Conformational Response of 30S-bound IF3 to A-Site Binders Streptomycin and Kanamycin
Abstract
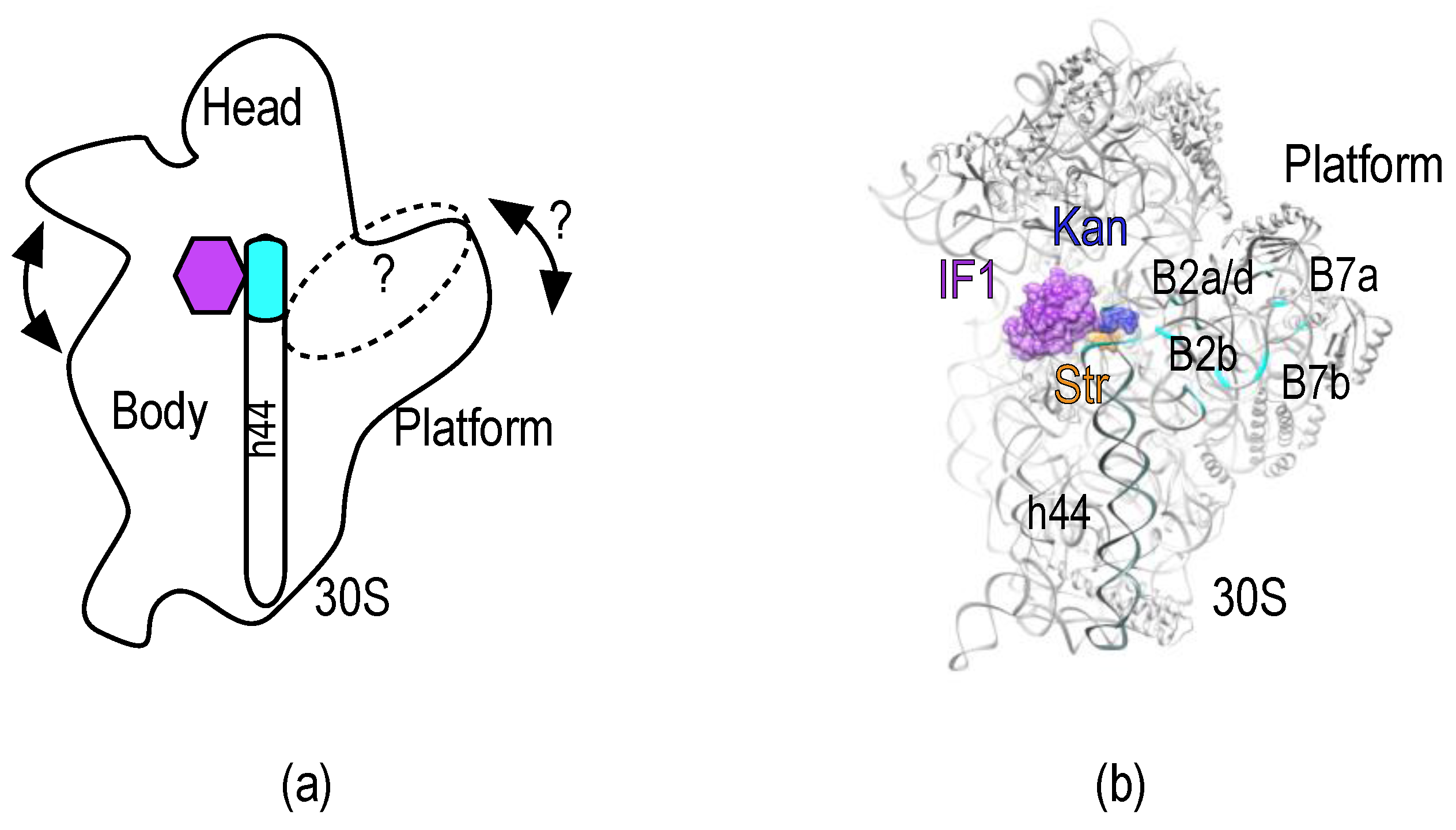
:1. Introduction
2. Results
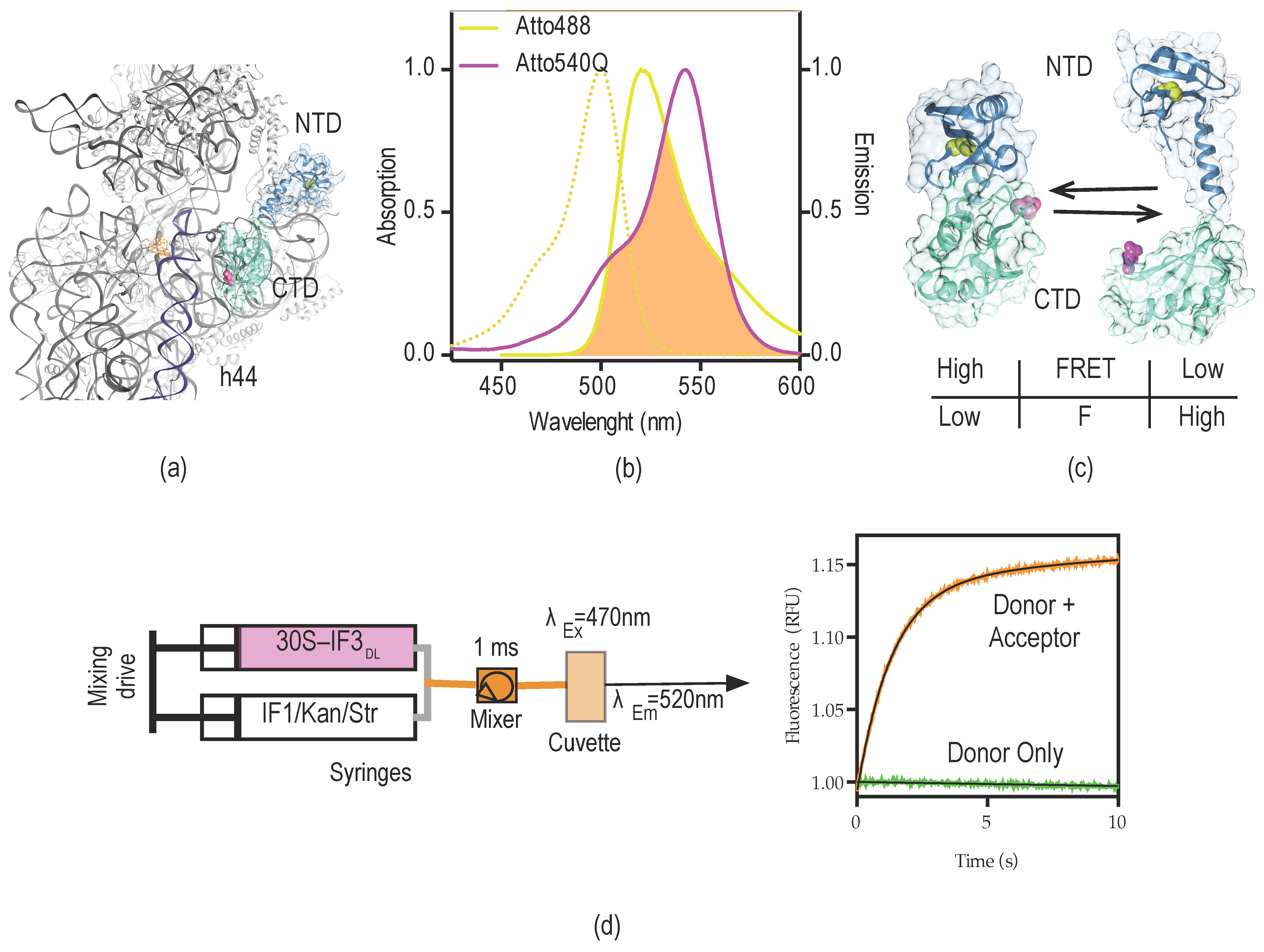
2.1. Experimental Outline
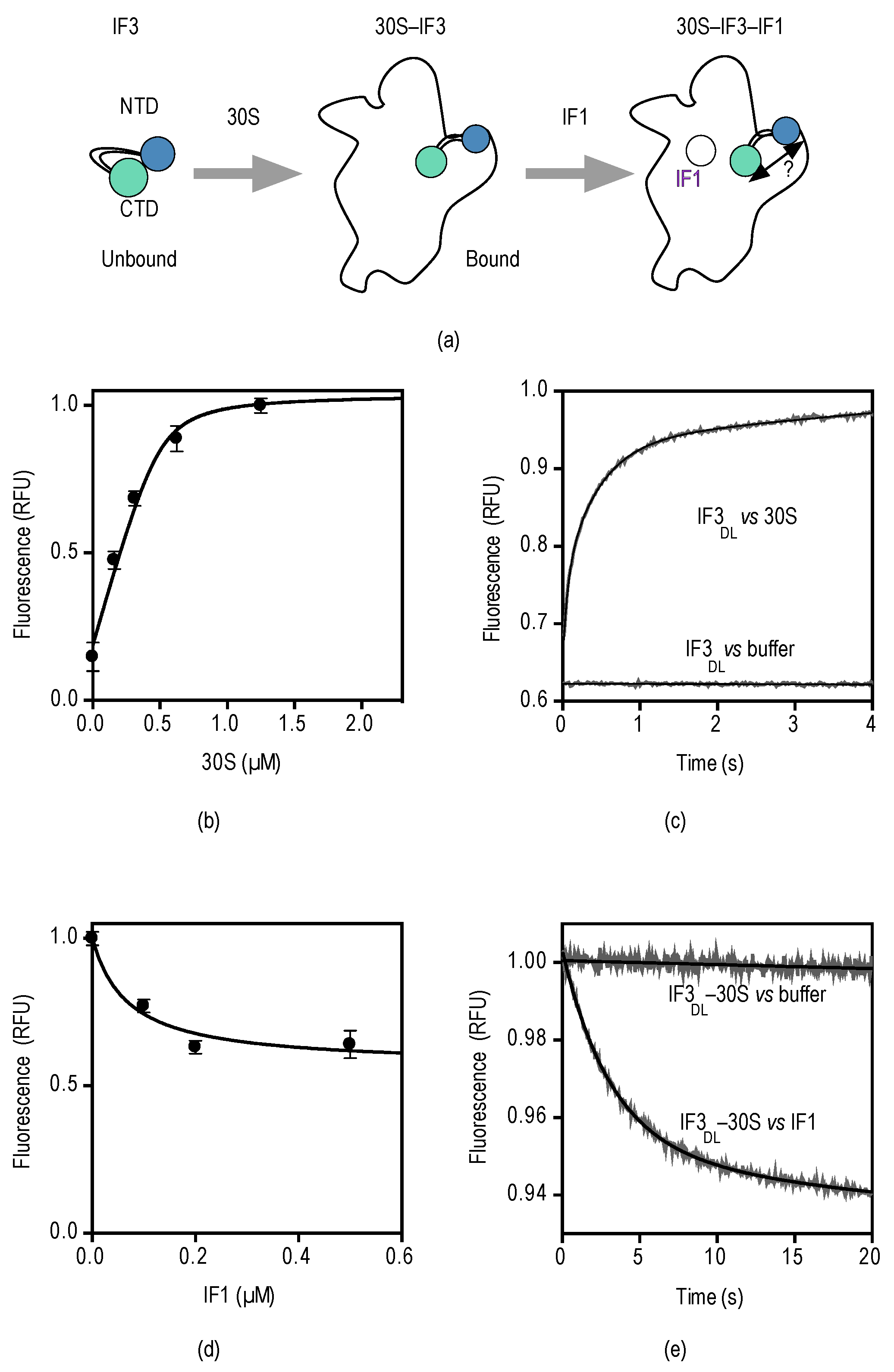
2.2. Probing the Sensing Limits of IF3DL
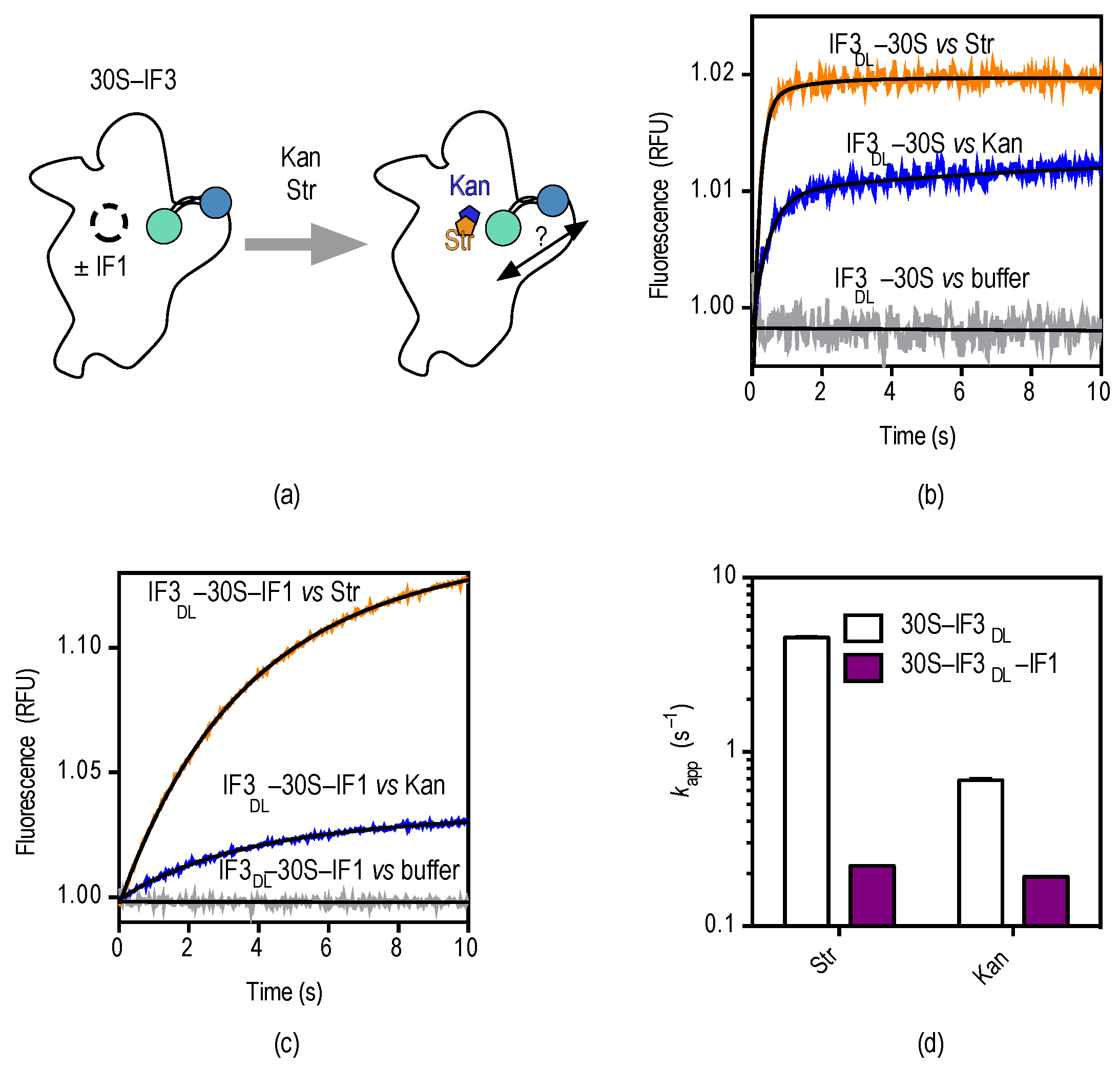
2.3. Counter Effects between IF1 and Streptomycin/Kanamycin
3. Discussion
4. Materials and Methods
4.1. Escherichia coli Strains, Expression Vectors, Cell Growth, and Protein Expression Induction
4.2. IF1, IF3, and 30S Subunits Purification
4.3. Double Labeling of IF3 with Atto-Tec Dyes
4.4. Equilibrium Binding Measurements
4.5. Stopped-Flow Measurements and Analysis
4.6. Data Analysis
4.7. Structural Models
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| MDR | Multidrug-resistant |
| XDR | Extensively drug-resistant |
| TB | Tuberculosis |
| IF3 | Initiation factor 3 |
| IF1 | Initiation factor 1 |
| CDC | Centers for Disease Control and Prevention |
| 30S | Minor ribosomal subunit |
| IC | Initiation complexes |
| SD | Shine–Dalgarno sequence |
| NTD | N-terminal domain |
| CTD | C-terminal domain |
| FRET | Fluorescence Resonance Energy Transfer |
| IF3DL | Double-labeled IF3 |
| Pre-IC | Pre-initiation complex |
| Initiatior tRNA | fMet-tRNAfMet |
| KD | Dissociation constant |
| kapp | Apparent rate constant |
| SEM | Standard error of the mean |
| F | fluorescent amplitude |
| LB | Luria–Bertoni medium |
| IPTG | Isopropyl β-d-1-thiogalactopyranoside |
| SDS-PAGE | Sodium-dodecyl-sulfate-Polyacrylamide Gel Electrophoresis |
| HEPES | 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid |
| TCEP | tris(2-carboxyethyl)phosphine |
References
- Taylor, L.H.; Latham, S.M.; Woolhouse, M.E.J. Risk factors for human disease emergence. Philos. Trans. R. Soc. B Biol. Sci. 2001, 356, 983–989. [Google Scholar] [CrossRef] [PubMed]
