Electronic and Thermoelectric Properties of V2O5, MgV2O5, and CaV2O5
Abstract
:1. Introduction
2. Methods
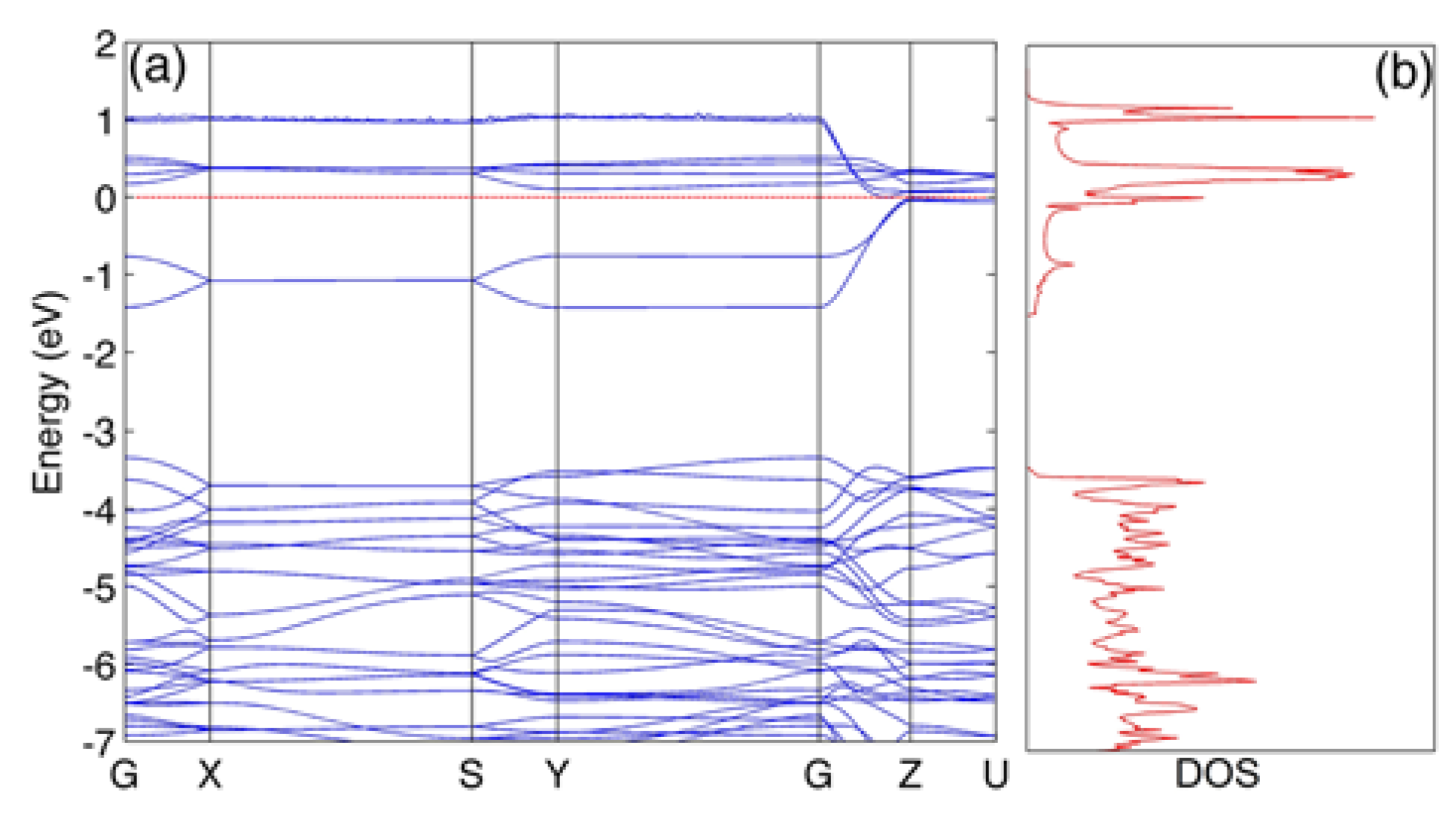
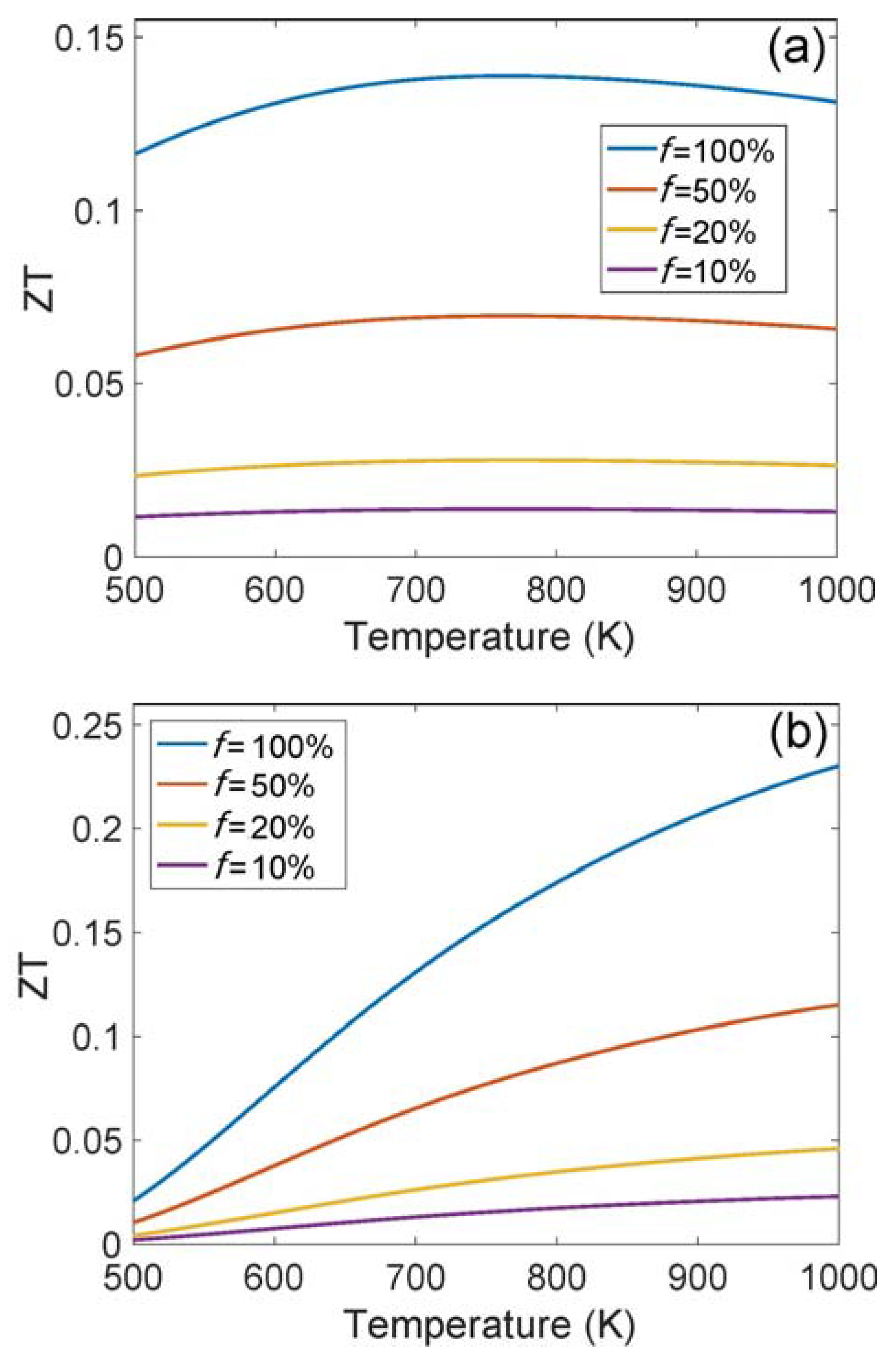
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- DiSalvo, F.J. Thermoelectric Cooling and Power Generation. Science 1999, 285, 703. [Google Scholar] [CrossRef]
- Xiong, S.; Latour, B.; Ni, Y.; Volz, S.; Chalopin, Y. Efficient phonon blocking in SiC antiphase superlattice nanowires. Phys. Rev. B 2015, 91, 224307. [Google Scholar] [CrossRef]
- Xiong, S.; Ma, J.; Volz, S.; Dumitrică, T. Thermally-Active Screw Dislocations in Si Nanowires and Nanotubes. Small 2014, 10, 1756–1760. [Google Scholar] [CrossRef]
- He, J.; Tritt, T.M. Advances in thermoelectric materials research: Looking back and moving forward. Science 2017, 357, eaak9997. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.-G.; Shi, X.; Zhao, L.-D.; Zou, J. High-performance SnSe thermoelectric materials: Progress and future challenge. Prog. Mater. Sci. 2018, 97, 283–346. [Google Scholar] [CrossRef] [Green Version]
- Shi, X.-L.; Tao, X.; Zou, J.; Chen, Z.-G. High-Performance Thermoelectric SnSe: Aqueous Synthesis, Innovations, and Challenges. Adv. Sci. 2020, 7, 1902923. [Google Scholar] [CrossRef] [Green Version]
- Ohta, H.; Kim, S.; Mune, Y.; Mizoguchi, T.; Nomura, K.; Ohta, S.; Nomura, T.; Nakanishi, Y.; Ikuhara, Y.; Hirano, M.; et al. Giant thermoelectric Seebeck coefficient of a two-dimensional electron gas in SrTiO3. Nat. Mater. 2007, 6, 129–134. [Google Scholar] [CrossRef]
- Rhyee, J.-S.; Lee, K.H.; Lee, S.M.; Cho, E.; Kim, S.I.; Lee, E.; Kwon, Y.S.; Shim, J.H.; Kotliar, G. Peierls distortion as a route to high thermoelectric performance in In4Se3-δ crystals. Nature 2009, 459, 965–968. [Google Scholar] [CrossRef]
- Venkatasubramanian, R.; Siivola, E.; Colpitts, T.; O’Quinn, B. Thin-film thermoelectric devices with high room-temperature figures of merit. Nature 2001, 413, 597–602. [Google Scholar] [CrossRef]
- Srinivasan, B.; Gellé, A.; Gucci, F.; Boussard-Pledel, C.; Fontaine, B.; Gautier, R.; Halet, J.-F.; Reece, M.J.; Bureau, B. Realizing a stable high thermoelectric zT ∼ 2 over a broad temperature range in Ge1−x−yGaxSbyTe via band engineering and hybrid flash-SPS processing. Inorg. Chem. Front. 2019, 6, 63–73. [Google Scholar] [CrossRef]
