Genomic, Transcriptomic and Metabolomic Studies of Two Well-Characterized, Laboratory-Derived Vancomycin-Intermediate Staphylococcus aureus Strains Derived from the Same Parent Strain
Abstract
:1. Introduction
2. Results
2.1. Mutations in Strains 13136p−m+V5 and 13136p−m+V20 Compared to 13136p−m+
| SNP | Amino acid change | Locus | Gene Name | Known or Predicted Gene Product | Mutation Present In: | Function of Predicted Gene Product Based on Published Studies and Similarities to Other Proteins, and Known Stress-Response Associations [references] | ||
|---|---|---|---|---|---|---|---|---|
| 13136p−m+V5 | 13136p−m+V20 | |||||||
| 223 | G→G | K→E | SACOL1005 | pepF | Oligoendopeptidase F | + | - | Cytoplasmic endopeptidase releasing amino acids from internalized peptides; involved in protein turnover [19] |
| 143 | C→T | A→V | SACOL1231 | stp1 | Eukaryotic-like serine / threonine phosphatase | + | - | Influences the regulation of virulence, cell wall structure, autolysis, and susceptibility to some cell wall-active antibiotics [20] |
| 554 | G→A | S→F | SACOL1600 | comGB | ComGB, competence protein | + | - | A membrane protein associated with the SOS response that transports exogenous DNA into the Gram-positive cell [21] |
| 126 | A→C | E→D | SACOL0593 | fusA | Translation elongation factor G | - | + | Protein synthesis roles in tRNA translocation during elongation and post-termination ribosome dissociation; upregulated by acid adaptation [22,23] |
| 121 | G→T | G→C | SACOL0593 | + | + | |||
| 6596 | G→A | A→V | SACOL2150 | fmtB | FmtB protein | + | + | β-lactam resistance-related surface protein with cell wall anchoring and spanning domains, putatively involved in cell wall biosynthesis, cell adhesion, biofilms [6] |
| 171 | C→A | M→I | SACOL2217 | infA | Translation initiation factor IF-1 | + | + | RNA-binding protein that binds the 30S ribosomal subunit, required for correct translation initiation, and downregulated by cell wall-active antibiotics [5,24] |
| 311 | A→T | Y→F | SACOL1319 | glpF | Glycerol uptake facilitator protein | + | + | Housekeeping gene product that transports glycerol or small uncharged molecules into the cell; down-regulated by oxidative stress [25] |
| 353 | G→C | G→P | SACOL0339 | ssb1 | Prophage L54a single-stranded DNA-binding protein | + | + | One of a family of proteins that bind with high affinity to ssDNA intermediates during DNA replication, recombination and repair [26,27,28] |
| 352 | G→C | G→P | SACOL0339 | + | + | |||
| 350 | A→C | N→P | SACOL0339 | - | + | |||
| 349 | A→C | N→P | SACOL0339 | - | + | |||
| 345 | A→C | Q→P | SACOL0339 | - | + | |||
| 344 | A→C | Q→P | SACOL0339 | - | + | |||
| 329 | A→C | K→T | SACOL0339 | - | + | |||
| 670 | C→T | L→F | SACOL0810 | tarO | glycosyl transferase, group 4 family protein (TarO) | - | + | Teichoic acid synthesis enzyme; inactivation increases autolysis rates, reduces β-lactam resistance, and disrupts sepatation and cell separation [29] |
| 11 | G→A | A→V | SACOL1495 | dinG | Damage inducible gene G (DinG) | - | + | 3'→5' ssDNA and ssRNA exonuclease; proposed functions: R-loop resolution, other unspecified recombination repair systems, anitviral defense [30] |
| 191 | C→T | C→Y | SACOL1690 | apt | Adenine phosphoribosyl transferase | - | + | Enzyme in an adenine recycling pathway upregulated by acid adaptation, the stringent response, and in some organisms polyamine metabolism [23,31] |
| 574 | A→C | F→V | SACOL2451 | none | Amino acid ABC transporter binding protein | - | + | Homolog of OpuBC, the glycine betaine/choline binding lipoprotein of an osmoprotectant uptake system [32] |
2.2. Comparison of Transcriptional Profiles between 13136p−m+, 13136p−m+V5 and 13136p−m+V20
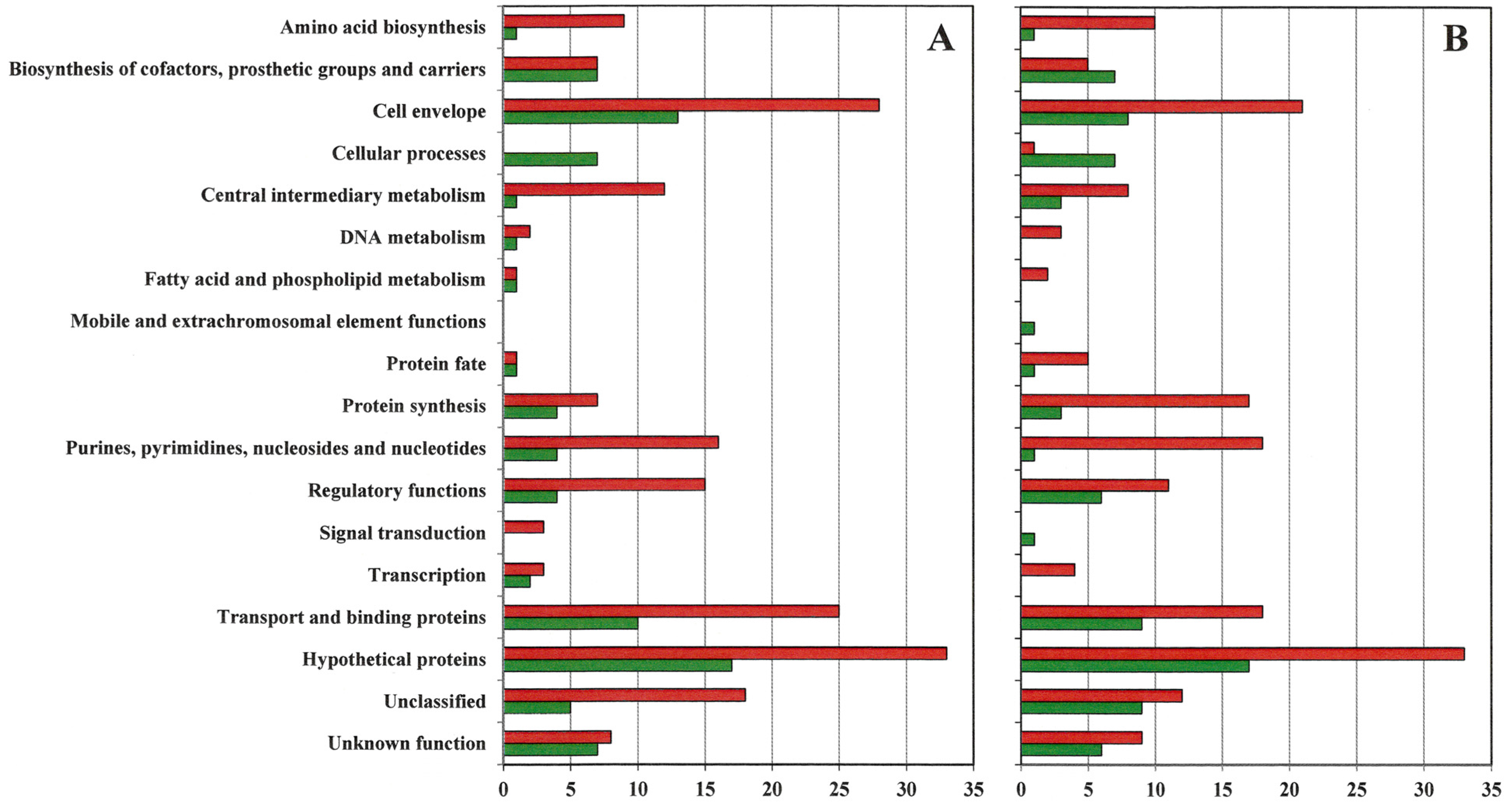
2.3. Genes with at Least One Eight-Fold Expression Change among VISA and VSSA
| Locus ID | Gene | Protein | 13136p−m+V5 vs. VSSA | 13136p−m+V20 vs. VSSA | VISA V20 vs. VISA V5 | VISA-Related Categories | Stress Response Association | Gene and Protein Functional Role Comments [references] |
|---|---|---|---|---|---|---|---|---|
| SAV2009 | sec3 | enterotoxin typeC3 | −24.4 | −21.5 | 2.9 | Virulence Factor-Associated | Secreted exotoxin, same as gene sea in MRSA strain COL [11] | |
| SACOL0907 | seb | staphylococcal enterotoxin B | −17.2 | −18.3 | −1.1 | Nutritional | Secreted cytotoxin upregulated during the stringent response [45] | |
| SACOL1871 | epiG | epidermin immunity protein F | −11.2 | 11.2 | Nutritional | Virulence factor upregulated during the stringent response [45] | ||
| SAV2472 | NA | short chain dehydrogenase | −8.5 | −5.2 | 3.3 | Capsular biosynthesis enzyme from the SDR protein super-family [46] | ||
| SAV0372 | NA | predicted PepSY family membrane peptidase propeptide | −4.1 | −10.5 | −6.4 | Unknown function but likely to have a protease inhibitory function based on homology to peptidase propeptides | ||
| SAV1046 | sspC | cysteine protease | −3.4 | −8.6 | −5.2 | Secreted virulence factor Staphostatin B that inhibits sspB2-encoded serine protease Staphopain B activity [47] | ||
| SACOL2026 | agrA | accessory gene regulator protein A | −2.8 | −9 | −6.2 | Response regulator of the agr operon which generally upregulates secreted proteins and downregulates cell surface proteins [48] | ||
| SACOL0096 | sarS | staphylococcal accessory regulator S | 8.9 | 8.9 | Nutritional | SarA global regulator family that generally upregulates virulence factor genes; sarS is downregulated by agr and upregulated by Rot and during the stringent response [45,48,49] | ||
| SAV0111 | spa | Immunoglobulin G binding protein A precursor | 11.5 | 11.5 | General | Cell surface adhesion protein upregulated by heat shock and downregulated by cell wall-active antibiotics [5,47,50] | ||
| SAV1764 | rot | repressor of toxins Rot | 12.6 | 12.6 | Virulence Factor-Associated | Homolog of the SarA transcriptional regulator that often exerts effects opposite of agr [48] | ||
| SAV0320 | geh | glycerol ester hydrolase | 2.7 | 12 | 9.3 | rot-regulated lipase translated as an inactive, truncated form due bacteriophage L54a integration [10,49] | ||
| SAV2637 | aur | zinc metalloproteinase aureolysin | 8.2 | −8.2 | Secreted virulence factor [51] | |||