- Zumla, A.; Raviglione, M.; Hafner, R.; von Reyn, C.F. Tuberculosis. N. Engl. J. Med. 2013, 368, 745–755. [Google Scholar] [CrossRef] [PubMed]
- Blumberg, H.M.; Burman, W.J.; Chaisson, R.E.; Daley, C.L.; Etkind, S.C.; Friedman, L.N.; Fujiwara, P.; Grzemska, M.; Hopewell, P.C.; Iseman, M.D.; et al. Centers for Disease Control and Prevention/Infectious Diseases Society of America: Treatment of tuberculosis. Am. J. Respir. Crit. Care Med. 2003, 167, 603–662. [Google Scholar] [PubMed]
- Horsburgh, C.R.; Feldman, S.; Ridzon, R. Infectious Diseases Society of America Practice guidelines for the treatment of tuberculosis. Clin. Infect. Dis. 2000, 31, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Mingeot-Leclercq, M.P.; Glupczynski, Y.; Tulkens, P.M. Aminoglycosides: Activity and resistance. Antimicrob. Agents Chemother. 1999, 43, 727–737. [Google Scholar] [PubMed]
- Pestka, S. [28] The use of inhibitors in studies of protein synthesis. Methods Enzymol. 1974, 30, 261–282. [Google Scholar] [PubMed]
- Misumi, M.; Tanaka, N. Mechanism of inhibition of translocation by kanamycin and viomycin: A comparative study with fusidic acid. Biochem. Biophys. Res. Commun. 1980, 92, 647–654. [Google Scholar] [CrossRef]
- Gorini, L.; Jacoby, G.A.; Breckenridge, L. Ribosomal ambiguity. Cold Spring Harb. Symp. Quant. Biol. 1966, 31, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Lodmell, J.S.; Dahlberg, A.E. A conformational switch in Escherichia coli 16S ribosomal RNA during decoding of messenger RNA. Science 1997, 277, 1262–1267. [Google Scholar] [CrossRef] [PubMed]
- Blomberg, C.; Johansson, J.; Liljenström, H. Error propagation in E. coli protein synthesis. J. Theor. Biol. 1985, 113, 407–423. [Google Scholar] [CrossRef]
- Fast, R.; Eberhard, T.H.; Ruusala, T.; Kurland, C.G. Does streptomycin cause an error catastrophe? Biochimie 1987, 69, 131–136. [Google Scholar] [CrossRef]
- Luzzatto, L.; Apirion, D.; Schlessinger, D. Streptomycin action: Greater inhibition of Escherichia coli ribosome function with exogenous than with endogenous messenger ribonucleic acid. J. Bacteriol. 1969, 99, 206–209. [Google Scholar] [PubMed]