- Srinivasan, B.; Berthebaud, D.; Mori, T. Is LiI a Potential Dopant Candidate to Enhance the Thermoelectric Performance in Sb-Free GeTe Systems? A Prelusive Study. Energies 2020, 13, 643. [Google Scholar] [CrossRef] [Green Version]
- Zeier, W.G.; Schmitt, J.; Hautier, G.; Aydemir, U.; Gibbs, Z.M.; Felser, C.; Snyder, G.J. Engineering half-Heusler thermoelectric materials using Zintl chemistry. Nat. Rev. Mater. 2016, 1, 16032. [Google Scholar] [CrossRef]
- Fu, C.; Zhu, T.; Pei, Y.; Xie, H.; Wang, H.; Snyder, G.J.; Liu, Y.; Liu, Y.; Zhao, X. High Band Degeneracy Contributes to High Thermoelectric Performance in p-Type Half-Heusler Compounds. Adv. Energy Mater. 2014, 4, 1400600. [Google Scholar] [CrossRef]
- Tang, Y.; Hanus, R.; Chen, S.-w.; Snyder, G.J. Solubility design leading to high figure of merit in low-cost Ce-CoSb3 skutterudites. Nat. Commun. 2015, 6, 7584. [Google Scholar] [CrossRef] [Green Version]
- Olvera, A.A.; Moroz, N.A.; Sahoo, P.; Ren, P.; Bailey, T.P.; Page, A.A.; Uher, C.; Poudeu, P.F.P. Partial indium solubility induces chemical stability and colossal thermoelectric figure of merit in Cu2Se. Energy Environ. Sci. 2017, 10, 1668–1676. [Google Scholar] [CrossRef]
- Xiong, S.; Yang, K.; Kosevich, Y.A.; Chalopin, Y.; D’Agosta, R.; Cortona, P.; Volz, S. Classical to Quantum Transition of Heat Transfer between Two Silica Clusters. Phys. Rev. Lett. 2014, 112, 114301. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Wang, Z.; Srinivasan, B.; Wang, Z.; Zhang, J.; Li, K.; Boussard-Pledel, C.; Troles, J.; Bureau, B.; Wei, L. Ultraflexible Glassy Semiconductor Fibers for Thermal Sensing and Positioning. Acs Appl. Mater. Interfaces 2019, 11, 2441–2447. [Google Scholar] [CrossRef]
- Srinivasan, B.; Cui, S.; Prestipino, C.; Gellé, A.; Boussard-Pledel, C.; Ababou-Girard, S.; Trapananti, A.; Bureau, B.; Di Matteo, S. Possible Mechanism for Hole Conductivity in Cu–As–Te Thermoelectric Glasses: A XANES and EXAFS Study. J. Phys. Chem. C 2017, 121, 14045–14050. [Google Scholar] [CrossRef]
- Zhou, Y.; Xiong, S.; Zhang, X.; Volz, S.; Hu, M. Thermal transport crossover from crystalline to partial-crystalline partial-liquid state. Nat. Commun. 2018, 9, 4712. [Google Scholar] [CrossRef] [Green Version]
- Chain, E.E. Optical properties of vanadium dioxide and vanadium pentoxide thin films. Appl. Opt. 1991, 30, 2782–2787. [Google Scholar] [CrossRef]