| SAV2667 | icaD | intercellular adhesion protein D | 15.9 | −15.9 | Oxidative | Cell surface virulence factor downregulated by oxidative stress [25] | ||
| SACOL2689 | icaA | intercellular adhesion protein A | 25.2 | −25.2 | Oxidative | Cell surface virulence factor downregulated by oxidative stress [25] | ||
| SAS0236 | scdA | cell wall metabolism protein ScdA | −2 | 9.5 | 11.5 | Cell Wall Metabolism | Oxidative | ScdA is involved in peptidoglycan cross-linking and cell division, and upregulated by oxidative stress [25] |
| SACOL0034 | mecR1 | methicillin-resistance MecR1 regulatory protein | 38.5 | −38.5 | Cell Wall-Active Antibiotics | Integral membrane metalloprotease acting as a beta-lactam sensing signal transducer [52] | ||
| SAV0041 | mecA | penicillin binding protein 2A | 48.5 | −48.5 | Nutritional & Cell Wall-Active Antibiotics | Alternative PBP imparting mec-mediated resistance to beta-lactam antibiotics | ||
| SACOL2147 | NA | transcriptional antiterminator, BglG family/DNA-binding protein | −12 | −4.4 | 7.6 | Central Intermediary Metabolism | Oxidative | Regulatory functions; downregulated by oxidative stress [25] |
| SACOL1573 | NA | integrase/recombinase, core domain family | −10.6 | −12.1 | −1.5 | DNA Damage | Pseudogene not located within a prophage or pathogenicity island | |
| SAV2328 | NA | dehydrogenase | −9.9 | −4.4 | 5.5 | Unknown function; from the SDR protein super-family [53] | ||
| SAV2182 | asp23 | alkaline shock protein 23 | −9.8 | −19.7 | −9.9 | Nutritional & General | σB-regulated general stress response gene upregulated in VISA, by alkaline or heat shock, and during the stringent response [45,50,54] | |
| SACOL1114 | NA | Mn2+/Fe2+ transporter, NRAMP family | −9.5 | −9.4 | 0.1 | Oxidative & Nutritional | Gene upregulated by oxidative stress - Mn2+ and Fe2+ are important for oxidative stress resistance, and Mn may have a role in virulence related competition with hosts for limited nutrient [25,55] | |
| SAV1074 | purD | phosphoribosylamine-glycine ligase | −9.5 | −2.3 | 7.2 | Nutritional | Purine ribonucleotide biosynthesis gene downregulated during the stringent response [45] | |
| SAV1072 | purN | phosphoribosylglycinamide formyltransferase | −9.5 | −2.5 | 7 | Purine ribonucleotide biosynthesis gene [56] | ||
| SAV2185 | NA | glycine betaine transporter opuD homolog | −9.2 | −14.5 | −5.3 | Osmotic & General | The opuCABCD operon is upregulated by osmotic stress and part of the general stress response [57] | |
| SAS0678 | NA | glutamine amidotransferase class-I protein | −8.6 | −5.4 | 3.2 | Subunit of anthranilate synthase, an enzyme from the glutamate-consuming folate biosynthetic pathway | ||
| SACOL0872 | NA | OsmC/Ohr family protein | −8.5 | −5.7 | 2.8 | Osmotic | Membrane protein of unknown function induced by osmotic stress | |
| SAV1071 | purM | phosphoribosylaminoimidazole synthetase | −8.4 | −2.2 | 6.2 | Nutritional | Purine ribonucleotide biosynthesis gene downregulated during the stringent response [45] | |
| SACOL0630 | NA | amino acid permease | −6 | −9.5 | −3.5 | Transmembrane amino acid transporter protein | ||
| SACOL2428 | bioD | dethiobiotin synthase | 8.7 | 8.7 | Biotin biosynthesis enzyme; competition for biotin may play an important role in phagosome escape [58] | |||
| SACOL0032 | maoC | (R)-specific enoyl-CoA hydratase | 23.7 | −23.7 | Amino acid degradation enzyme in aerobic phenylalanine/phenylacetate catabolism [59] | |||
| SACOL0866 | NA | hypothetical protein | −12.9 | −3.8 | 9.1 | Hypothetical Proteins | ||
| SAR0592 | NA | hypothetical protein | −12.3 | −15.8 | −3.5 | |||
| SAV0823 | NA | hypothetical protein | −11.3 | −3.1 | 8.2 | |||
| SAR2275 | NA | hypothetical protein | −9.8 | −20.4 | −10.6 | |||
| SACOL2547 | NA | hypothetical protein | −9.5 | 4.2 | 13.7 | |||
| SACOL2720 | NA | hypothetical protein | −9.4 | −7 | 2.4 | |||
| SAS2396a | NA | hypothetical protein | −8.3 | −6.4 | 1.9 | |||
| SAV2565 | NA | hypothetical protein | −8.2 | −2.3 | 5.9 | |||
| SAS2047 | NA | hypothetical protein | −8.1 | −3.9 | 4.2 | |||
| SACOL2174 | NA | hypothetical protein | −7.4 | −20.2 | −12.8 | |||
| SACOL1679 | NA | hypothetical protein | −7.3 | −10.3 | −3 | |||
| SACOL2175 | NA | hypothetical protein | −6.8 | −17.3 | −10.5 | |||
| SACOL1680 | NA | hypothetical protein | −6.3 | −8.4 | −2.1 | |||
| SAV2474 | NA | hypothetical protein | −5.5 | −10.8 | −5.3 | |||
| SACOL0912 | NA | hypothetical protein | −5.4 | −8.3 | −2.9 | |||
| SACOL1574 | NA | hypothetical protein | −5.4 | −8.6 | −3.2 | |||
| SACOL0908 | NA | hypothetical protein | −3.8 | −8.8 | −5 | |||
| SAS0281 | NA | hypothetical protein | 10 | 3.6 | −6.4 | |||
| SACOL0625 | NA | hypothetical protein | 11.2 | 2.5 | −8.7 | |||
| SACOL0067 | NA | hypothetical protein | 12.6 | 5 | −7.6 | |||
| SAV2556 | NA | hypothetical protein | 12.8 | 3.6 | −9.2 |
2.4. Metabolomics
| Metabolite Class | Metabolite | Metabolite relative concentration per 10 mg dry weight (mean ± SD) | Metabolite Relative Concentration Fold-Change | ||||
|---|---|---|---|---|---|---|---|
| VSSA 13136p−m+ | 13136p−m+V5 | 13136p−m+V20 | VSSA → V5 | VSSA → V20 | V5 → V20 | ||
| Amines & Polyamines | 2-Amino-4,6-dihydroxypyrimidine | 1.4 ± 0.2 | 1.9 ± 0.4 | 1.3 ± 0.3 | 1.4 | −1.1 | −1.4 |
| 4,5-Dimethyl-2,6-hydroxypyrimidine | 0.3 ± 0.1 | 0.3 ± 0.0 | 0.2 ± 0.0 | −1.5 | −1.5 | ||
| 5-Methylthioadenosine | 2.7 ± 0.2 | 2.4 ± 0.6 | 4.6 ± 1.0 | −1.3 | 1.7 | 1.9 | |
| Adenine | 181.5 ± 21.5 | 138.5 ± 27.2 | 74.4 ± 5.5 | −1.3 | −2.4 | −1.9 | |
| Adenosine | 125.0 ± 21.5 | 300.9 ± 94.4 | 353.6 ± 10.3 | 2.4 | 2.8 | 1.2 | |
| Adenosine-5-monophosphate | ND | ND | 5.7 ± 1.2 | >100 | >100 | ||
| Cytosine | 5.5 ± 0.6 | 5.7 ± 1.7 | 3.3 ± 0.3 | −1.7 | −1.7 | ||
| Dihydroorotic acid | ND | 3.8 ± 0.7 | 171.8 ± 27.9 | >100 | >100 | 45.2 | |
| Ethanolamine | 2.7 ± 0.7 | 4.0 ± 0.8 | 1.4 ± 0.1 | 1.5 | −2 | −2.9 | |
| Glucosamine | ND | 6.9 ± 1.5 | 129.4 ± 30.5 | >100 | >100 | 18.8 | |
| Guanine | 16.2 ± 1.2 | 2.7 ± 0.8 | 1.7 ± 0.3 | −5 | −10 | −1.6 | |
| Guanosine | 10.9 ± 2.3 | ND | 42.7 ± 8.6 | <−100 | 3.9 | >100 | |
| Hydroxylamine | 1.0 ± 0.1 | 4.3 ± 0.8 | 1.9 ± 0.2 | 4.3 | 1.9 | −2.3 | |
| Hypoxanthine | ND | ND | 0.1 ± 0.0 | >10 | >10 | ||
| Inosine | ND | ND | 3.3 ± 0.7 | >100 | >100 | ||