- Carter, A.P.; Clemons, W.M.; Brodersen, D.E.; Morgan-Warren, R.J.; Hartsch, T.; Wimberly, B.T.; Ramakrishnan, V. Crystal structure of an initiation factor bound to the 30S ribosomal subunit. Science 2001, 291, 498–501. [Google Scholar] [CrossRef] [PubMed]
- Demirci, H.; Murphy, F.; Murphy, E.; Gregory, S.T.; Dahlberg, A.E.; Jogl, G. A structural basis for streptomycin-induced misreading of the genetic code. Nat. Commun. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- François, B.; Russell, R.J.M.; Murray, J.B.; Aboul-ela, F.; Masquida, B.; Vicens, Q.; Westhof, E. Crystal structures of complexes between aminoglycosides and decoding A site oligonucleotides: Role of the number of rings and positive charges in the specific binding leading to miscoding. Nucleic Acids Res. 2005, 33, 5677–5690. [Google Scholar] [CrossRef] [PubMed]
- Carter, A.P.; Clemons, W.M.; Brodersen, D.E.; Morgan-Warren, R.J.; Wimberly, B.T.; Ramakrishnan, V. Functional insights from the structure of the 30S ribosomal subunit and its interactions with antibiotics. Nature 2000, 407, 340–348. [Google Scholar] [PubMed]
- Moazed, D.; Samaha, R.R.; Gualerzi, C.O.; Noller, H.F. Specific protection of 16S rRNA by translational initiation factors. J. Mol. Biol. 1995, 248, 207–210. [Google Scholar] [CrossRef]
- Hussain, T.; Llácer, J.L.; Wimberly, B.T.; Kieft, J.S.; Ramakrishnan, V. Large-scale movements of IF3 and tRNA during bacterial translation initiation. Cell 2016, 167, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Fabbretti, A.; Pon, C.L.; Hennelly, S.P.; Hill, W.E.; Lodmell, J.S.; Gualerzi, C.O. The real-time path of translation factor IF3 onto and off the ribosome. Mol. Cell 2007, 25, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Dallas, A.; Noller, H.F. Interaction of translation initiation factor 3 with the 30S ribosomal subunit. Mol. Cell 2001, 8, 855–864. [Google Scholar] [CrossRef]