- Scanlon, D.O.; Walsh, A.; Morgan, B.J.; Watson, G.W. An ab initio Study of Reduction of V2O5 through the Formation of Oxygen Vacancies and Li Intercalation. J. Phys. Chem. C 2008, 112, 9903–9911. [Google Scholar] [CrossRef]
- Park, H.K. V2O5 Xerogel Films as Intercalation Hosts for Lithium. J. Electrochem. Soc. 1995, 142, 1068. [Google Scholar] [CrossRef]
- Wu, Q.-H.; Thißen, A.; Jaegermann, W. Photoelectron spectroscopic study of Na intercalation into V2O5 thin films. Solid State Ion. 2004, 167, 155–163. [Google Scholar] [CrossRef]
- Iwanaga, S.; Marciniak, M.; Darling, R.B.; Ohuchi, F.S. Thermopower and electrical conductivity of sodium-doped V2O5 thin films. J. Appl. Phys. 2007, 101, 123709. [Google Scholar] [CrossRef]
- Chumakov, Y.; Santos, J.R.; Ferreira, I.; Termentzidis, K.; Pokropivny, A.; Xiong, S.Y.; Cortona, P.; Volz, S. Thermoelectric transport in V2O5 thin films. J. Phys. Conf. Ser. 2012, 395, 012016. [Google Scholar] [CrossRef]
- Bianchi, C.; Loureiro, J.; Duarte, P.; Marques, J.; Figueira, J.; Ropio, I.; Ferreira, I. V2O5 Thin Films for Flexible and High Sensitivity Transparent Temperature Sensor. Adv. Mater. Technol. 2016, 1, 1600077. [Google Scholar] [CrossRef]
- Santos, R.; Loureiro, J.; Nogueira, A.; Elangovan, E.; Pinto, J.V.; Veiga, J.P.; Busani, T.; Fortunato, E.; Martins, R.; Ferreira, I. Thermoelectric properties of V2O5 thin films deposited by thermal evaporation. Appl. Surf. Sci. 2013, 282, 590–594. [Google Scholar] [CrossRef]
- Loureiro, J.; Santos, J.R.; Nogueira, A.; Wyczisk, F.; Divay, L.; Reparaz, S.; Alzina, F.; Sotomayor Torres, C.M.; Cuffe, J.; Montemor, F.; et al. Nanostructured p-type Cr/V2O5 thin films with boosted thermoelectric properties. J. Mater. Chem. A 2014, 2, 6456–6462. [Google Scholar] [CrossRef]
- Kordonis, K. Wärmetransport in eindimensionalen Spinsystemen. Ph.D Thesis, Universität zu Köln, Köln, Germany, 2007. [Google Scholar]
- Shklover, V.; Haibach, T.; Ried, F.; Nesper, R.; Novák, P. Crystal Structure of the Product of Mg2+Insertion into V2O5Single Crystals. J. Solid State Chem. 1996, 123, 317–323. [Google Scholar] [CrossRef]
- Millet, P.; Satto, C.; Sciau, P.; Galy, J. MgV2O5 and δLixV2O5: A Comparative Structural Investigation. J. Solid State Chem. 1998, 136, 56–62. [Google Scholar] [CrossRef]