| Nicotinamide | 19.0 ± 1.7 | 13.0 ± 1.6 | 25.9 ± 1.1 | −1.4 | 1.4 | 2 | |
| Nicotinic acid | 4.0 ± 0.9 | 0.5 ± 0.1 | 9.5 ± 1.5 | −8 | 2.4 | 19 | |
| Orotic acid | 1.7 ± 0.1 | 1.5 ± 0.1 | 38.7 ± 1.3 | −1.1 | 23 | 25.8 | |
| Putrescine | 63.3 ± 3.7 | 63.5 ± 6.5 | 11.8 ± 1.8 | −5.4 | −5.4 | ||
| Spermidine | 42.1 ± 5.7 | 4.5 ± 0.5 | 3.7 ± 0.6 | −9.4 | −11.4 | −1.2 | |
| Thymine | 15.0 ± 2.4 | 4.4 ± 1.1 | 3.4 ± 0.7 | −3.4 | −4.4 | −1.3 | |
| Uracil | 23.7 ± 2.1 | 4.4 ± 0.7 | 6.5 ± 0.8 | −5.4 | −3.6 | 1.5 | |
| Urea | 5.6 ± 0.5 | 11.4 ± 2.2 | 3.7 ± 0.6 | 2 | −1.5 | −3.1 | |
| Uridine | 9.3 ± 1.0 | 57.6 ± 7.4 | 13.2 ± 0.9 | 6.2 | 1.4 | −4.4 | |
| Amino Acids | 4-hydroxyproline | 5.5 ± 0.6 | 6.3 ± 1.7 | 3.9 ± 1.1 | 1.1 | −1.4 | −1.6 |
| Alanine | 498.0 ± 47.1 | 525.3 ± 67.1 | 493.2 ± 41.0 | 1.1 | −1.1 | ||
| Asparagine | 18.9 ± 3.0 | 36.8 ± 4.5 | 14.7 ± 2.5 | 1.1 | −1.3 | −2.5 | |
| Aspartic acid | 975.4 ± 127.0 | 2203.8 ± 446.5 | 2303.0 ± 397.1 | 2.3 | 2.4 | ||
| Cystathionine | 4.5 ± 0.8 | ND | ND | <−100 | <−100 | ||
| Glutamic acid | 20.5 ± 4.0 | 284.7 ± 76.8 | 308.5 ± 36.4 | 14 | 15 | 1.1 | |
| Glycine | 33.8 ± 10.3 | 45.7 ± 6.2 | 109.5 ± 9.8 | 1.4 | 3.2 | 2.4 | |
| Homocysteine | 1.5 ± 0.3 | 3.7 ± 0.8 | 5.7 ± 1.0 | 2.5 | 3.8 | 1.5 | |
| Homoserine | 1.0 ± 0.2 | ND | 0.6 ± 0.1 | <−100 | −1.7 | >100 | |
| Isoleucine | 26.5 ± 3.5 | 36.0 ± 7.1 | 88.9 ± 9.9 | 1.4 | 3.4 | 2.4 | |
| Leucine | 118.1 ± 24.2 | 330.4 ± 40.9 | 451.9 ± 68.2 | 2.8 | 3.8 | 1.4 | |
| Lysine | 354.5 ± 39.9 | 276.8 ± 20.6 | 121.9 ± 27.5 | −1.3 | −2.9 | −2.3 | |
| Methionine | 10.4 ± 2.1 | 11.7 ± 2.8 | 8.5 ± 1.2 | 1.1 | −1.2 | −1.4 | |
| N-Acetylglutamic acid | ND | ND | 25.6 ± 1.5 | >100 | >100 | ||
| O-Acetyl-serine | 5.4 ± 1.3 | 1.0 ± 0.2 | ND | −5.4 | <−100 | <−100 | |
| Ornithine | 30.8 ± 4.8 | 16.6 ± 3.1 | 1.2 ± 0.2 | −2 | −26 | −13.8 | |
| Phenylalanine | 165.9 ± 29.1 | 237.0 ± 30.3 | 116.5 ± 9.3 | 1.4 | −1.4 | −2 | |
| Proline | 10.9 ± 1.5 | 154.6 ± 30.0 | 239.8 ± 8.0 | 14 | 22 | 1.6 | |
| Pyroglutamic acid | 1017.3 ± 159.3 | 1017.6 ± 136.4 | 1659.0 ± 164.2 | 1.6 | 1.6 | ||
| Serine | 11.5 ± 1.3 | 7.2 ± 1.4 | 9.5 ± 2.9 | −1.6 | −1.2 | 1.3 | |
| Threonine | 52.8 ± 10.6 | 20.0 ± 8.5 | 7.8 ± 1.2 | −2.6 | −6.8 | −2.6 | |
| Tryptophan | 29.7 ± 4.8 | 8.5 ± 1.3 | 11.9 ± 3.0 | −3.5 | −2.5 | 1.4 | |
| Tyrosine | 33.6 ± 7.4 | 40.8 ± 5.7 | 26.3 ± 2.2 | 1.2 | −1.3 | −1.6 | |
| Valine | 63.1 ± 14.3 | 152.9 ± 25.8 | 170.6 ± 35.3 | 2.4 | 2.7 | 1.1 | |
| β-Alanine | 57.4 ± 7.6 | 272.3 ± 42.6 | 95.1 ± 12.9 | 4.7 | 1.7 | −2.9 | |
| Polar Organic Acids | 2-Hydroxyglutaric acid | 6.1 ± 0.4 | 2.5 ± 0.3 | 2.8 ± 0.2 | −2.4 | −2.2 | 1.1 |
| 2-Phosphoglycerate | 14.6 ± 2.0 | 7.5 ± 1.9 | 3.0 ± 0.6 | −2 | −5 | −2.5 | |
| 3-Hydroxybenzoic acid | 0.3 ± 0.0 | 0.4 ± 0.1 | 0.5 ± 0.0 | 1.3 | 1.7 | 1.3 | |
| 3-Phosphoglycerate | 242.6 ± 45.1 | 62.7 ± 10.9 | 40.1 ± 7.5 | −3.9 | −6 | −1.6 | |
| Aminomalonic acid | 0.6 ± 0.1 | 0.21 ± 0.1 | 0.7 ± 0.1 | 2.9 | 1.2 | 3.3 | |
| Benzoic acid | 1.6 ± 0.1 | 1.9 ± 0.2 | 1.6 ± 0.4 | 1.2 | −1.2 | ||
| cis-Aconitic acid | ND | 2.1 ± 0.2 | ND | >100 | <−100 | ||
| Citramalic acid | 14.8 ± 2.7 | 19.1 ± 4.1 | 27.3 ± 5.4 | 1.3 | 1.8 | 1.4 | |
| Citric acid | 15.6 ± 2.0 | 243.7 ± 22.6 | 35.1 ± 8.4 | 16 | 2.3 | −6.9 | |
| Fumaric acid | 19.8 ± 4.5 | 1.5 ± 0.4 | 28.6 ± 4.0 | −12.5 | 1.4 | 19.1 | |
| Glucaric acid | 1.2 ± 0.3 | 2.7 ± 0.4 | 3.0 ± 0.1 | 2.3 | 2.6 | 1.1 | |
| Gluconic acid | 0.6 ± 0.1 | 11.8 ± 2.2 | 5.8 ± 2.1 | 20 | 10 | −2 | |
| Glyceric acid | 7.0 ± 0.9 | 6.9 ± 1.3 | 3.7 ± 0.2 | −2 | −1.9 | ||
| Glycolic acid | 5.2 ± 0.6 | 5.1 ± 0.9 | 20.2 ± 1.8 | 3.9 | 4 | ||
| Lactic acid | 358.9 ± 52.4 | 320.6 ± 36.2 | 682.9 ± 60.6 | −1.1 | 1.9 | 2.1 | |
| Malic acid | 3.4 ± 0.6 | ND | 12.9 ± 2.3 | <−100 | 3.7 | >100 | |
| Monomethylphosphate | 104.8 ± 24.0 | 161.3 ± 2.0 | 258.1 ± 17.9 | 1.5 | 2.5 | 1.6 | |
| Oxyphosphinyloxyacetate | 3.8 ± 0.1 | 3.4 ± 0.3 | 6.6 ± 1.0 | −1.1 | 1.7 | 1.9 | |
| Pantothenate | ND | ND | 2.2 ± 0.0 | >100 | >100 | ||
| Phenylpyruvic acid | ND | ND | 3.4 ± 0.4 | >100 | >100 | ||
| Pyruvic acid | 58.1 ± 10.5 | 38.9 ± 15.8 | 31.4 ± 4.8 | −1.4 | −2 | −1.2 | |
| Succinic acid | 36.1 ± 8.7 | 31.6 ± 8.2 | 42.9 ± 9.6 | −1.1 | 1.2 | 1.4 | |
| α-Glycerophosphate | 824.3 ± 131.3 | 1487.9 ± 121.8 | 920.7 ± 76.9 | 1.8 | 1.1 | −1.6 | |
| α-Ketoglutaric acid | 30.7 ± 6.9 | 11.1 ± 2.1 | 18.2 ± 2.7 | −2.8 | −1.7 | 1.6 | |
| β-Lactate | 3.6 ± 0.8 | 2.0 ± 0.2 | 8.5 ± 1.7 | −1.7 | 2.3 | 4.3 | |
| β-Phenyllactic acid | 3.0 ± 0.6 | 1.7 ± 0.2 | 7.3 ± 0.7 | −1.7 | 2.4 | 4.3 | |
| Sugars | Arabitol | 6.3 ± 1.3 | 12.0 ± 2.3 | 4.8 ± 0.7 | 1.9 | −1.3 | −2.5 |
| Fructose | 40.5 ± 7.5 | 4.0 ± 0.8 | 1.8 ± 0.2 | −10 | −23 | −2.2 | |
| Fructose-6-P | 6.7 ± 2.0 | 5.2 ± 1.0 | 3.0 ± 0.7 | −1.3 | −2.5 | −1.7 | |
| Galactitol | ND | 2.1 ± 0.2 | 2.0 ± 0.3 | >100 | >100 | −1.1 | |
| Galactopyranose | 2.2 ± 0.5 | 3.4 ± 0.7 | 2.0 ± 0.1 | 1.5 | −1.1 | −1.7 | |
| Galactose | 5.1 ± 1.3 | 7.9 ± 0.8 | 1.5 ± 0.3 | 1.5 | −3.3 | −5.3 | |
| Glucose-1-P | 20.3 ± 5.2 | 13.8 ± 3.4 | 2.3 ± 0.6 | −1.4 | −10 | −6 | |
| Glucose-6-P | 13.6 ± 0.7 | 1.6 ± 0.3 | 0.5 ± 0.1 | −8.5 | −27.2 | −3.2 | |
| Glycerol | 569.8 ± 71.4 | 668.7 ± 76.0 | 611.0 ± 103.9 | 1.2 | 1.1 | −1.1 | |
| Glycerol-2-P | 28.2 ± 7.4 | 12.1 ± 3.0 | 7.6 ± 1.5 | −2.5 | −3.3 | −1.6 | |
| Inositol | 3.1 ± 1.0 | 12.8 ± 1.1 | 0.1 ± 0.0 | 4.1 | −31 | −128 | |
| Inositol, -chiro- | 0.8 ± 0.1 | 1.5 ± 0.3 | 1.9 ± 0.1 | 1.8 | 2.2 | 1.3 | |
| Mannitol | 178.1 ± 15.6 | 29.5 ± 7.2 | 32.3 ± 3.1 | −6 | −5.5 | 1.1 | |
| Mannitol-P | 36.6 ± 3.2 | 9.0 ± 1.3 | 14.3 ± 2.8 | −4.1 | −2.6 | 1.6 | |
| Mannose | 7.2 ± 1.0 | 12.8 ± 2.4 | 1.2 ± 0.2 | 1.8 | −6 | −10.7 | |
| Mannose-6-P | 13.2 ± 2.9 | ND | ND | <−100 | <−100 | ||
| Ribitol | 122.9 ± 22.0 | 24.2 ± 4.4 | 76.2 ± 15.4 | −5 | −1.6 | 3.1 | |
| Ribose | 47.9 ± 9.4 | 42.0 ± 7.1 | 25.0 ± 3.3 | −1.1 | −2 | −1.7 | |
| Ribose-5-P | 4.3 ± 0.5 | 30.6 ± 1.8 | 12.5 ± 2.0 | 7.2 | 2.9 | −2.4 | |
| Sedoheptulose | 2.3 ± 0.7 | 2.2 ± 0.0 | ND | <−100 | <−100 | ||
| Sedoheptulose-7-P | 1.0 ± 0.0 | ND | ND | <−100 | <−100 | ||
| Sucrose | 14.5 ± 2.5 | 1.4 ± 0.7 | 6.4 ± 0.2 | −10 | −2.3 | 4.6 | |
| Trehalose | 2.5 ± 0.3 | 17.6 ± 0.7 | 3.4 ± 0.8 | 7 | 1.4 | −5.2 | |
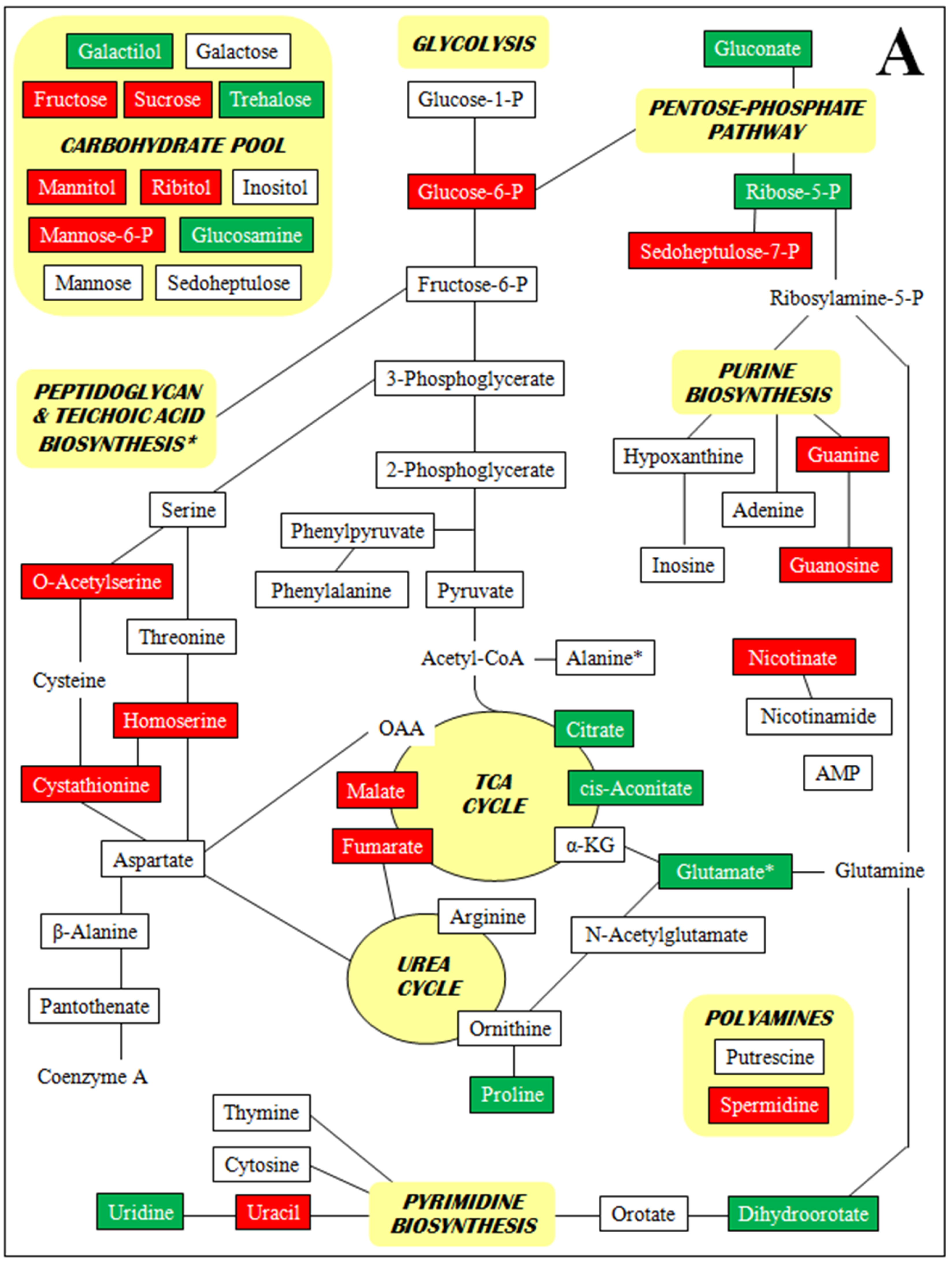
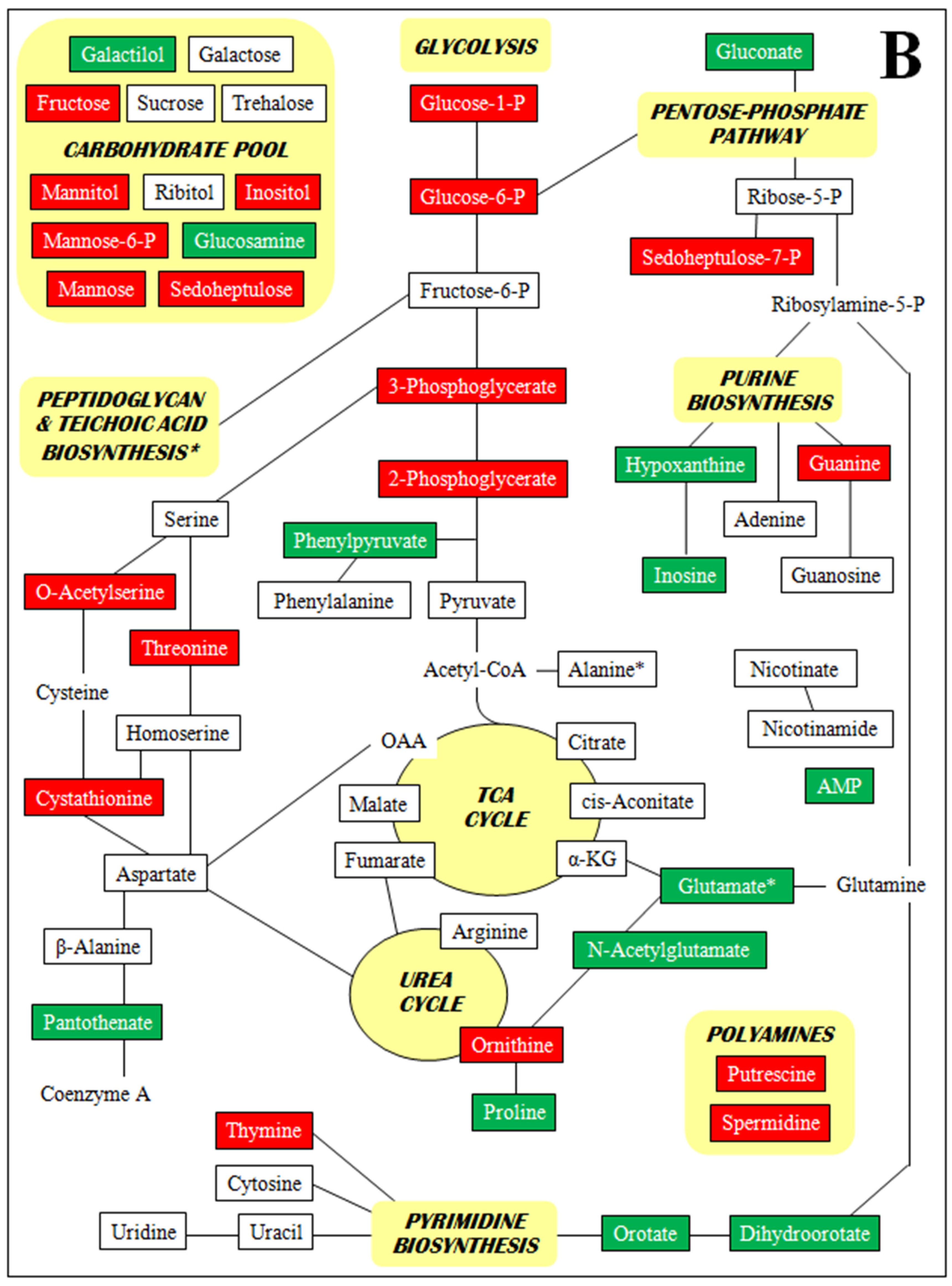
