Anti-Biofilm and Immunomodulatory Activities of Peptides That Inhibit Biofilms Formed by Pathogens Isolated from Cystic Fibrosis Patients
Abstract
:1. Introduction
2. Results
2.1. MICs vs. Cystic Fibrosis Isolates
| Strains (Colonial Morphology or Genomovar) | MIC (μg/mL) | |||
|---|---|---|---|---|
| 1018 | LL-37 | 1037 | Polymyxin B | |
| P. aeruginosa PA01 | 16 | 32 | 304 | 2 |
| P. aeruginosa PA14 | 16 | 32 | 304 | 2 |
| P. aeruginosa 3195 (Classic) | 16 | 64 | 304 | 2 |
| P. aeruginosa C2773 (Classic) | 16 | 256 | 152 | 4 |
| P. aeruginosa 1172 (Classic) | 16 | >128 | 304 | 2 |
| P. aeruginosa 7632 (Classic) | 16 | 256 | 304 | 2 |
| P. aeruginosa 3330 (Classic) | 16 | >128 | 304 | 2 |
| P. aeruginosa 4020 (Classic) | 16 | >128 | 304 | 2 |
| P. aeruginosa C4276 (Classic) | 32 | 256 | 608 | 2 |
| P. aeruginosa 2631 (Classic) | 32 | >256 | 608 | 4 |
| P. aeruginosa C4278 (Mucoid) | 16 | >256 | 304 | 2 |
| P. aeruginosa 4608 (Mucoid) | 32 | >128 | 304 | 2 |