- Onoda, M.; Nishiguchi, N. LETTER TO THE EDITOR: Crystal Structure and Spin Gap State of CaV2O5. J. Solid State Chem. 1996, 127, 359–362. [Google Scholar] [CrossRef]
- Delmas, C.; Cognac-Auradou, H.; Cocciantelli, J.M.; Ménétrier, M.; Doumerc, J.P. The LixV2O5 system: An overview of the structure modifications induced by the lithium intercalation. Solid State Ion. 1994, 69, 257–264. [Google Scholar] [CrossRef]
- Chakrabarti, A.; Hermann, K.; Druzinic, R.; Witko, M.; Wagner, F.; Petersen, M. Geometric and electronic structure of vanadium pentoxide: A density functional bulk and surface study. Phys. Rev. B 1999, 59, 10583–10590. [Google Scholar] [CrossRef] [Green Version]
- Goclon, J.; Grybos, R.; Witko, M.; Hafner, J. Relative stability of low-index V2O5 surfaces: A density functional investigation. J. Phys. Condens. Matter 2009, 21, 095008. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.R.; Guo, G.Y. Structural, electronic and magnetic properties of V2O5−x: An ab initio study. J. Chem. Phys. 2009, 130, 214704. [Google Scholar] [CrossRef]
- Ganduglia-Pirovano, M.V.; Sauer, J. Stability of reduced ${\mathrm{V}}_{2}{\mathrm{O}}_{5}(001)$ surfaces. Phys. Rev. B 2004, 70, 045422. [Google Scholar] [CrossRef]
- Spitaler, J.; Sherman, E.Y.; Ambrosch Draxl, C.; Hohenadler, M.; Michel, F.; Aichhorn, M.; Evertz, H.G. Ab-initio Studies of the Vanadium Ladder Compounds NaV2O5, CaV2O5 and MgV2O5. APS 2004, 2004, D25-002. [Google Scholar]
- Laubach, S.; Schmidt, P.C.; Thißen, A.; Fernandez-Madrigal, F.J.; Wu, Q.-H.; Jaegermann, W.; Klemm, M.; Horn, S. Theoretical and experimental determination of the electronic structure of V2O5, reduced V2O5−x and sodium intercalated NaV2O5. Phys. Chem. Chem. Phys. 2007, 9, 2564–2576. [Google Scholar] [CrossRef]
- Jovanović, A.; Dobrota, A.S.; Rafailović, L.D.; Mentus, S.V.; Pašti, I.A.; Johansson, B.; Skorodumova, N.V. Structural and electronic properties of V2O5 and their tuning by doping with 3d elements – modelling using the DFT+U method and dispersion correction. Phys. Chem. Chem. Phys. 2018, 20, 13934–13943. [Google Scholar] [CrossRef] [Green Version]
- Gonze, X.; Amadon, B.; Antonius, G.; Arnardi, F.; Baguet, L.; Beuken, J.-M.; Bieder, J.; Bottin, F.; Bouchet, J.; Bousquet, E.; et al. The Abinitproject: Impact, environment and recent developments. Comput. Phys. Commun. 2020, 248, 107042. [Google Scholar] [CrossRef]