- Julián, P.; Milon, P.; Agirrezabala, X.; Lasso, G.; Gil, D.; Rodnina, M.V.; Valle, M. The Cryo-EM structure of a complete 30S translation initiation complex from Escherichia coli. PLoS Biol. 2011, 9, e1001095. [Google Scholar] [CrossRef] [PubMed]
- Pon, C.L.; Pawlik, R.T.; Gualerzi, C. The topographical localization of IF3 on Escherichia coli 30S ribosomal subunits as a clue to its way of functioning. FEBS Lett. 1982, 137, 163–167. [Google Scholar] [CrossRef]
- Biou, V.; Shu, F.; Ramakrishnan, V. X-ray crystallography shows that translational initiation factor IF3 consists of two compact alpha/beta domains linked by an alpha-helix. EMBO J. 1995, 14, 4056–4064. [Google Scholar] [PubMed]
- Garcia, C.; Fortier, P.L.; Blanquet, S.; Lallemand, J.Y.; Dardel, F. Solution structure of the ribosome-binding domain of E. coli translation initiation factor IF3. Homology with the U1A protein of the eukaryotic spliceosome. J. Mol. Biol. 1995, 254, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Moreau, M.; de Cock, E.; Fortier, P.L.; Garcia, C.; Albaret, C.; Blanquet, S.; Lallemand, J.Y.; Dardel, F. Heteronuclear NMR studies of E. coli translation initiation factor IF3. Evidence that the inter-domain region is disordered in solution. J. Mol. Biol. 1997, 266, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Gualerzi, C.O.; Pon, C.L. Initiation of mRNA translation in bacteria: Structural and dynamic aspects. Cell. Mol. Life Sci. 2015, 72, 4341–4367. [Google Scholar] [CrossRef] [PubMed]
- De Cock, E.; Springer, M.; Dardel, F. The interdomain linker of Escherichia coli initiation factor IF3: A possible trigger of translation initiation specificity. Mol. Microbiol. 1999, 32, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Milon, P.; Konevega, A.L.; Gualerzi, C.O.; Rodnina, M.V. Kinetic checkpoint at a late step in translation initiation. Mol. Cell 2008, 30, 712–720. [Google Scholar] [CrossRef] [PubMed]
