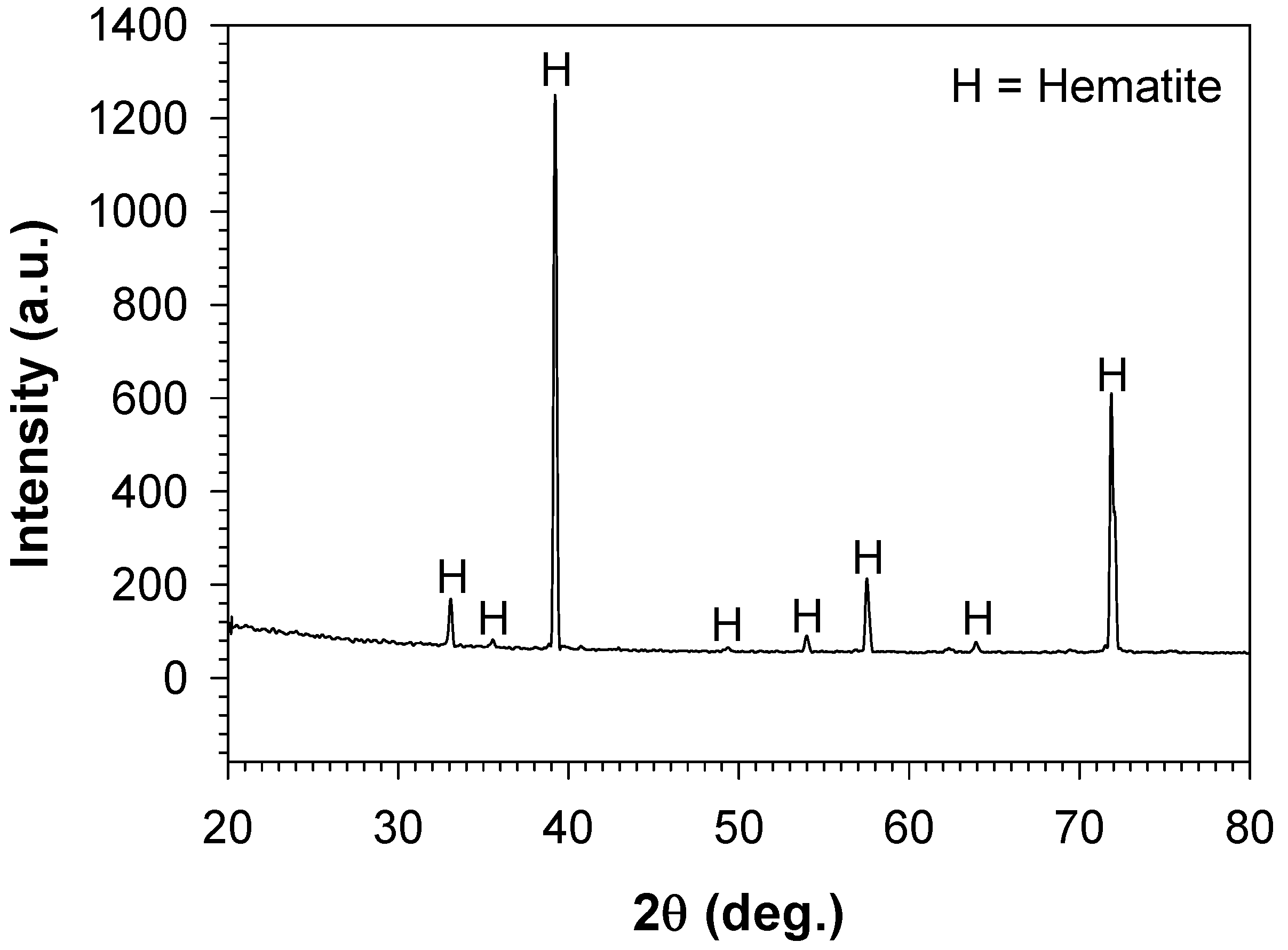
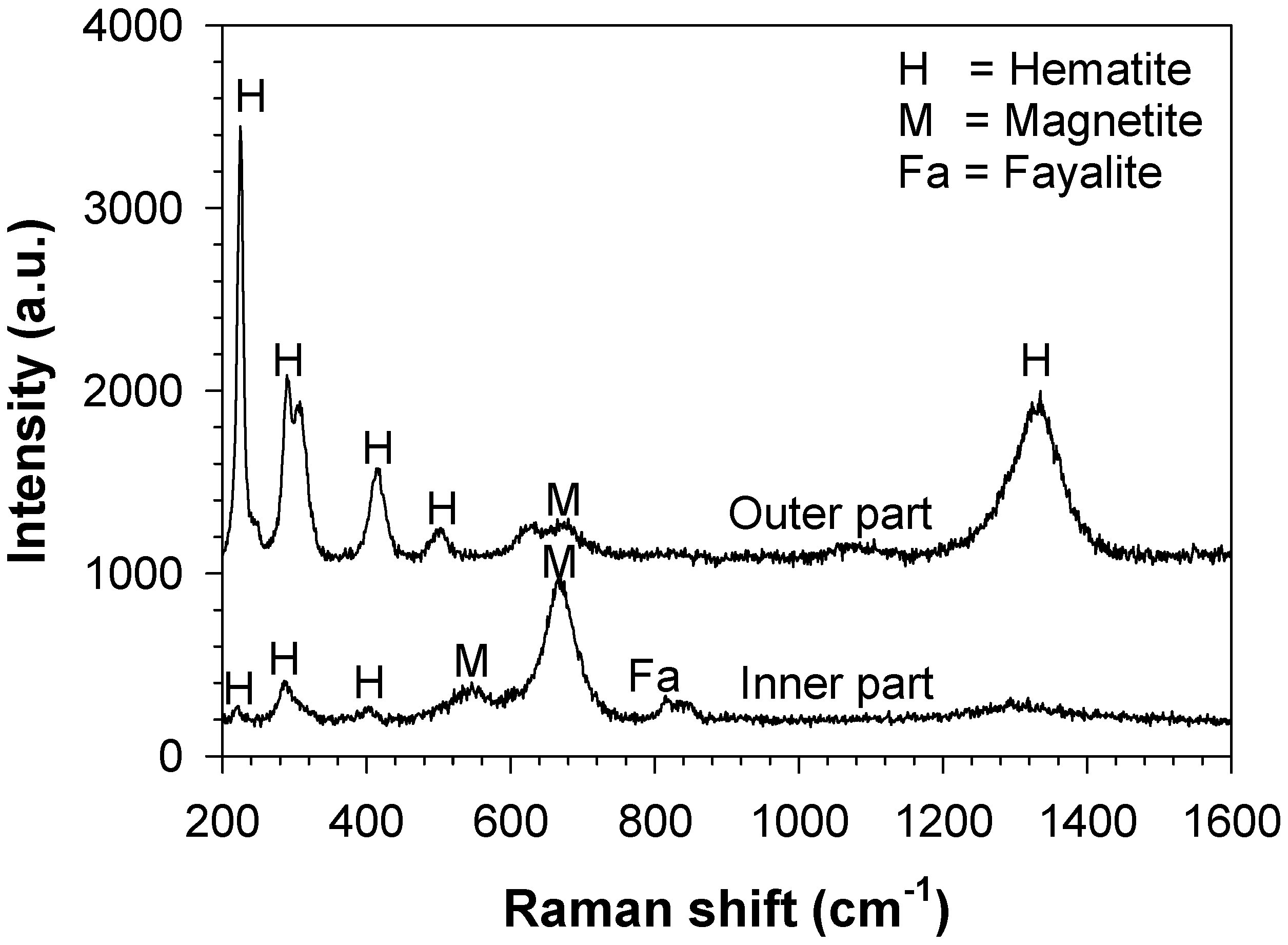
3.2. Characterisation of the Bare Cast Iron after Oxidation
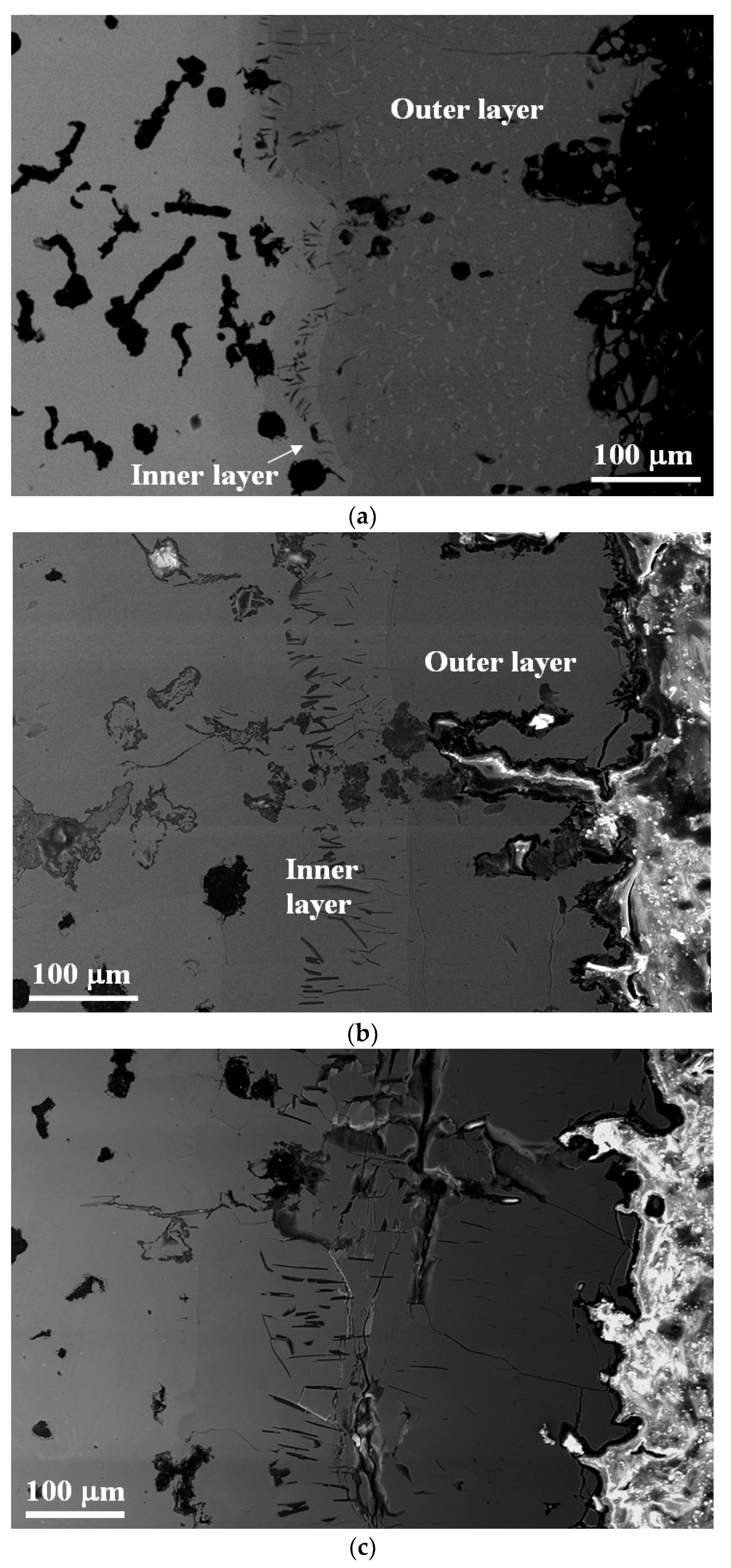
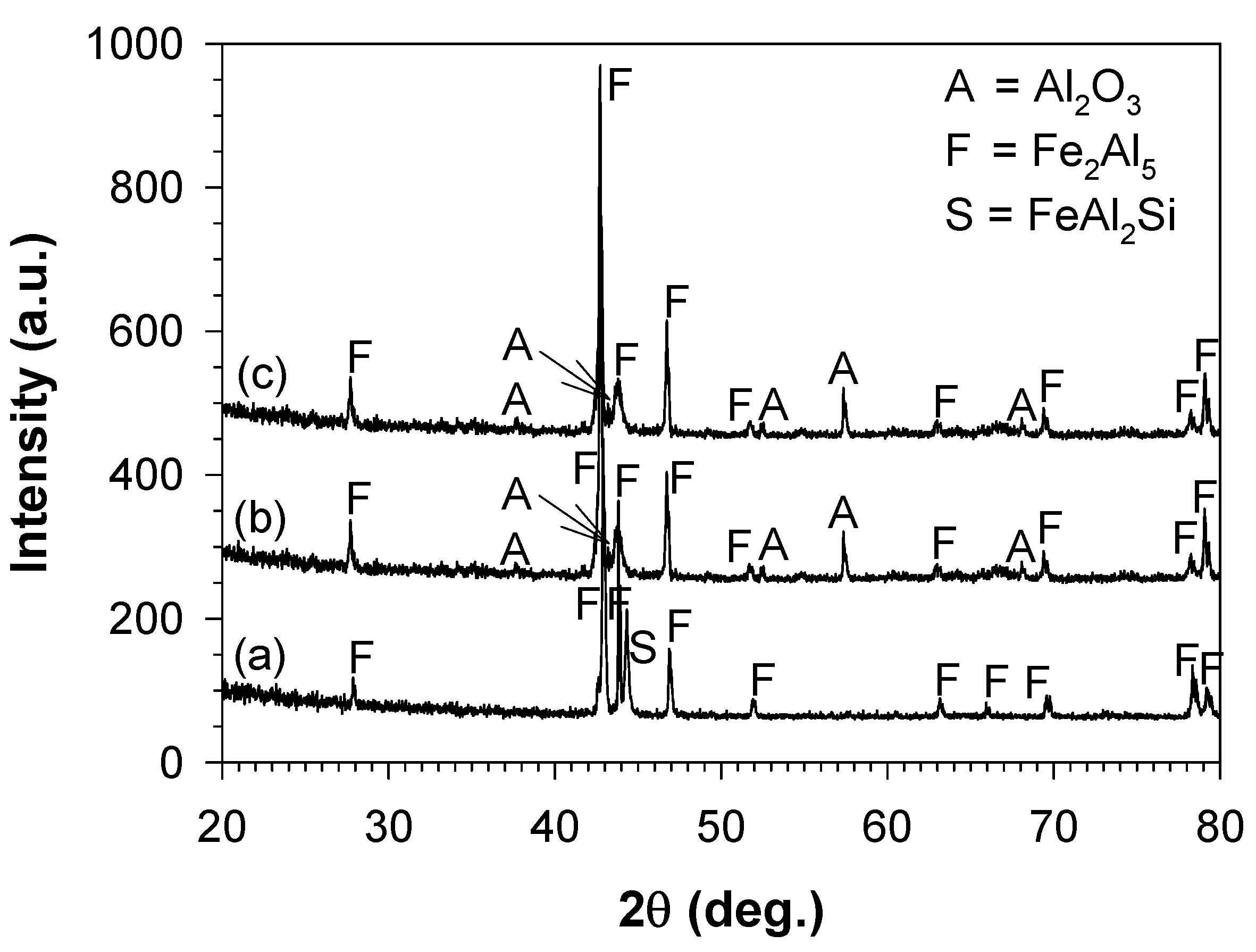
3.3. Characterisation of the Aluminide-Coated Cast Iron before Oxidation
3.4. Characterisation of the Aluminide-Coated Cast Iron after Oxidation
4. Discussion
4.1. Oxidation Kinetics
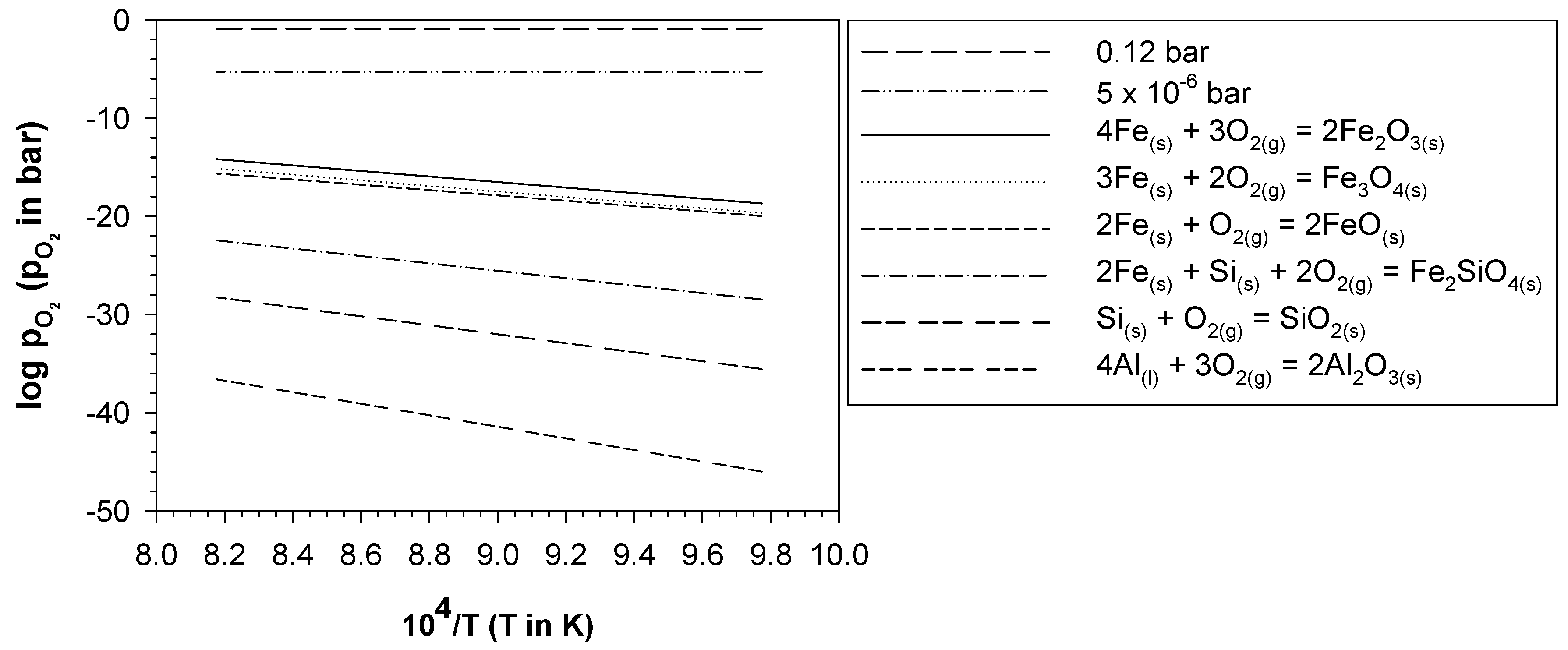
4.2. Formation of Thermal Oxide Scales on the Bare Cast Iron
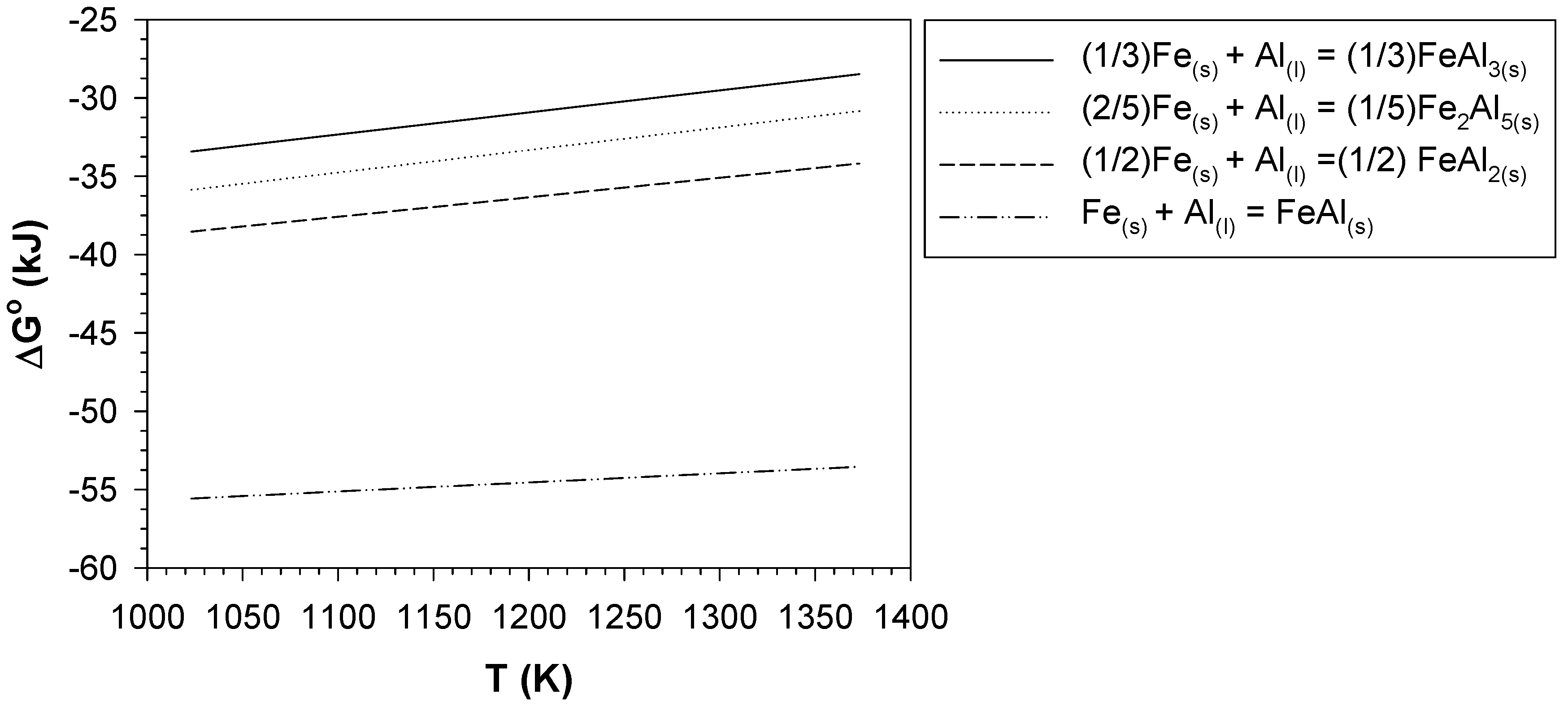
4.3. Formation of the Aluminide Layer after Aluminising
4.4. Evolution of the Aluminide Layer during Oxidation
5. Conclusions
- The cyclic oxidation kinetics of the bare cast iron in N2–12O2–10H2O and the aluminide-coated cast iron in N2–12O2–10H2O and N2–12O2 at 850 °C were parabolic, indicating that their oxidation rates reduce when the period of oxidation is longer. Furthermore, aluminising can reduce the oxidation rate of the studied cast iron in both dry and humidified atmospheres.
- The presence of water vapour in the atmosphere increased the oxidation rate of the aluminide-coated cast iron and helped thicken the aluminide layer. The higher hardness value of the coated sample oxidised in humidified atmosphere was also observed. This indicated the possible dissolution of hydrogen in the aluminide layer, which related to the enhanced oxidation rate and the thickening of the aluminide layer.
Author Contributions
Funding
Conflicts of Interest
References
- Tholence, F.; Norell, M. High Temperature Corrosion of Cast Alloys in Exhaust Environments I-Ductile Cast Irons. Oxid. Met. 2007, 69, 13–36. [Google Scholar] [CrossRef]
- Brady, M.P.; Muralidharan, G.; Leonard, D.N.; Haynes, J.A.; Weldon, R.G.; England, R.D. Long-term oxidation of candidate cast iron and stainless steel exhaust system alloys from 650 to 800 °C in air with water vapour. Oxid. Met. 2014, 82, 359–381. [Google Scholar] [CrossRef]