| P. aeruginosa 2639 (Mucoid) | 32 | >128 | 608 | 2 |
| P. aeruginosa 7633 (Entire) | 16 | >128 | 304 | 2 |
| P. aeruginosa 2955 (Dwarf) | 50 | -a | - | - |
| B. multivorans D2661 (Genomovar II) | >512 | >512 | >608 | >128 |
| B. cenocepacia 4813 (IIIa) | >256 | >256 | >608 | >128 |
| B. cenocepacia C5424 (IIIa) | 128 | >256 | >608 | >128 |
| B. cenocepacia CEP0055 (IIIb) | 128 | >512 | >608 | >128 |
| B. cenocepacia CEP509 (IIIb) | >256 | >512 | >608 | >128 |
| B. stabilis C6061 (IV) | 64 | >512 | 304 | >128 |
| B. dolosa CEP0021 (VI) | >256 | >512 | >608 | >128 |
| B. ambifaria CEP0996 (VII) | 64 | >512 | >608 | >128 |
2.2. Anti-Biofilm Activity vs. Cystic Fibrosis Isolates
| Strains (Colonial Morphology or Genomovar) | Biofilm Formation a | Inhibition by 10 µg/mL 1018 |
|---|---|---|
| P. aeruginosa PA01 | 0.79 ± 0.22 | 51% |
| P. aeruginosa PA14 | 0.82 ± 0.21 | 49% |
| P. aeruginosa 3195 (Classic) | 0.99 ± 0.16 | 37% |
| P. aeruginosa C2773 (Classic) | 0.89 ± 0.17 | 60% |