- Hennelly, S.P.; Antoun, A.; Ehrenberg, M.; Gualerzi, C.O.; Knight, W.; Lodmell, J.S.; Hill, W.E. A time-resolved investigation of ribosomal subunit association. J. Mol. Biol. 2005, 346, 1243–1258. [Google Scholar] [CrossRef] [PubMed]
- Garcia, C.; Fortier, P.L.; Blanquet, S.; Lallemand, J.Y.; Dardel, F. 1H and 15N resonance assignments and structure of the N-terminal domain of Escherichia coli initiation factor 3. Eur. J. Biochem. 1995, 228, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Pon, C.; Cannistraro, S.; Giovane, A.; Gualerzi, C.O. Structure-function relationship in Escherichia coli initiation factors. Environment of the Cys residue and evidence for a hydrophobic region in initiation factor IF3 by fluorescence and ESR spectroscopy. Arch. Biochem. Biophys. 1982, 217, 47–57. [Google Scholar] [CrossRef]
- Pon, C.L.; Gualerzi, C.O. Effect of initiation factor 3 binding on the 30S ribosomal subunits of Escherichia coli. Proc. Natl. Acad. Sci. USA 1974, 71, 4950–4954. [Google Scholar] [CrossRef] [PubMed]
- Milon, P.; Maracci, C.; Filonava, L.; Gualerzi, C.O.; Rodnina, M.V. Real-time assembly landscape of bacterial 30S translation initiation complex. Nat. Struct. Mol. Biol. 2012, 19, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Milon, P.; Carotti, M.; Konevega, A.L.; Wintermeyer, W.; Rodnina, M.V.; Gualerzi, C.O. The ribosome-bound initiation factor 2 recruits initiator tRNA to the 30S initiation complex. EMBO Rep. 2010, 11, 312–316. [Google Scholar] [CrossRef] [PubMed]
- Tsai, A.; Petrov, A.; Marshall, R.A.; Korlach, J.; Uemura, S.; Puglisi, J.D. Heterogeneous pathways and timing of factor departure during translation initiation. Nature 2012, 487, 390–393. [Google Scholar] [CrossRef] [PubMed]
- Qin, D.; Fredrick, K. Control of translation initiation involves a factor-induced rearrangement of helix 44 of 16S ribosomal RNA. Mol. Microbiol. 2009, 71, 1239–1249. [Google Scholar] [CrossRef] [PubMed]