- Pedrazzini, S.; Kiseeva, K.; Escoube, R.; Gardner, H.M.; Douglas, J.O.; Radecka, A.; Mignanelli, P.M.; Hughes, G.M.; Chapman, G.; Edmondson, P.D.; et al. In-Service Oxidation and Microstructural Evolution of a Nickel Superalloy in a Formula 1 Car Exhaust. Oxid. Met. 2017, 89, 375–394. [Google Scholar] [CrossRef] [Green Version]
- Yamagato, H. The Science and Technology of Materials in Automotive Engines; Woodhead Publishing Limited: Cambridge, UK, 2005; pp. 248–260. [Google Scholar]
- Al-Arkawazi, S.A.F. Analyzing and predicting the relation between air–fuel ratio (AFR), lambda (λ) and the exhaust emissions percentages and values of gasoline-fueled vehicles using versatile and portable emissions measurement system tool. SN Appl. Sci. 2019, 1, 1370. [Google Scholar] [CrossRef] [Green Version]
- Dawi, K.; Favergeon, J.; Moulin, G. High Temperature Corrosion of the Si-Mo Cast Iron in Exhaust Atmosphere. Mater. Sci. Forum 2008, 595, 743–751. [Google Scholar] [CrossRef]
- Lin, M.-B.; Wang, C.-J.; Volinsky, A.A. High Temperature Oxidation Behavior of Flake and Spheroidal Graphite Cast Irons. Oxid. Met. 2011, 76, 161–168. [Google Scholar] [CrossRef]
- Wang, X.; Fan, Y.; Zhao, X.; Du, A.; Ma, R.; Cao, X. Process and High-Temperature Oxidation Resistance of Pack-Aluminized Layers on Cast Iron. Mettals 2019, 9, 648. [Google Scholar] [CrossRef] [Green Version]
- Ekström, M.; Szakálos, P.; Jonsson, S. Influence of Cr and Ni on High-Temperature Corrosion Behavior of Ferritic Ductile Cast Iron in Air and Exhaust Gases. Oxid. Met. 2013, 80, 455–466. [Google Scholar] [CrossRef]
- Ekström, M. Oxidation and Corrosion Fatigue Aspects of Cast Exhaust Manifold. Ph.D. Thesis, KTH Royal Institute of Technology, Stockholm, Sweden, 2015. [Google Scholar]