| P. aeruginosa 1172 (Classic) | 0.92 ± 0.27 | 37% |
| P. aeruginosa 7632 (Classic) | 0.59 ± 0.12 | 19% |
| P. aeruginosa 3330 (Classic) | 0.84 ± 0.20 | 33% |
| P. aeruginosa 4020 (Classic) | 0.62 ± 0.16 | 53% |
| P. aeruginosa C4276 (Classic) | 0.62 ± 0.28 | 53% |
| P. aeruginosa 2631 (Classic) | 0.74 ± 0.27 | 44% |
| P. aeruginosa C4278 (Mucoid) | 0.73 ± 0.20 | 58% |
| P. aeruginosa 4608 (Mucoid) | 0.62 ± 0.09 | 67% |
| P. aeruginosa 2639 (Mucoid) | 0.93 ± 0.20 | 44% |
| P. aeruginosa 7633 (Entire) | 0.72 ± 0.16 | 49% |
| P. aeruginosa 2955 (Dwarf) | 0.64 ± 0.19 | 41% |
| B. multivorans D2661 (Genomovar II) | 0.75 ± 0.07 | 75% |
| B. cenocepacia 4813 (IIIa) | 0.94 ± 0.18 | 52% |
| B. cenocepacia C5424 (IIIa) | 0.75 ± 0.18 | 59% |
| B. cenocepacia CEP0055 (IIIb) | 0.33 ± 0.11 | 58% |
| B. cenocepacia CEP509 (IIIb) | 0.42 ± 0.08 | 61% |
| B. stabilis C6061 (IV) | 0.39 ± 0.07 | 53% |
| B. dolosa CEP0021 (VI) | 0.41 ± 0.06 | 55% |
| B. ambifaria CEP0996 (VII) | 0.29 ± 0.10 | 62% |