- Elvekrog, M.M.; Gonzalez, R.L. Conformational selection of translation initiation factor 3 signals proper substrate selection. Nat. Struct. Mol. Biol. 2013, 20, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Milon, P.; Konevega, A.L.; Peske, F.; Fabbretti, A.; Gualerzi, C.O.; Rodnina, M.V. Transient kinetics, fluorescence, and FRET in studies of initiation of translation in bacteria. Methods Enzymol. 2007, 430, 1–30. [Google Scholar] [PubMed]
- Wilson, D.N. The A-Z of bacterial translation inhibitors. Crit. Rev. Biochem. Mol. Biol. 2009, 44, 393–433. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.; GILBERT, W.; Gorini, L. Streptomycin, suppression, and the code. Proc. Natl. Acad. Sci. USA 1964, 51, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Gorini, L.; Gundersen, W.; Burger, M. Genetics of regulation of enzyme synthesis in the arginine biosynthetic pathway of Escherichia coli. Cold Spring Harb. Symp. Quant. Biol. 1961, 26, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Tsai, A.; Uemura, S.; Johansson, M.; Puglisi, E.V.; Marshall, R.A.; Aitken, C.E.; Korlach, J.; Ehrenberg, M.; Puglisi, J.D. The impact of aminoglycosides on the dynamics of translation elongation. Cell Rep. 2013, 3, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Gromadski, K.B.; Rodnina, M.V. Streptomycin interferes with conformational coupling between codon recognition and GTPase activation on the ribosome. Nat. Struct. Mol. Biol. 2004, 11, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Luzzatto, L.; Apirion, D.; Schlessinger, D. Mechanism of action of streptomycin in E. coli: Interruption of the ribosome cycle at the initiation of protein synthesis. Proc. Natl. Acad. Sci. USA 1968, 60, 873–880. [Google Scholar] [CrossRef] [PubMed]
- La Teana, A.; Gualerzi, C.O.; Brimacombe, R. From stand-by to decoding site. Adjustment of the mRNA on the 30S ribosomal subunit under the influence of the initiation factors. RNA 1995, 1, 772–782. [Google Scholar] [PubMed]