- Ebel, A.; Brou, S.Y.; Malard, B.; Lacaze, J.; Monceau, D.; Vaissière, L. High-Temperature Oxidation of a High Silicon SiMo Spheroidal Cast Iron in Air with In Situ Change in H2O Content. Mater. Sci. Forum 2018, 925, 353–360. [Google Scholar] [CrossRef] [Green Version]
- Tholence, F.; Norell, M. High Temperature Corrosion of Cast Alloys in Exhaust Environments. II—Cast Stainless Steels. Oxid. Met. 2007, 69, 37–62. [Google Scholar] [CrossRef]
- Inoue, Y.; Kikuchi, M. Present and future trends of stainless steel for automotive exhaust system. High-Temperature 2003, 950, 750. [Google Scholar]
- Zheng, L.; Peng, X.; Wang, F. Comparison of the dry and wet oxidation at 900 °C of η-Fe2Al5 and δ-Ni2Al3 coatings. Corros. Sci. 2011, 53, 597–603. [Google Scholar] [CrossRef]
- Agüero, A.; Baraibar, I.; González, V.; Muelas, R.; Plana, D. Corrosion resistance of novel coatings on ferritic seels for oxycombustuin-supercritical steam boilers: Preliminary results. Oxid. Met. 2016, 85, 263–281. [Google Scholar] [CrossRef]
- Agüero, A.; Gutiérrez, M.; Muelas, R. Aluminum Solid-Solution Coating for High-Temperature Corrosion Protection. Oxid. Met. 2017, 88, 145–154. [Google Scholar] [CrossRef]
- Houngninou, C.; Chevalier, S.; Larpin, J. Synthesis and characterisation of pack cemented aluminide coatings on metals. Appl. Surf. Sci. 2004, 236, 256–269. [Google Scholar] [CrossRef]
- Mao, S.-W.; Huang, H.-L.; Gan, D. Microstructure and aluminized stainless steel 310 after annealing. Surf. Coat. Technol. 2010, 205, 533–539. [Google Scholar] [CrossRef]
- Shirvani, K.; Firouzi, S.; Rashidghamat, A. Microstructures and cyclic oxidation behaviour of Pt-free and low-Pt NiAl coatings on the Ni-base superalloy Rene-80. Corros. Sci. 2012, 55, 378–384. [Google Scholar] [CrossRef]