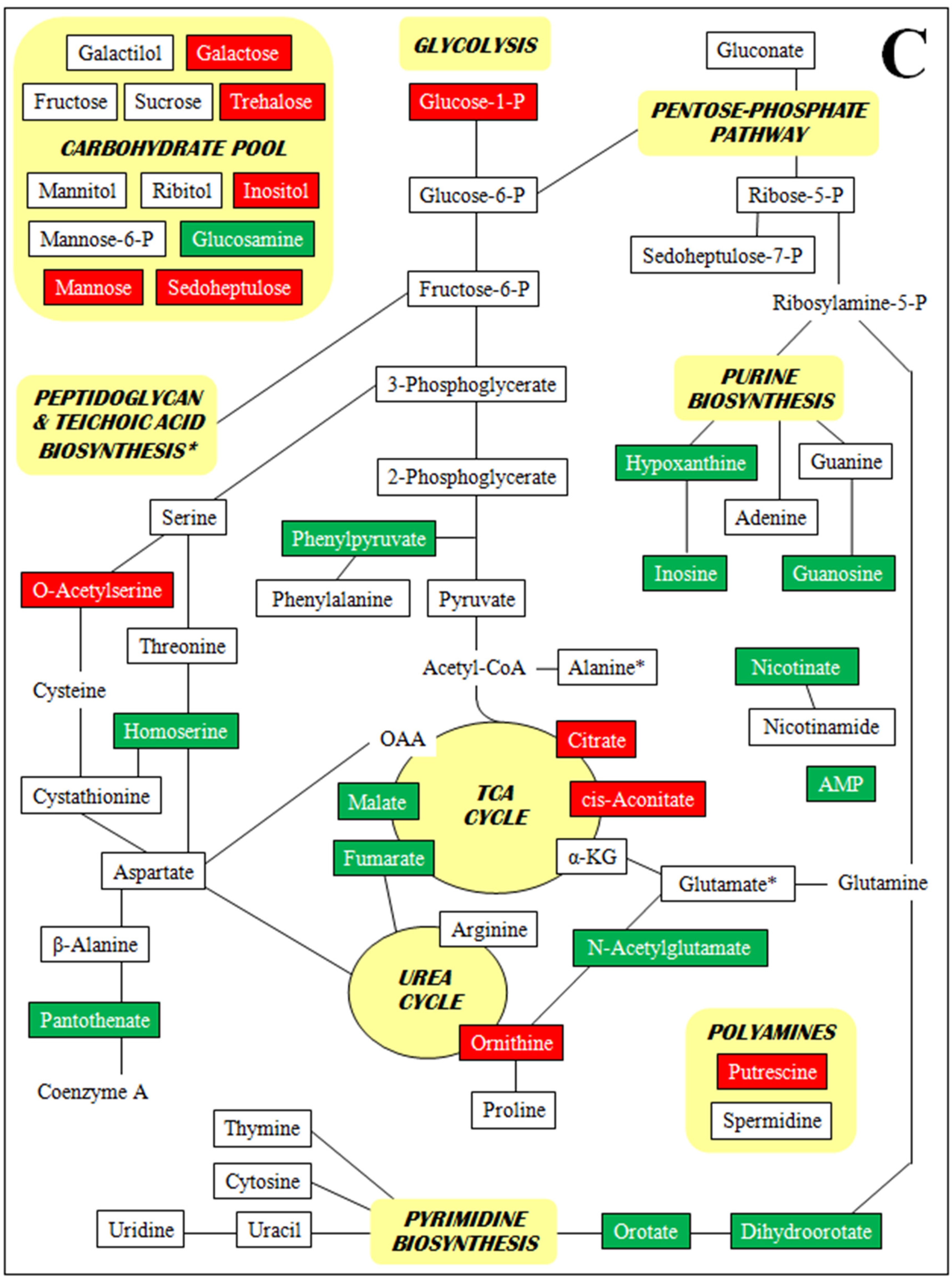
3. Discussion
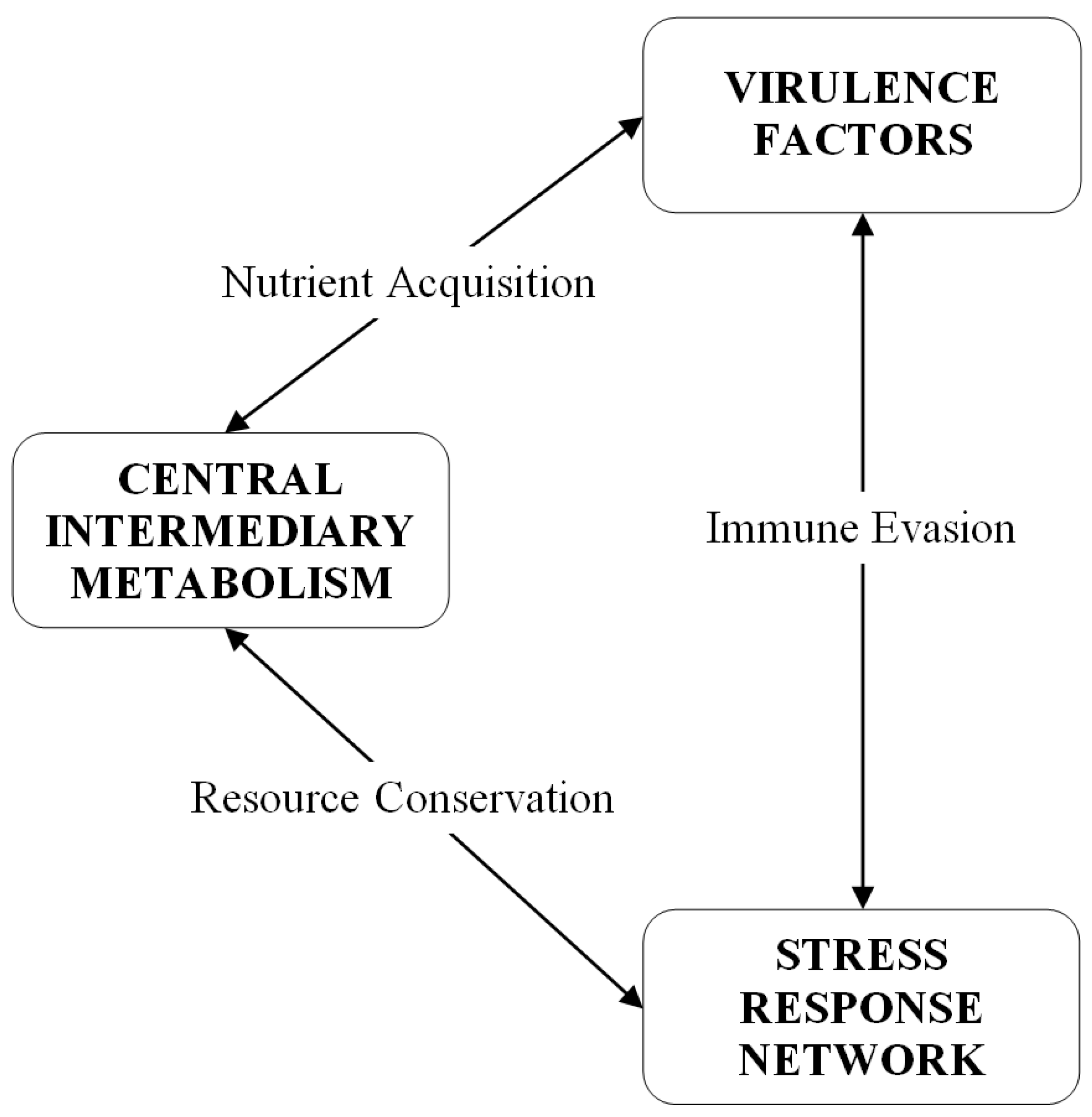
4. Experimental Section
4.1. Strains and Growth Conditions
4.2. Complete-Genome Comparisons
4.3. Transcriptional Profiling
4.4. Microarray Validation by Real-Time Reverse Transcription-PCR (RT-PCR)
4.5. Metabolomic Analysis
5. Conclusions
Supplementary Tables and Figures
- Supplementary Table S1. Two-fold or greater gene expression changes in VISA 13136p−m+V5 and 13136p−m+V20 versus parent VSSA 13136p−m+.
- Supplementary Table S2. Expression patterns by gene functional group in VISA 13136p−m+V5 and 13136p−m+V20 as number of genes upregulated and downregulated at least two-fold relative to gene expression in VSSA 13136p−m+.
- Supplementary Table S3. Concordance of expression patterns vs. parent VSSA 13136p−m+ between 13136p−m+V5 and 13136p−m+V20 for the 335 genes with expression data for both VISA.
- Supplementary Table S4. The 45 metabolites with at least one five-fold change among comparisons between 13136p−m+, 13136p−m+V5 and 13136p−m+V20.
- Supplementary Figure S1. Number of genes by functional group upregulated and downregulated at least eight-fold in VISA 13136p−m+V5 (A) and 13136p−m+V20 (B) relative to gene expression in VSSA parent 13136p−m+.
- Supplementary Figure S2. Metabolomic profiles by metabolite class (A–D).
- Supplementary File S1. Metabolomic analysis background information.
Acknowledgments
Conflicts of Interest
References
- Hiramatsu, K.; Aritaka, N.; Hanaki, H.; Kawasaki, S.; Hosoda, Y.; Hori, S.; Fukuchi, Y.; Kobayashi, I. Dissemination in Japanese hospitals of strains of Staphylococcus aureus heterogeneously resistant to vancomycin. Lancet 1997, 350, 1670–1673. [Google Scholar] [CrossRef] [PubMed]
- Pfeltz, R.F.; Wilkinson, B.J. The escalating challenge of vancomycin resistance in Staphylococcus aureus. Curr. Drug Targets Infect. Disord. 2004, 4, 273–294. [Google Scholar] [CrossRef] [PubMed]
- Howden, B.P.; Davies, J.K.; Johnson, P.D.; Stinear, T.P.; Grayson, M.L. Reduced vancomycin susceptibility in Staphylococcus aureus, including vancomycin-intermediate and heterogeneous vancomycin-intermediate strains: resistance mechanisms, laboratory detection, and clinical implications. Clin. Microbiol. Rev. 2010, 23, 99–139. [Google Scholar] [CrossRef] [PubMed]
- Howden, B.P.; Peleg, A.Y.; Stinear, T.P. The evolution of vancomycin intermediate Staphylococcus aureus (VISA) and heterogenous-VISA. Infect. Genet. Evol. 2013, 13, S1567–S1348. [Google Scholar] [PubMed]
- Utaida, S.; Dunman, P.M.; Macapagal, D.; Murphy, E.; Projan, S.J.; Singh, V.K.; Jayaswal, R.K.; Wilkinson, B.J. Genome-wide transcriptional profiling of the response of Staphylococcus aureus to cell-wall-active antibiotics reveals a cell-wall-stress stimulon. Microbiology 2003, 149, 2719–2732. [Google Scholar] [CrossRef] [PubMed]
- McAleese, F.; Wu, S.W.; Sieradzki, K.; Dunman, P.; Murphy, E.; Projan, S.; Tomasz, A. Overexpression of genes of the cell wall stimulon in clinical isolates of Staphylococcus aureus Exhibiting vancomycin-intermediate-S. aureus-type resistance to vancomycin. J. Bacteriol. 2006, 188, 1120–1133. [Google Scholar] [CrossRef] [PubMed]
- Pfeltz, R.F.; Singh, V.K.; Schmidt, J.L.; Batten, M.A.; Baranyk, C.S.; Nadakavukaren, M.J.; Jayaswal, R.K.; Wilkinson, B.J. Characterization of passage-selected vancomycin-resistant Staphylococcus aureus strains of diverse parental backgrounds. Antimicrob. Agents Chemother. 2000, 44, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Jevons, M.P. “Celbenin”—Resistant Staphylococci. Br. Med. J. 1961, 1, 124–125. [Google Scholar] [CrossRef]
- Dyke, K.G. Penicillinase production and intrinsic resistance to penicillins in methicillin-resistant cultures of Staphylococcus aureus. J. Med. Microbiol. 1969, 3, 261–278. [Google Scholar] [CrossRef]
- Lee, C.Y.; Iandolo, J.J. Integration of staphylococcal phage L54a occurs by site-specific recombination: Structural analysis of the attachment sites. Proc. Natl. Acad. Sci. USA 1986, 83, 5474–5478. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.R.; Fouts, D.E.; Archer, G.L.; Mongodin, E.F.; Deboy, R.T.; Ravel, J.; Paulsen, I.T.; Kolonay, J.F.; Brinkac, L.; Beanan, M.; et al. Insights on evolution of virulence and resistance from the complete genome analysis of an early methicillin-resistant Staphylococcus aureus strain and a biofilm-producing methicillin-resistant Staphylococcus epidermidis strain. J. Bacteriol. 2005, 187, 2426–2438. [Google Scholar] [CrossRef] [PubMed]
- Parker, M.T.; Jevons, M.P. A survey of methicillin resistance in Staphylococcus. Postgrad. Med. J. 1964, 40 (Suppl), 170–178. [Google Scholar] [CrossRef] [PubMed]
- Koehl, J.L.; Muthaiyan, A.; Jayaswal, R.K.; Ehlert, K.; Labischinski, H.; Wilkinson, B.J. Cell wall composition and decreased autolytic activity and lysostaphin susceptibility of glycopeptide-intermediate Staphylococcus aureus. Antimicrob. Agents Chemother. 2004, 48, 3749–3757. [Google Scholar] [CrossRef] [PubMed]
- Oshida, T.; Sugai, M.; Komatsuzawa, H.; Hong, Y.M.; Suginaka, H.; Tomasz, A. A Staphylococcus aureus autolysin that has an N-acetylmuramoyl-L-alanine amidase domain and an endo-β-N-acetylglucosaminidase domain. Cloning, sequence analysis, and characterization. Proc. Natl. Acad. Sci. USA 1995, 92, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Fiehn, O. Metabolomics—The link between genotypes and phenotypes. Plant. Mol. Biol. 2002, 48, 155–171. [Google Scholar] [CrossRef] [PubMed]
- Hanaki, H.; Labischinski, H.; Inaba, Y.; Kondo, N.; Murakami, H.; Hiramatsu, K. Increase in glutamine-non-amidated muropeptides in the peptidoglycan of vancomycin-resistant Staphylococcus aureus strain Mu50. J. Antimicrob. Chemother. 1998, 42, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Nelson, J.L.; Rice, K.C.; Slater, S.R.; Fox, P.M.; Archer, G.L.; Bayles, K.W.; Fey, P.D.; Kreiswirth, B.N.; Somerville, G.A. Vancomycin-intermediate Staphylococcus aureus strains have impaired acetate catabolism: Implications for polysaccharide intercellular adhesin synthesis and autolysis. Antimicrob. Agents Chemother. 2007, 51, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Alexander, E.L.; Gardete, S.; Bar, H.Y.; Wells, M.T.; Tomasz, A.; Rhee, K.Y. Intermediate-type vancomycin resistance (VISA) in genetically-distinct Staphylococcus aureus isolates is linked to specific, reversible metabolic alterations. PLOS ONE 2014, 9, e97137. [Google Scholar] [CrossRef] [PubMed]