- La Teana, A.; Pon, C.L.; Gualerzi, C.O. Translation of mRNAs with degenerate initiation triplet AUU displays high initiation factor 2 dependence and is subject to initiation factor 3 repression. Proc. Natl. Acad. Sci. USA 1993, 90, 4161–4165. [Google Scholar] [CrossRef] [PubMed]
- Grigoriadou, C.; Marzi, S.; Pan, D.; Gualerzi, C.O.; Cooperman, B.S. The translational fidelity function of IF3 during transition from the 30 S initiation complex to the 70 S initiation complex. J. Mol. Biol. 2007, 373, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Moazed, D.; Noller, H.F. Interaction of antibiotics with functional sites in 16S ribosomal RNA. Nature 1987, 327, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Fabbretti, A.; Schedlbauer, A.; Brandi, L.; Kaminishi, T.; Giuliodori, A.M.; Garofalo, R.; Ochoa-Lizarralde, B.; Takemoto, C.; Yokoyama, S.; Connell, S.R.; et al. Inhibition of translation initiation complex formation by GE81112 unravels a 16S rRNA structural switch involved in P-site decoding. Proc. Natl. Acad. Sci. USA 2016, 113, E2286–E2295. [Google Scholar] [CrossRef] [PubMed]
- Fabbretti, A.; Gualerzi, C.O.; Brandi, L. How to cope with the quest for new antibiotics. FEBS Lett. 2011, 585, 1673–1681. [Google Scholar] [CrossRef] [PubMed]
- Brandi, L.; Fabbretti, A.; Milon, P.; Carotti, M.; Pon, C.L.; Gualerzi, C.O. Methods for identifying compounds that specifically target translation. Methods Enzymol. 2007, 431, 229–267. [Google Scholar] [PubMed]
- Fabbretti, A.; He, C.-G.; Gaspari, E.; Maffioli, S.; Brandi, L.; Spurio, R.; Sosio, M.; Jabes, D.; Donadio, S. A derivative of the thiopeptide GE2270A highly selective against Propionibacterium acnes. Antimicrob. Agents Chemother. 2015, 59, 4560–4568. [Google Scholar] [CrossRef] [PubMed]