- Mollard, M.; Rannou, B.; Bouchaud, B.; Balmain, J.; Bonnet, G.; Pedraza, F. Comparative degradation of nickel aluminized by slurry and by pack cementation under isothermal conditions. Corros. Sci. 2013, 66, 118–124. [Google Scholar] [CrossRef]
- Dutta, R.S.; Banerjee, R.H.; Dey, G.K. An XPS Study with Depth Profiling for the Surface Oxide Layer Formed on Aluminides Produced on Superalloy 690 Substrates. Oxid. Met. 2017, 89, 699–711. [Google Scholar] [CrossRef]
- El-Helaly, M.; Elzomor, M.A.; Ahmed, M.H.; Youssef, A.O. Effect of Zirconium Addition on High-Temperature Cyclic Oxidation of Diffusion Chromo-Aluminized Ni-Base Superalloy. Oxid. Met. 2018, 91, 159–175. [Google Scholar] [CrossRef]
- Nowak, W.J.; Tomków, M.; Wierzba, P.; Gancarczyk, K.; Wierzba, B. The Role of Substrate Surface Roughness on in-Pack Aluminization Kinetics of Ni-Base Superalloy. J. Manuf. Mater. Process. 2020, 4, 15. [Google Scholar] [CrossRef] [Green Version]
- Tuck, C.; Odgers, M.; Sachs, K. The oxidation of iron at 950°C in oxygen/water vapour mixtures. Corros. Sci. 1969, 9, 271–285. [Google Scholar] [CrossRef]
- Wouters, Y.; Galerie, A.; Petit, J.-P. Thermal oxidation of titanium by water vapour. Solid State Ion. 1997, 104, 89–96. [Google Scholar] [CrossRef]
- Galerie, A.; Wouters, Y.; Caillet, M. The Kinetic Behaviour of Metals in Water Vapour at High Temperatures: Can General Rules Be Proposed? Mater. Sci. Forum 2001, 369, 231–238. [Google Scholar] [CrossRef]
- Saunders, S.; Monteiro, M.; Rizzo, F. The oxidation behaviour of metals and alloys at high temperatures in atmospheres containing water vapour: A review. Prog. Mater. Sci. 2008, 53, 775–837. [Google Scholar] [CrossRef]
- Young, D.J.; Zurek, J.; Singheiser, L.; Quadakkers, W.J. Temperature dependence of oxide scale formation on high-Cr ferritic steels in Ar–H2–H2O. Corros. Sci. 2011, 53, 2131–2141. [Google Scholar] [CrossRef]