- Kanamaru, K.; Stephenson, S.; Perego, M. Overexpression of the PepF oligopeptidase inhibits sporulation initiation in Bacillus subtilis. J. Bacteriol. 2002, 184, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.F.; Goss, L.; Dworkin, J. Eukaryote-like serine/threonine kinases and phosphatases in bacteria. Microbiol. Mol. Biol. Rev. 2011, 75, 192–212. [Google Scholar]
- Ogura, M.; Tanaka, T. Bacillus subtilis comZ (yjzA) negatively affects expression of comG but not comK. J. Bacteriol. 2000, 182, 4992–4994. [Google Scholar] [CrossRef] [PubMed]
- Suematsu, T.; Yokobori, S.; Morita, H.; Yoshinari, S.; Ueda, T.; Kita, K.; Takeuchi, N.; Watanabe, Y. A bacterial elongation factor G homologue exclusively functions in ribosome recycling in the spirochaete Borrelia burgdorferi. Mol. Microbiol. 2010, 75, 1445–1454. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.J.; Tsai, T.Y.; Pan, T.M. Physiological response and protein expression under acid stress of Escherichia coli O157:H7 TWC01 isolated from Taiwan. J. Agric. Food Chem. 2007, 5, 7182–7191. [Google Scholar] [CrossRef]
- Kapralou, S.; Fabbretti, A.; Garulli, C.; Spurio, R.; Gualerzi, C.O.; Dahlberg, A.E.; Pon, C.L. Translation initiation factor IF1 of Bacillus stearothermophilus and Thermus thermophilus substitute for Escherichia coli IF1 in vivo and in vitro without a direct IF1-IF2 interaction. Mol. Microbiol. 2008, 70, 1368–1377. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.; Small, D.A.; Toghrol, F.; Bentley, W.E. Global transcriptome analysis of Staphylococcus aureus response to hydrogen peroxide. J. Bacteriol. 2006, 188, 1648–1659. [Google Scholar] [CrossRef] [PubMed]
- Maul, R.W.; Sanders, L.H.; Lim, J.B.; Benitez, R.; Sutton, M.D. Role of Escherichia coli DNA polymerase I in conferring viability upon the dnaN159 mutant strain. J. Bacteriol. 2007, 189, 4688–4695. [Google Scholar] [CrossRef] [PubMed]
- Sobral, R.G.; Jones, A.E.; Des Etages, S.G.; Dougherty, T.J.; Peitzsch, R.M.; Gaasterland, T.; Ludovice, A.M.; de Lencastre, H.; Tomasz, A. Extensive and genome-wide changes in the transcription profile of Staphylococcus aureus induced by modulating the transcription of the cell wall synthesis gene murF. J. Bacteriol. 2007, 189, 2376–2391. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.H.; Lee, C.Y. Nucleotide sequence and genetic characterization of staphylococcal bacteriophage L54a int and xis genes. J. Bacteriol. 1989, 171, 4146–4153. [Google Scholar] [PubMed]
- Campbell, J.; Singh, A.K.; Santa Maria, J.P., Jr.; Kim, Y.; Brown, S.; Swoboda, J.G.; Mylonakis, E.; Wilkinson, B.J.; Walker, S. Synthetic lethal compound combinations reveal a fundamental connection between wall teichoic acid and peptidoglycan biosynthesis in Staphylococcus aureus. ACS Chem. Biol. 2011, 6, 106–116. [Google Scholar] [CrossRef] [PubMed]
- McRobbie, A.M.; Meyer, B.; Rouillon, C.; Petrovic-Stojanovska, B.; Liu, H.; White, M.F. Staphylococcus aureus DinG, a helicase that has evolved into a nuclease. Biochem. J. 2012, 442, 77–84. [Google Scholar] [PubMed]
- Singh, V.; Evans, G.B.; Lenz, D.H.; Mason, J.M.; Clinch, K.; Mee, S.; Painter, G.F.; Tyler, P.C.; Furneaux, R.H.; Lee, J.E.; et al. Femtomolar transition state analogue inhibitors of 5'-methylthioadenosine/S-adenosylhomocysteine nucleosidase from Escherichia coli. J. Biol. Chem. 2005, 280, 18265–18273. [Google Scholar] [CrossRef] [PubMed]
- Kappes, R.M.; Kempf, B.; Kneip, S.; Boch, J.; Gade, J.; Meier-Wagner, J.; Bremer, E. Two evolutionarily closely related ABC transporters mediate the uptake of choline for synthesis of the osmoprotectant glycine betaine in Bacillus subtilis. Mol. Microbiol. 1999, 32, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Wehmeier, L.; Schäfer, A.; Burkovski, A.; Krämer, R.; Mechold, U.; Malke, H.; Pühler, A.; Kalinowski, J. The role of the Corynebacterium glutamicum rel gene in (p)ppGpp metabolism. Microbiology 1998, 144, 1853–1862. [Google Scholar] [CrossRef] [PubMed]
- Wendrich, T.M.; Marahiel, M.A. Cloning and characterization of a relA/spoT homologue from Bacillus subtilis. Mol. Microbiol. 1997, 26, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Chakraburtty, R.; White, J.; Takano, E.; Bibb, M. Cloning, characterization and disruption of a (p)ppGpp synthetase gene (relA) of Streptomyces coelicolor A3(2). Mol. Microbiol. 1996, 19, 357–368. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, A.J.; Lindsay, J.A. Genetic variation in Staphylococcus aureus surface and immune evasion genes is lineage associated: Implications for vaccine design and host-pathogen interactions. BMC Microbiol. 2010, 10, e173. [Google Scholar] [CrossRef]
- Ythier, M.; Resch, G.; Waridel, P.; Panchaud, A.; Gfeller, A.; Majcherczyk, P.; Quadroni, M.; Moreillon, P. Proteomic and transcriptomic profiling of Staphylococcus aureus surface LPXTG-proteins: Correlation with agr genotypes and adherence phenotypes. Mol. Cell Proteomics 2012, 11, 1123–1139. [Google Scholar] [CrossRef] [PubMed]
- Komatsuzawa, H.; Ohta, K.; Sugai, M.; Fujiwara, T.; Glanzmann, P.; Berger-Bachi, B.; Suginaka, H. Tn551-mediated insertional inactivation of the fmtB gene encoding a cell wall-associated protein abolishes methicillin resistance in Staphylococcus aureus. J. Antimicrob. Chemother. 2000, 45, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Ohlsen, K.; Donat, S. The impact of serine/threonine phosphorylation in Staphylococcus aureus. Int. J. Med. Microbiol. 2010, 300, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Beltramini, A.M.; Mukhopadhyay, C.D.; Pancholi, V. Modulation of cell wall structure and antimicrobial susceptibility by a Staphylococcus aureus eukaryote-like serine/threonine kinase and phosphatase. Infect. Immun. 2009, 77, 1406–1416. [Google Scholar] [CrossRef] [PubMed]
- Renzoni, A.; Andrey, D.O.; Jousselin, A.; Barras, C.; Monod, A.; Vaudaux, P.; Lew, D.; Kelley, W.L. Whole genome sequencing and complete genetic analysis reveals novel pathways to glycopeptide resistance in Staphylococcus aureus. PLOS ONE 2011, 6, e21577. [Google Scholar] [CrossRef] [PubMed]