2.3. Microscopic Imaging of Anti-Biofilm Activity
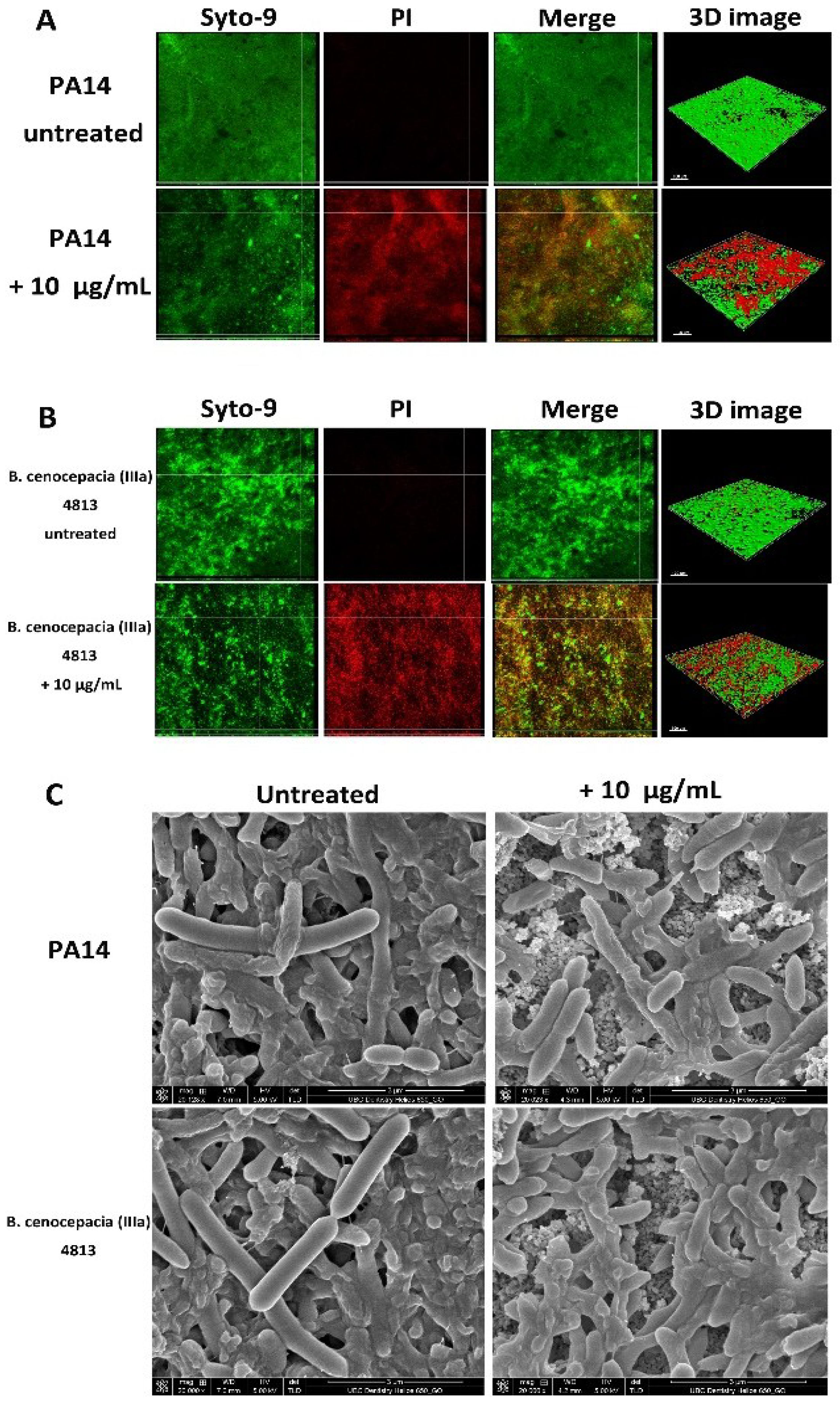
| Bacterial Strains | Proportion of Dead Cells (%) | |
|---|---|---|
| Untreated | 10 µg/mL 1018 | |
| P. aeruginosa PA14 | 7 ± 4 | 48 ± 17 a |
| B. cenocepacia (IIIa) 4813 | 6 ± 3 | 42 ± 13 a |
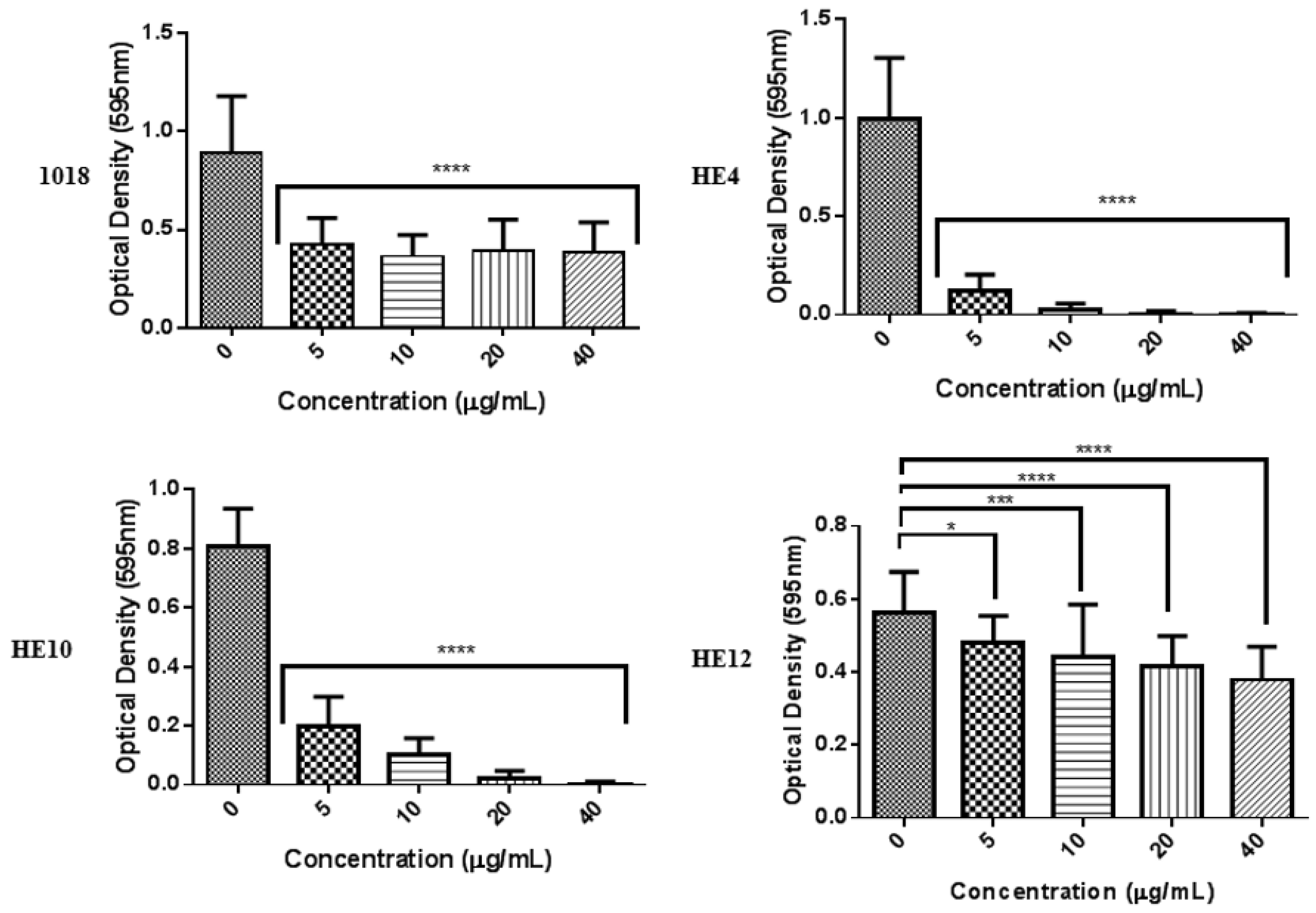
2.4. Derivatives of 1018: Anti-Biofilm and Immunomodulatory Activity
| Peptide | Sequence a | Modification | Anti-Biofilm Activity (% Decrease in Biofilm) | |
|---|---|---|---|---|
| P. aeruginosa PA14 | B. cenocepacia 4813 | |||
| 1018 | VRLIVAVRIWRR | Parent | 99% | 52% |
| HE1 | RRWIRVAVILRV | Reverse sequence | 93% | 73% |
| HE2 | VRLIRAVRAWRV | Break hydrophobicity; maintain charge | 42% | 24% |
| HE3 | VRWARVARILRV | Reverse of HE2 | 31% | 21% |
| HE4 | VRLIWAVRIWRR | Substitute in another W for V | 99% | - b |
| HE10 | VRLI--VRIWRR | Truncate to remove hydrophobic patch | 39% | - |
| HE12 | RFKRVARVIW | Scrambled smaller peptide | 10% | - |