- Gonze, X.; Jollet, F.; Abreu Araujo, F.; Adams, D.; Amadon, B.; Applencourt, T.; Audouze, C.; Beuken, J.M.; Bieder, J.; Bokhanchuk, A.; et al. Recent developments in the ABINIT software package. Comput. Phys. Commun. 2016, 205, 106–131. [Google Scholar] [CrossRef] [Green Version]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [Green Version]
- Anisimov, V.I.; Aryasetiawan, F.; Lichtenstein, A.I. First-principles calculations of the electronic structure and spectra of strongly correlated systems: The LDA+U method. J. Phys. Condens. Matter 1997, 9, 767–808. [Google Scholar] [CrossRef] [Green Version]
- Tkatchenko, A.; Scheffler, M. Accurate Molecular Van Der Waals Interactions from Ground-State Electron Density and Free-Atom Reference Data. Phys. Rev. Lett. 2009, 102, 073005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madsen, G.K.H.; Singh, D.J. BoltzTraP. A code for calculating band-structure dependent quantities. Comput. Phys. Commun. 2006, 175, 67–71. [Google Scholar] [CrossRef] [Green Version]
- Hieu, N.V.; Lichtman, D. Bandgap radiation induced photodesorption from V2O5 powder and vanadium oxide surfaces. J. Vac. Sci. Technol. 1981, 18, 49–53. [Google Scholar] [CrossRef]
- Cogan, S.F.; Nguyen, N.M.; Perrotti, S.J.; Rauh, R.D. Optical properties of electrochromic vanadium pentoxide. J. Appl. Phys. 1989, 66, 1333–1337. [Google Scholar] [CrossRef]
- Moshfegh, A.Z.; Ignatiev, A. Formation and characterization of thin film vanadium oxides: Auger electron spectroscopy, X-ray photoelectron spectroscopy, X-ray diffraction, scanning electron microscopy, and optical reflectance studies. Thin Solid Film. 1991, 198, 251–268. [Google Scholar] [CrossRef]
- Isobe, M.; Ueda, Y.; Takizawa, K.; Goto, T. Observation of a Spin Gap in MgV2O5 from High Field Magnetization Measurements. J. Phys. Soc. Jpn. 1998, 67, 755–758. [Google Scholar] [CrossRef]
- Iwase, H.; Isobe, M.; Ueda, Y.; Yasuoka, H. Observation of Spin Gap in CaV2O5 by NMR. J. Phys. Soc. Jpn. 1996, 65, 2397–2400. [Google Scholar] [CrossRef]
- Korotin, M.A.; Anisimov, V.I.; Saha-Dasgupta, T.; Dasgupta, I. Electronic structure and exchange interactions of the ladder vanadates CaV2O5and MgV2O5. J. Phys. Condens. Matter 1999, 12, 113–124. [Google Scholar] [CrossRef]