- Passalacqua, K.D.; Satola, S.W.; Crispell, E.K.; Read, T.D. A mutation in the PP2C phosphatase gene in a Staphylococcus aureus USA300 clinical isolate with reduced susceptibility to vancomycin and daptomycin. Antimicrob. Agents Chemother. 2012, 13, 5212–5223. [Google Scholar] [CrossRef]
- Cameron, D.R.; Ward, D.V.; Kostoulias, X.; Howden, B.P.; Moellering, R.C.J.; Eliopoulos, G.; Peleg, A.Y. The serine/threonine phosphatase Stp1 contributes to reduced susceptibility to vancomycin and virulence in Staphylococcus aureus. J. Infect. Dis. 2012, 205, 1677–1687. [Google Scholar] [CrossRef] [PubMed]
- Hatzios, S.K.; Baer, C.E.; Rustad, T.R.; Siegrist, M.S.; Pang, J.M.; Ortega, C.; Alber, T.; Grundner, C.; Sherman, D.R.; Bertozzi, C.R. Osmosensory signaling in Mycobacterium tuberculosis mediated by a eukaryotic-like Ser/Thr protein kinase. Proc. Natl. Acad. Sci. USA 2013, 110, E5069–E5077. [Google Scholar] [CrossRef] [PubMed]
- Reiss, S.; Pané-Farré, J.; Fuchs, S.; François, P.; Liebeke, M.; Schrenzel, J.; Lindequist, U.; Lalk, M.; Wolz, C.; Hecker, M.; et al. Global analysis of the Staphylococcus aureus response to mupirocin. Antimicrob. Agents Chemother. 2012, 56, 787–804. [Google Scholar] [CrossRef] [PubMed]
- Miyafusa, T.; Caaveiro, J.M.; Tanaka, Y.; Tsumoto, K. Crystal structure of the enzyme CapF of Staphylococcus aureus reveals a unique architecture composed of two functional domains. Biochem. J. 2012, 443, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Lamamy, C.; Berthelot, A.; Bertrand, X.; Valentin, A.S.; Dos Santos, S.; Thiais, S.; Morange, V.; Girard, N.; Donnio, P.Y.; Quentin, R.; et al. Bloodstream Infection Study Group of the Réseau des Hygiénistes du Centre. CC9 livestock-associated Staphylococcus aureus emerges in bloodstream infections in French patients unconnected with animal farming. Clin. Infect. Dis. 2013, 56, e83–e86. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, A.; Månsson, M.; Bojer, M.S.; Gram, L.; Larsen, T.O.; Novick, R.P.; Frees, D.; Frøkiær, H.; Ingmer, H. Solonamide B inhibits quorum sensing and reduces Staphylococcus aureus mediated killing of human neutrophils. PLOS ONE 2014, 9, e84992. [Google Scholar] [CrossRef] [PubMed]
- Saïd-Salim, B.; Dunman, P.M.; McAleese, F.M.; Macapagal, D.; Murphy, E.; McNamara, P.J.; Arvidson, S.; Foster, T.J.; Projan, S.J.; Kreiswirth, B.N. Global regulation of Staphylococcus aureus genes by Rot. J. Bacteriol. 2003, 185, 610–619. [Google Scholar] [CrossRef] [PubMed]
- Shrihari, R.Y.; Singh, N.P. Multiplex reverse transcription polymerase chain reaction to study the expression of virulence and stress response genes in Staphylococcus aureus. J. Food Sci. 2012, 77, M95–M101. [Google Scholar] [CrossRef] [PubMed]
- Banbula, A.; Potempa, J.; Travis, J.; Fernandez-Catalán, C.; Mann, K.; Huber, R.; Bode, W.; Medrano, F. Amino-acid sequence and three-dimensional structure of the Staphylococcus aureus metalloproteinase at 1.72 A resolution. Structure 1998, 6, 1185–1193. [Google Scholar] [CrossRef] [PubMed]
- Arêde, P.; Botelho, T.; Guevara, T.; Usón, I.; Oliveira, D.C.; Gomis-Rüth, F.X. Structure-function studies of the staphylococcal methicillin resistance antirepressor MecR2. J. Biol. Chem. 2013, 288, 21267–21278. [Google Scholar] [CrossRef] [PubMed]
- Kavanagh, K.L.; Jörnvall, H.; Persson, B.; Oppermann, U. Medium- and short-chain dehydrogenase/reductase gene and protein families: The SDR superfamily: Functional and structural diversity within a family of metabolic and regulatory enzymes. Cell. Mol. Life Sci. 2008, 65, 3895–3906. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Liu, Y.; Zhao, C.; Xiao, D.; Zhang, J.; Zhang, F.; Chen, M.; Wang, H. Comparative proteomics-based identification of genes associated with glycopeptide resistance in clinically derived heterogeneous vancomycin-intermediate Staphylococcus aureus strains. PLOS ONE 2013, 8, e66880. [Google Scholar] [CrossRef] [PubMed]
- Haemig, H.A.; Moen, P.J.; Brooker, R.J. Evidence that highly conserved residues of transmembrane segment 6 of Escherichia coli MntH are important for transport activity. Biochemistry 2010, 49, 4662–4671. [Google Scholar] [CrossRef] [PubMed]
- Mongodin, E.; Finan, J.; Climo, M.W.; Rosato, A.; Gill, S.; Archer, G.L. Microarray transcription analysis of clinical Staphylococcus aureus isolates resistant to vancomycin. J. Bacteriol. 2003, 185, 4638–4643. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Hall, J.W.; Yang, J.; Ji, Y. The essential yhcSR two-component signal transduction system directly regulates the lac and opuCABCD operons of Staphylococcus aureus. PLOS ONE 2012, 7, e50608. [Google Scholar] [CrossRef] [PubMed]
- Napier, B.A.; Meyer, L.; Bina, J.E.; Miller, M.A.; Sjöstedt, A.; Weiss, D.S. Link between intraphagosomal biotin and rapid phagosomal escape in Francisella. Proc. Natl. Acad. Sci. USA 2012, 109, 18084–18089. [Google Scholar] [CrossRef] [PubMed]
- Teufel, R.; Gantert, C.; Voss, M.; Eisenreich, W.; Haehnel, W.; Fuchs, G. Studies on the mechanism of ring hydrolysis in phenylacetate degradation: A metabolic branching point. J. Biol. Chem. 2011, 286, 11021–11034. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Bachhawat, A.K. Pyroglutamic acid: Throwing light on a lightly studied metabolite. Curr. Sci. 2012, 102, 288–297. [Google Scholar]
- Ledala, N.; Zhang, B.; Seravalli, J.; Powers, R.; Somerville, G.A. The influence of iron and aeration on Staphylococcus aureus growth, metabolism, and transcription. J. Bacteriol. 2014, 196, 2178–2189. [Google Scholar] [CrossRef] [PubMed]