- Chandra-ambhorn, S.; Ngamkham, K.; Jiratthanakul, N. Effects of Process Parameters on Mechanical Adhesion of Thermal Oxide Scales on Hot-Rolled Low Carbon Steels. Oxid. Met. 2013, 80, 61–72. [Google Scholar] [CrossRef]
- Chandra-ambhorn, S.; Nilsonthi, T.; Wouters, Y.; Galerie, A. Oxidation of simulated recycled steels with 0.23 and 1.03 wt.% Si in Ar–20%H2O at 900 °C. Corros. Sci. 2014, 87, 101–110. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Zhang, J.; Young, D.J. Water vapor effect of Fe–Cr and Fe–Cr–Ni alloys containing cerium and manganese in CO2 gas at 818 °C. Corros. Sci. 2014, 89, 220–235. [Google Scholar] [CrossRef]
- Zhang, Y.; Pint, B.A.; Haynes, J.A.; Tortorelli, P.F. The Effect of Water Vapor on the Oxidation Behavior of CVD Iron-Aluminide Coatings. Oxid. Met. 2004, 62, 103–120. [Google Scholar] [CrossRef]
- Wang, C.J.; Badaruddin, M.; Badaruddin, M. The dependence of high temperature resistance of aluminized steel exposed to water-vapour oxidation. Surf. Coat. Technol. 2010, 205, 1200–1205. [Google Scholar] [CrossRef]
- Xing, L.; Zheng, Y.; Cui, L.; Sun, M.; Shao, M.; Lu, G. Influence of water vapor on the oxidation behavior of aluminized coatings under low oxygen partial pressure. Corros. Sci. 2011, 53, 3978–3982. [Google Scholar] [CrossRef]
- Callister, W.D.; Rethwisch, D.G. Fundamentals of Materials Science and Engineering: An Integrated Approach, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2012; p. 552. [Google Scholar]
- Barin, I. Thermochemical Data of Pure Substances; Wiley: Hoboken, NJ, USA, 1995; p. 795. [Google Scholar]
- Young, D.J. High Temperature Oxidation and Corrosion of Metals; Elsevier: Amsterdam, The Netherlands, 2008; pp. 16–18. [Google Scholar]
- Kofstad, P. High Temperature Corrosion; Elsevier Applied Science: Essex, UK, 1988; pp. 17–18. [Google Scholar]