- Jones, E.C.; Christen, D.K.; Sales, B.C. Sign reversals in the vortex-state Hall effect in Y-Ba-Cu-O: Correlations with the normal-state Seebeck coefficient. Phys. Rev. B 1994, 50, 7234–7237. [Google Scholar] [CrossRef] [PubMed]
- Surnev, S.; Ramsey, M.G.; Netzer, F.P. Vanadium oxide surface studies. Prog. Surf. Sci. 2003, 73, 117–165. [Google Scholar] [CrossRef]
- Gorodynskyy, V.; Zdansky, K.; Pekárek, L.; Vacková, S. Temperature change of Hall and Seebeck coefficient sign in InP doped with transition metals. Semicond. Sci. Technol. 2003, 19, 203–207. [Google Scholar] [CrossRef]
- Yang, J.; Li, H.; Wu, T.; Zhang, W.; Chen, L.; Yang, J. Evaluation of Half-Heusler Compounds as Thermoelectric Materials Based on the Calculated Electrical Transport Properties. Adv. Funct. Mater. 2008, 18, 2880–2888. [Google Scholar] [CrossRef]
- Xiong, S.; Selli, D.; Neogi, S.; Donadio, D. Native surface oxide turns alloyed silicon membranes into nanophononic metamaterials with ultralow thermal conductivity. Phys. Rev. B 2017, 95, 180301. [Google Scholar] [CrossRef] [Green Version]
- Ouyang, B.; Xiong, S.; Yang, Z.; Jing, Y.; Wang, Y. MoS2 heterostructure with tunable phase stability: Strain induced interlayer covalent bond formation. Nanoscale 2017, 9, 8126–8132. [Google Scholar] [CrossRef] [Green Version]





| Lattice Parameter | V2O5 | MgV2O5 | CaV2O5 | |||
|---|---|---|---|---|---|---|
| Exp. [30] | Current Work | Exp. [31] | Current Work | Exp. [32] | Current Work | |
| a | 11.544 | 11.530 (−0.12%) | 11.010 | 10.896 (−1.03%) | 11.351 | 11.525 (+1.53%) |
| b | 3.571 | 3.577 (+0.17%) | 3.693 | 3.789 (+2.06%) | 3.604 | 3.609 (+0.14%) |
| c | 4.383 | 4.483 (+2.28%) | 9.958 | 10.173 (+2.16%) | 4.893 | 4.962 (+1.41%) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheng, X.; Li, Z.; Cheng, Y. Electronic and Thermoelectric Properties of V2O5, MgV2O5, and CaV2O5. Coatings 2020, 10, 453. https://doi.org/10.3390/coatings10050453
Sheng X, Li Z, Cheng Y. Electronic and Thermoelectric Properties of V2O5, MgV2O5, and CaV2O5. Coatings. 2020; 10(5):453. https://doi.org/10.3390/coatings10050453
Chicago/Turabian StyleSheng, Xiaofei, Zhuhong Li, and Yajuan Cheng. 2020. "Electronic and Thermoelectric Properties of V2O5, MgV2O5, and CaV2O5" Coatings 10, no. 5: 453. https://doi.org/10.3390/coatings10050453