- Liebeke, M.; Meyer, H.; Donat, S.; Ohlsen, K.; Lalk, M. A metabolomic view of Staphylococcus aureus and its ser/thr kinase and phosphatase deletion mutants: involvement in cell wall biosynthesis. Chem. Biol. 2010, 17, 820–830. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Nandakumar, R.; Sadykov, M.R.; Madayiputhiya, N.; Luong, T.T.; Gaupp, R.; Lee, C.Y. RpiR homologues may link Staphylococcus aureus RNAIII synthesis and pentose phosphate pathway regulation. J. Bacteriol. 2011, 193, 6187–6196. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane-Khadka, R.; Cantore, S.A.; Riordan, J.T.; Delgado, A.; Norman, A.E.; Dueñas, S.; Zaman, S.; Horan, S.; Wilkinson, B.J.; Gustafson, J.E. sarA inactivation reduces vancomycin-intermediate and ciprofloxacin resistance expression by Staphylococcus aureus. Int. J. Antimicrob. Agents 2009, 34, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Lithgow, J.K.; Ingham, E.; Foster, S.J. Role of the hprT-ftsH locus in Staphylococcus aureus. Microbiology 2004, 150, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, M.; Hishinuma, T.; Katayama, Y.; Cui, L.; Kapi, M.; Hiramatsu, K. Mutation of RNApolymerase beta subunit (rpoB) promotes hVISA-to-VISA phenotypic conversion of strain Mu3. Antimicrob. Agents Chemother. 2011, 55, 4188–4195. [Google Scholar] [CrossRef] [PubMed]
- Keaton, M.A.; Rosato, R.R.; Plata, K.B.; Singh, C.R.; Rosato, A.E. Exposure of clinical MRSA heterogeneous strains to β-lactams redirects metabolism to optimize energy production through the TCA cycle. PLOS ONE 2013, 8, e71025. [Google Scholar] [CrossRef] [PubMed]
- Dordel, J.; Kim, C.; Chung, M.; Pardos de la Gándara, M.; Holden, M.T.; Parkhill, J.; de Lencastre, H.; Bentley, S.D.; Tomasz, A. Novel determinants of antibiotic resistance: Identification of mutated loci in highly methicillin-resistant subpopulations of methicillin-resistant Staphylococcus aureus. MBio 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Mwangi, M.; Chung, M.; Milheiriço, C.; de Lencastre, H.; Tomasz, A. The mechanism of heterogeneous beta-lactam resistance in MRSA: Key role of the stringent stress response. PLOS ONE 2013, 8, e82814. [Google Scholar] [CrossRef] [PubMed]
- Dalebroux, Z.D.; Svensson, S.L.; Gaynor, E.C.; Swanson, M.S. ppGpp Conjures bacterial virulence. Microbiol. Mol. Biol. Rev. 2010, 74, 171–199. [Google Scholar] [CrossRef] [PubMed]
- Moreira, B.; Boyle-Vavra, S.; deJonge, B.L.; Daum, R.S. Increased production of penicillin-binding protein 2, increased detection of other penicillin-binding proteins, and decreased coagulase activity associated with glycopeptide resistance in Staphylococcus aureus. Antimicrob. Agents Chemother. 1997, 41, 1788–1793. [Google Scholar] [PubMed]
- Delaune, A.; Poupel, O.; Mallet, A.; Coic, Y.M.; Msadek, T.; Dubrac, S. Peptidoglycan crosslinking relaxation plays an important role in Staphylococcus aureus WalKR-dependent cell viability. PLOS ONE 2011, 6, e17054. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, M.; Kuroda, H.; Oshima, T.; Takeuchi, F.; Mori, H.; Hiramatsu, K. Two-component system VraSR positively modulates the regulation of cell-wall biosynthesis pathway in Staphylococcus aureus. Mol. Microbiol. 2003, 49, 807–821. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Lian, J.Q.; Neoh, H.M.; Reyes, E.; Hiramatsu, K. DNA microarray-based identification of genes associated with glycopeptide resistance in Staphylococcus aureus. Antimicrob. Agents Chemother. 2005, 49, 3404–3413. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Cha, D.J.; Lai, Y.; Villaruz, A.E.; Sturdevant, D.E.; Otto, M. The antimicrobial peptide-sensing system aps of Staphylococcus aureus. Mol. Microbiol. 2007, 66, 1136–1147. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, M.; Cui, L.; Kim, J.; Hiramatsu, K. Comprehensive identification of mutations responsible for heterogeneous vancomycin-intermediate Staphylococcus aureus (hVISA)-to-VISA conversion in laboratory-generated VISA strains derived from hVISA clinical strain Mu3. Antimicrob. Agents Chemother. 2013, 12, 5843–5853. [Google Scholar] [CrossRef]
- Ye, Z.H.; Buranen, S.L.; Lee, C.Y. Sequence analysis and comparison of int and xis genes from staphylococcal bacteriophages L54a and phi 11. J. Bacteriol. 1990, 172, 2568–2575. [Google Scholar] [PubMed]
- Novick, R.P.; Christie, G.E.; Penadés, J.R. The phage-related chromosomal islands of Gram-positive bacteria. Nat. Rev. Microbiol. 2010, 8, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, A.; Banerjee, R.; Pani, B.; Sen, U.; Sen, R. The moonlighting function of bacteriophage P4 capsid protein, Psu, as a transcription antiterminator. Bacteriophage 2013, 3, e25657. [Google Scholar] [CrossRef] [PubMed]
- Bae, B.; Davis, E.; Brown, D.; Campbell, E.A.; Wigneshweraraj, S.; Darst, S.A. Phage T7 Gp2 inhibition of Escherichia coli RNA polymerase involves misappropriation of σ70 domain 1.1. Proc. Natl. Acad. Sci. USA 2013, 110, 19772–19777. [Google Scholar] [CrossRef] [PubMed]
- Sjöström, J.E.; Philipson, L. Role of the phi 11 phage genome in competence of Staphylococcus aureus. J. Bacteriol. 1974, 119, 19–32. [Google Scholar] [PubMed]
- Kuroda, H.; Kuroda, M.; Cui, L.; Hiramatsu, K. Subinhibitory concentrations of beta-lactam induce haemolytic activity in Staphylococcus aureus through the SaeRS two-component system. FEMS Microbiol. Lett. 2007, 268, 98–105. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, J.O.; Langevin, M.J.; Price, C.T.; Blevins, J.S.; Smeltzer, M.S.; Gustafson, J.E. Effects of sarA inactivation on the intrinsic multidrug resistance mechanism of Staphylococcus aureus. FEMS Microbiol. Lett. 2004, 237, 297–302. [Google Scholar] [PubMed]
- Tang, J.; Chen, J.; Li, H.; Zeng, P.; Li, J. Characterization of adhesin genes, staphylococcal nuclease, hemolysis, and biofilm formation among Staphylococcus aureus strains isolated from different sources. Foodborne Pathog. Dis. 2013, 10, 757–763. [Google Scholar] [CrossRef] [PubMed]
- Laarman, A.J.; Ruyken, M.; Malone, C.L.; van Strijp, J.A.; Horswill, A.R.; Rooijakkers, S.H. Staphylococcus aureus metalloprotease aureolysin cleaves complement C3 to mediate immune evasion. J. Immunol. 2011, 186, 6445–6453. [Google Scholar] [CrossRef] [PubMed]
- Ingavale, S.S.; van Wamel, W.; Cheung, A.L. Characterization of RAT, an autolysis regulator in Staphylococcus aureus. Mol. Microbiol. 2003, 48, 1451–1466. [Google Scholar] [CrossRef] [PubMed]
- Komatsuzawa, H.; Ohta, K.; Fujiwara, T.; Choi, G.H.; Labischinski, H.; Sugai, M. Cloning and sequencing of the gene, fmtC, which affects oxacillin resistance in methicillin-resistant Staphylococcus aureus. FEMS Microbiol. Lett. 2001, 203, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Koprivnjak, T.; Mlakar, V.; Swanson, L.; Fournier, B.; Peschel, A.; Weiss, J.P. Cation-induced transcriptional regulation of the dlt operon of Staphylococcus aureus. J. Bacteriol. 2006, 188, 3622–3630. [Google Scholar] [CrossRef] [PubMed]