- Zhang, Z.; Gesmundo, F.; Hou, P.; Niu, Y. Criteria for the formation of protective Al2O3 scales on Fe–Al and Fe–Cr–Al alloys. Corros. Sci. 2006, 48, 741–765. [Google Scholar] [CrossRef]
- Yang, X.; Peng, X.; Wang, F. Effect of annealing treatment on the oxidation of an electrodeposited alumina-forming Ni–Al nanocomposite. Corros. Sci. 2008, 50, 3227–3232. [Google Scholar] [CrossRef]
- Badini, C.; Laurella, F. Oxidation of FeCrAl alloy: Influence of temperature and atmosphere on scale growth rate and mechanism. Surf. Coat. Technol. 2001, 135, 291–298. [Google Scholar] [CrossRef]
- Cueff, R.; Buscail, H.; Caudron, E.; Issartel, C.; Riffard, F. Oxidation of alumina formers at 1173 K: Effect of yttrium ion implantation and yttrium allying addition. Corros. Sci. 2003, 45, 1815–1831. [Google Scholar] [CrossRef]
- Cueff, R.; Buscail, H.; Caudron, E.; Riffard, F.; Issartel, C.; El Messki, S. Effect of reactive element oxide coating on the high temperature oxidation behaviour of FeCrAl alloys. App. Surf. Sci. 2004, 229, 233–241. [Google Scholar] [CrossRef]
- Maréchal, L.; Lesage, B.; Huntz, A.M.; Molins, R. Oxidation behaviour of ODS Fe–Cr–Al alloys: Aluminium depletion and life time. Oxid. Met. 2003, 60, 1–27. [Google Scholar] [CrossRef]
- Chandra-ambhorn, S.; Jutilarptavorn, A.; Rojhirunsakool, T. High temperature oxidation of irons without and with 0.06 wt% Sn in dry and humidified oxygen. Corros. Sci. 2019, 148, 355–365. [Google Scholar] [CrossRef]
- Chandra-ambhorn, S.; Thublaor, T.; Pascal, C. CHAPTER 1 Thermodynamics and Kinetics of the High Temperature Oxidation of Stainless Steels. Solid State Phenom. 2020, 300, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Sarrazin, P.; Galerie, A.; Fouletier, J. Les Mécanismes de la Corrosion Sèche: Une Approach Cinétique; EDS Sciences: Les Ulis, France, 2000; pp. 8–11. [Google Scholar]