- Brandi, L.; Maffioli, S.; Donadio, S.; Quaglia, F.; Sette, M.; Milon, P.; Gualerzi, C.O.; Fabbretti, A. Structural and functional characterization of the bacterial translocation inhibitor GE82832. FEBS Lett. 2012, 586, 3373–3378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fabbretti, A.; Brandi, L.; Petrelli, D.; Pon, C.L.; Castanedo, N.R.; Medina, R.; Gualerzi, C.O. The antibiotic Furvina(R) targets the P-site of 30S ribosomal subunits and inhibits translation initiation displaying start codon bias. Nucleic Acids Res. 2012, 40, 10366–10374. [Google Scholar] [CrossRef] [PubMed]
- Kaminishi, T.; Schedlbauer, A.; Fabbretti, A.; Brandi, L.; Ochoa-Lizarralde, B.; He, C.-G.; Milon, P.; Connell, S.R.; Gualerzi, C.O.; Fucini, P. Crystallographic characterization of the ribosomal binding site and molecular mechanism of action of Hygromycin A. Nucleic Acids Res. 2015, 43, 10015–10025. [Google Scholar] [CrossRef] [PubMed]
- Guex, N.; Peitsch, M.C. SWISS-MODEL and the Swiss-PdbViewer: An environment for comparative protein modeling. Electrophoresis 1997, 18, 2714–2723. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Ban, N.; Beckmann, R.; Cate, J.H.D.; Dinman, J.D.; Dragon, F.; Ellis, S.R.; Lafontaine, D.L.J.; Lindahl, L.; Liljas, A.; Lipton, J.M.; et al. A new system for naming ribosomal proteins. Curr. Opin. Struct. Biol. 2014, 24, 165–169. [Google Scholar] [CrossRef] [PubMed]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chulluncuy, R.; Espiche, C.; Nakamoto, J.A.; Fabbretti, A.; Milón, P. Conformational Response of 30S-bound IF3 to A-Site Binders Streptomycin and Kanamycin. Antibiotics 2016, 5, 38. https://doi.org/10.3390/antibiotics5040038
Chulluncuy R, Espiche C, Nakamoto JA, Fabbretti A, Milón P. Conformational Response of 30S-bound IF3 to A-Site Binders Streptomycin and Kanamycin. Antibiotics. 2016; 5(4):38. https://doi.org/10.3390/antibiotics5040038
Chicago/Turabian StyleChulluncuy, Roberto, Carlos Espiche, Jose Alberto Nakamoto, Attilio Fabbretti, and Pohl Milón. 2016. "Conformational Response of 30S-bound IF3 to A-Site Binders Streptomycin and Kanamycin" Antibiotics 5, no. 4: 38. https://doi.org/10.3390/antibiotics5040038