- Townsend, D.E.; Wilkinson, B.J. Proline transport in Staphylococcus aureus: A high affinity system and a low affinity system involved in osmoregulation. J. Bacteriol. 1992, 174, 2702–2710. [Google Scholar] [PubMed]
- Li, C.; Sun, F.; Cho, H.; Yelavarthi, V.; Sohn, C.; He, C.; Schneewind, O.; Bae, T. CcpA mediates proline auxotrophy and is required for Staphylococcus aureus pathogenesis. J. Bacteriol. 2010, 192, 3883–3892. [Google Scholar] [CrossRef] [PubMed]
- Bouchereau, A.; Aziz, A.; Larher, F.; Martin-Tanguy, J. Polyamines and environmental challenges: Recent developments. Plant Sci. 1999, 140, 103–125. [Google Scholar] [CrossRef]
- Thomas, T.; Thomas, T.J. Polyamine in cell growth and cell death: Molecular mechanisms and therapeutic applications. Cell. Mol. Life Sci. 2001, 58, 244–258. [Google Scholar] [CrossRef] [PubMed]
- Joshi, G.S.; Spontak, J.S.; Klapper, D.G.; Richardson, A.R. Arginine catabolic mobile element encoded speG abrogates the unique hypersensitivity of Staphylococcus aureus to exogenous polyamines. Mol. Microbiol. 2011, 82, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Potter, A.J.; Paton, J.C. Spermidine biosynthesis and transport modulates pneumococcal autolysis. J. Bacteriol. 2014. [Google Scholar] [CrossRef]
- Graham, J.E.; Wilkinson, B.J. Staphylococcus aureus osmoregulation: Roles for choline, glycine betaine, proline, and taurine. J. Bacteriol. 1992, 174, 2711–2716. [Google Scholar] [PubMed]
- Hiramatsu, K. Vancomycin-resistant Staphylococcus aureus: A new model of antibiotic resistance. Lancet Infect. Dis. 2001, 1, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Sieradzki, K.; Tomasz, A. Inhibition of cell wall turnover and autolysis by vancomycin in a highly vancomycin-resistant mutant of Staphylococcus aureus. J. Bacteriol. 1997, 179, 2557–2566. [Google Scholar] [PubMed]
- Cui, L.; Murakami, H.; Kuwahara-Arai, K.; Hanaki, H.; Hiramatsu, K. Contribution of a thickened cell wall and its glutamine nonamidated component to the vancomycin resistance expressed by Staphylococcus aureus Mu50. Antimicrob. Agents Chemother. 2000, 44, 2276–2285. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.T.; Manso, A.S.; Gaspar, P.; Pinho, M.G.; Neves, A.R. Effect of oxygen on glucose metabolism: Utilization of lactate in Staphylococcus aureus as revealed by in vivo NMR studies. PLOS ONE 2013, 8, e58277. [Google Scholar] [CrossRef] [PubMed]
- Friedman, L.; Alder, J.D.; Silverman, J.A. Genetic changes that correlate with reduced susceptibility to daptomycin in Staphylococcus aureus. Antimicrob. Agents Chemother. 2006, 50, 2137–2145. [Google Scholar] [CrossRef] [PubMed]
- Albert, T.J.; Dailidiene, D.; Dailide, G.; Norton, J.E.; Kalia, A.; Richmond, T.A.; Molla, M.; Singh, J.; Green, R.D.; Berg, D.E. Mutation discovery in bacterial genomes: Metronidazole resistance in Helicobacter pylori. Nat. Meth. 2005, 2, 951–953. [Google Scholar] [CrossRef]
- Muthaiyan, A.; Silverman, J.A.; Jayaswal, R.K.; Wilkinson, B.J. Transcriptional profiling reveals that daptomycin induces the Staphylococcus aureus cell wall stress stimulon and genes responsive to membrane depolarization. Antimicrob. Agents Chemother. 2008, 52, 980–990. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Rubio, A.; Jayaswal, R.K.; Silverman, J.A.; Wilkinson, B.J. Additional routes to Staphylococcus aureus daptomycin resistance as revealed by comparative genome sequencing, transcriptional profiling, and phenotypic studies. PLOS ONE 2013, 8, e58469. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Ulanov, A.V.; Li, Z.; Jayaswal, R.K.; Wilkinson, B.J. Metabolomes of the psychrotolerant bacterium Listeria monocytogenes 10403S grown at 37 °C and 8 °C. Int. J. Food Microbiol. 2011, 148, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Howden, B.P.; McEvoy, C.E.; Allen, D.L.; Chua, K.; Gao, W.; Harrison, P.F.; Bell, J.; Coombs, G.; Wood, V.B.; Porter, J.L.; et al. Evolution of multidrug resistance during Staphylococcus aureus infection involves mutation of the essential two component regulator WalKR. PLOS Pathog. 2011, 7, e1002359. [Google Scholar] [CrossRef] [PubMed]
- Gardete, S.; Kim, C.; Hartmann, B.M.; Mwangi, M.; Roux, C.M.; Dunman, P.M.; Chambers, H.F.; Tomasz, A. Genetic pathway in acquisition and loss of vancomycin resistance in a methicillin resistant Staphylococcus aureus (MRSA) strain of clonal type USA300. PLOS Pathog. 2012, 13, e1002505. [Google Scholar] [CrossRef]
- Pandya, U.; Sinha, M.; Luxon, B.A.; Watson, D.A.; Niesel, D.W. Global transcription profiling and virulence potential of Streptococcus pneumoniae after serial passage. Gene 2009, 443, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Somerville, G.A.; Beres, S.B.; Fitzgerald, J.R.; DeLeo, F.R.; Cole, R.L.; Hoff, J.S.; Musser, J.M. In vitro serial passage of Staphylococcus aureus: Changes in physiology, virulence factor production, and agr nucleotide sequence. J. Bacteriol. 2002, 184, 1430–1437. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hattangady, D.S.; Singh, A.K.; Muthaiyan, A.; Jayaswal, R.K.; Gustafson, J.E.; Ulanov, A.V.; Li, Z.; Wilkinson, B.J.; Pfeltz, R.F. Genomic, Transcriptomic and Metabolomic Studies of Two Well-Characterized, Laboratory-Derived Vancomycin-Intermediate Staphylococcus aureus Strains Derived from the Same Parent Strain. Antibiotics 2015, 4, 76-112. https://doi.org/10.3390/antibiotics4010076
Hattangady DS, Singh AK, Muthaiyan A, Jayaswal RK, Gustafson JE, Ulanov AV, Li Z, Wilkinson BJ, Pfeltz RF. Genomic, Transcriptomic and Metabolomic Studies of Two Well-Characterized, Laboratory-Derived Vancomycin-Intermediate Staphylococcus aureus Strains Derived from the Same Parent Strain. Antibiotics. 2015; 4(1):76-112. https://doi.org/10.3390/antibiotics4010076
Chicago/Turabian StyleHattangady, Dipti S., Atul K. Singh, Arun Muthaiyan, Radheshyam K. Jayaswal, John E. Gustafson, Alexander V. Ulanov, Zhong Li, Brian J. Wilkinson, and Richard F. Pfeltz. 2015. "Genomic, Transcriptomic and Metabolomic Studies of Two Well-Characterized, Laboratory-Derived Vancomycin-Intermediate Staphylococcus aureus Strains Derived from the Same Parent Strain" Antibiotics 4, no. 1: 76-112. https://doi.org/10.3390/antibiotics4010076