- Kubaschewski, O.; Evans, E.L.; Alcock, C.B. Metallurgical Thermochemistry; Pergamon Press: Oxford, UK, 1951; pp. 336–343. [Google Scholar]
- Ahtoy, E. Effect of Alloying Elements (Si, P, Al, B) on Low Carbon Steel Oxidation in Process at High Temperatures: Mechanism and Modeling. Ph.D. Thesis, Institut Polytechnique de Grenoble, Grenoble, France, 2010. [Google Scholar]
- Nishimoto, T.; Honda, K.; Kondo, Y.; Uemara, K. Effects of Si content on the oxidation behaviour of Fe-Si alloys in air. Mater. Sci. Forum 2011, 696, 126–131. [Google Scholar] [CrossRef]
- Fukumoto, M.; Maeda, S.; Hayashi, S.; Narita, T. Effect of Water Vapor on the Oxidation Behavior of Fe–1.5Si in Air at 1073 and 1273 K. Oxid. Met. 2001, 55, 401–422. [Google Scholar] [CrossRef]
- Maitra, T.; Gupta, S. Intermetallic compound formation in Fe–Al–Si ternary system: Part II. Mater. Charact. 2002, 49, 293–311. [Google Scholar] [CrossRef]
- Dutta, M.; Singh, S.B. Effect of strip temperature on the formation of an Fe2Al5 inhibition layer during hot-dip galvanizing. Scr. Mater. 2009, 60, 643–646. [Google Scholar] [CrossRef]
- Wang, C.-J.; Chen, S.-M. The high-temperature oxidation behavior of hot-dipping Al–Si coating on low carbon steel. Surf. Coat. Technol. 2006, 200, 6601–6605. [Google Scholar] [CrossRef]
- Wang, X.H.; Zhou, Y.C. Oxidation behavior of Ti3AlC2 at 1000–1400 °C in air. Corros. Sci. 2003, 45, 891–907. [Google Scholar] [CrossRef]
- Lin, Z.; Li, M.; Wang, J.; Zhou, Y. Influence of water vapor on the oxidation behavior of Ti3AlC2 and Ti2AlC. Scr. Mater. 2008, 58, 29–32. [Google Scholar] [CrossRef]
- Hou, P.Y. Oxidation of metals and alloys, In Shreir’s Corrosion Volume I Basic Concepts, High Temperature Corrosion; Cottis, R.A., Graham, M., Lindsay, R., Lyon, S., Richardson, T., Scantlebury, D., Stott, H., Eds.; Elsevier: Amsterdam, The Netherlands, 2010; pp. 195–239. [Google Scholar]
- Djukic, M.B.; Zeravcic, V.S.; Bakic, G.; Sedmak, A.; Rajicic, B. Hydrogen Embrittlement of Low Carbon Structural Steel. Procedia Mater. Sci. 2014, 3, 1167–1172. [Google Scholar] [CrossRef] [Green Version]
- Stępień, K.; Kupka, M. Effect of hydrogen on room-temperature hardness of B2 FeAl alloys. Scr. Mater. 2008, 59, 999–1001. [Google Scholar] [CrossRef]
- Kirchheim, R. Reducing grain boundary, dislocation line and vacancy formation energies by solute segregation. I. theoretical background. Acta Mater. 2007, 55, 5129–5138. [Google Scholar] [CrossRef]
- Cizek, J.; Lukáč, F.; Prochazka, I.; Vlček, M.; Jirásková, Y.; Švec, P.; Janičkovič, D. Positive effect of hydrogen-induced vacancies on mechanical alloying of Fe and Al. J. Alloy. Compd. 2015, 629, 22–26. [Google Scholar] [CrossRef]
- Fähnle, M.; Mayer, J.; Meyer, B. Theory of atomic defects and diffusion in ordered compounds, and application to B2-FeAl. Intermetallics 1999, 7, 315–323. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kerdbua, P.; Bidabadi, M.H.S.; Chandra-ambhorn, W.; Chandra-ambhorn, S. High Temperature Corrosion Behaviour of Aluminide-Coated Cast Iron for an Exhaust Manifold Application. Coatings 2020, 10, 705. https://doi.org/10.3390/coatings10080705
Kerdbua P, Bidabadi MHS, Chandra-ambhorn W, Chandra-ambhorn S. High Temperature Corrosion Behaviour of Aluminide-Coated Cast Iron for an Exhaust Manifold Application. Coatings. 2020; 10(8):705. https://doi.org/10.3390/coatings10080705
Chicago/Turabian StyleKerdbua, Panya, Mohammad Hassan Shirani Bidabadi, Walairat Chandra-ambhorn, and Somrerk Chandra-ambhorn. 2020. "High Temperature Corrosion Behaviour of Aluminide-Coated Cast Iron for an Exhaust Manifold Application" Coatings 10, no. 8: 705. https://doi.org/10.3390/coatings10080705