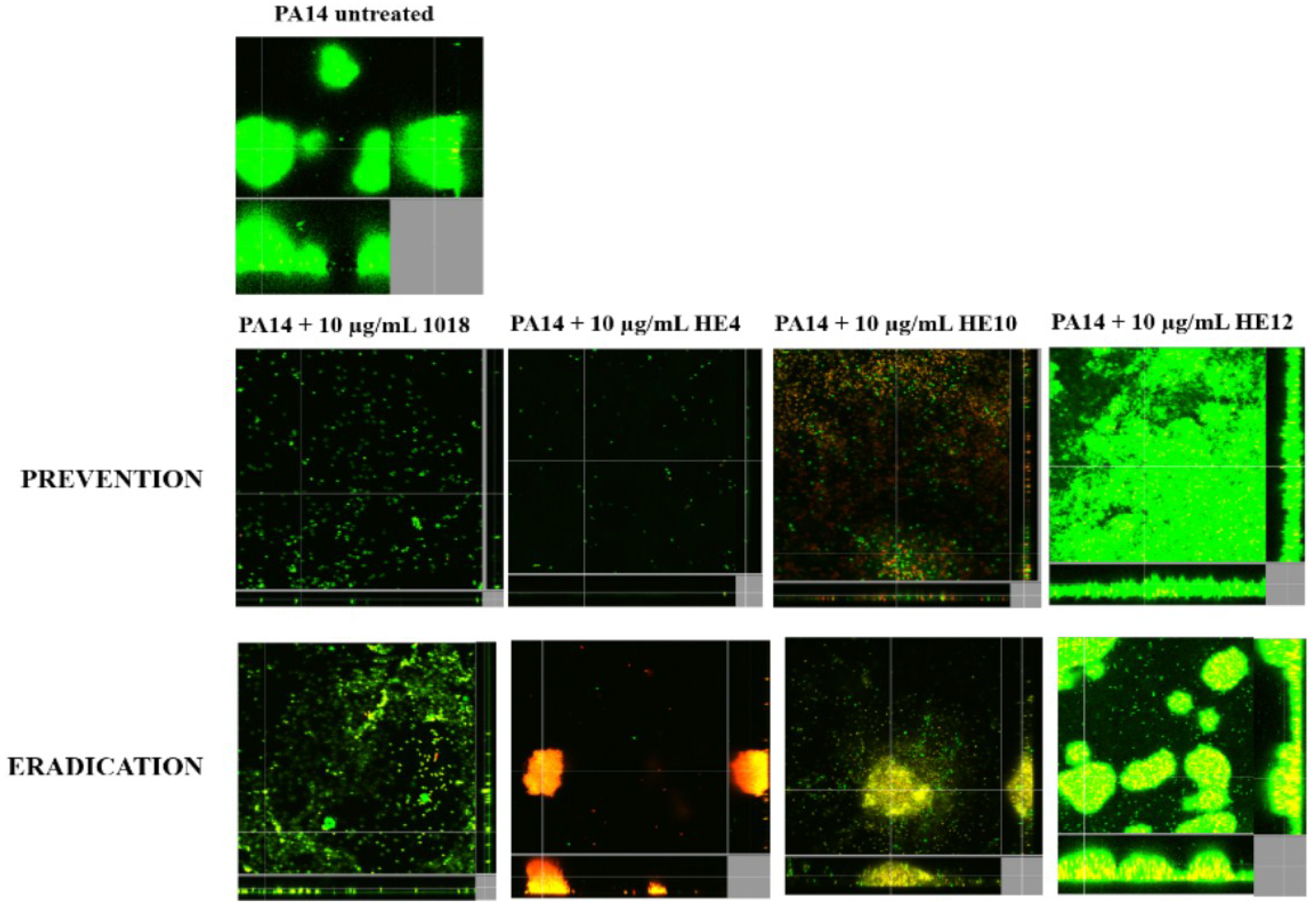
3. Discussion
4. Experimental
4.1. Peptide Synthesis
4.2. Bacteria, Growth, and Assessment of Minimal Inhibitory Concentrations (MIC)
4.3. Biofilm 96-Well Plate Assays
4.4. Culturing Biofilms Using the Hydroxyapatite Biofilm Model
4.5. Anti-Biofilm Effect of Peptide 1018 Evaluated Using the Hydroxyapatite Biofilm Model
4.6. Confocal Laser Scanning Microscopy
4.7. Scanning Electron Microscopy Examination of Hydroxyapatite-Grown Biofilms
4.8. Biofilm Cultivation in Flow Cell Chambers and Microscopy
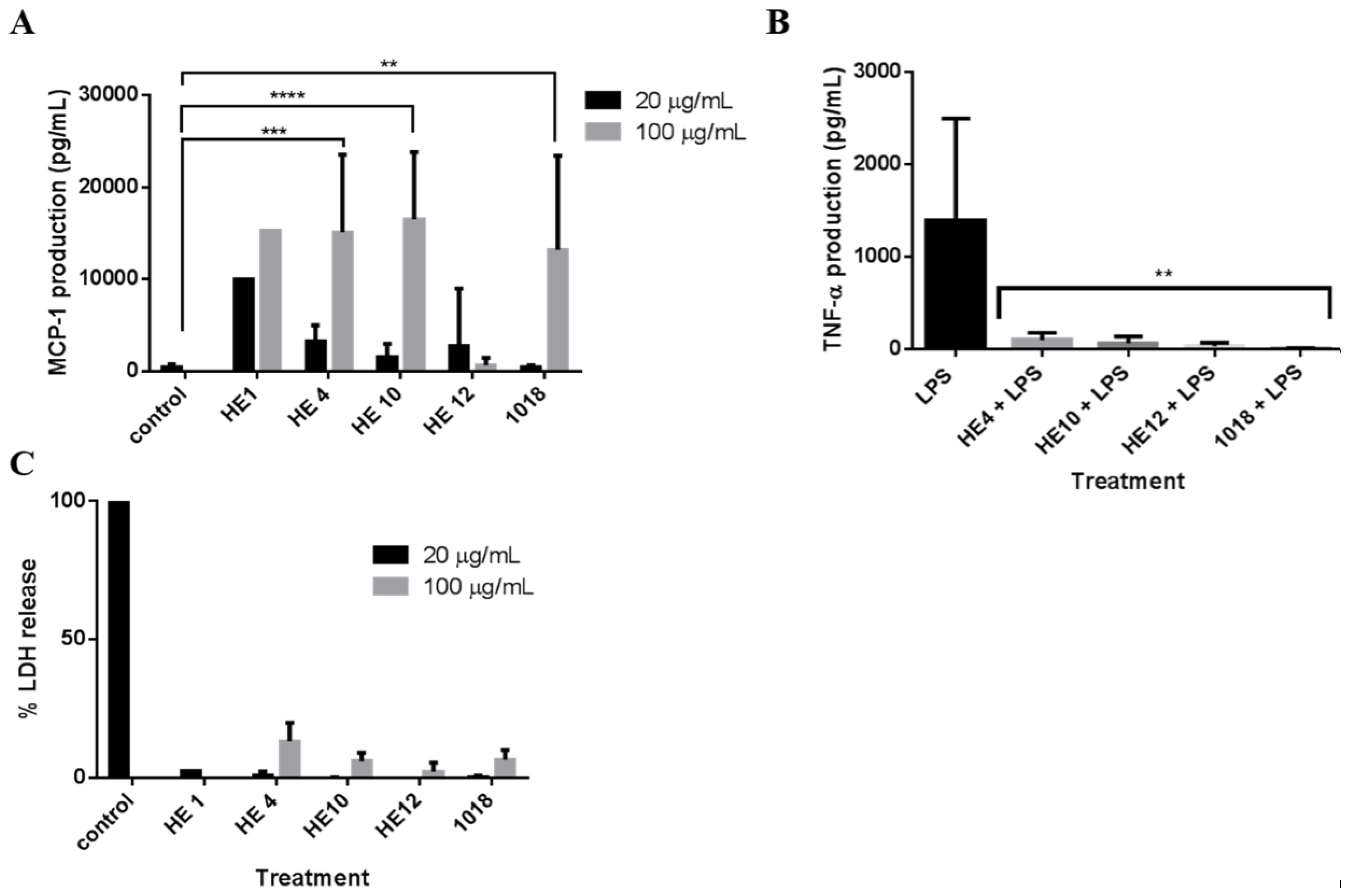
4.9. Immune Modulation Studies
4.10. Cytotoxicity Assessment
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interests
References
- López, D.; Vlamakis, H.; Kolter, R. Biofilms. Cold Spring Harb. Perspect. Biol. 2010, 2, a000398. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente-Núñez, C.; Reffuveille, F.; Fernández, L.; Hancock, R.E.W. Bacterial biofilm development as a multicellular adaptation: Antibiotic resistance and new therapeutic strategies. Curr. Opin. Microbiol. 2013, 16, 580–589. [Google Scholar]
- Moreau-Marquis, S.; Stanton, B.A.; O’Toole, G.A. Pseudomonas aeruginosa biofilm formation in the cystic fibrosis airway. Pulm. Pharmacol. Ther. 2008, 21, 595–599. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.K.; Schaefer, A.L.; Parsek, M.R.; Moninger, T.O.; Welsh, M.J.; Greenberg, E.P. Quorum-sensing signals indicate that cystic fibrosis lungs are infected with bacterial biofilms. Nature 2000, 407, 762–764. [Google Scholar] [CrossRef] [PubMed]
- Breidenstein, E.B.M.; de la Fuente-Núñez, C.; Hancock, R.E.W. Pseudomonas aeruginosa: All roads lead to resistance. Trends. Microbiol. 2011, 19, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, L.R.; D’Argenio, D.A.; MacCoss, M.J.; Zhang, Z.; Jones, R.A.; Miller, S.I. Aminoglycoside antibiotics induce bacterial biofilm formation. Nature 2005, 436, 1171–1175. [Google Scholar] [CrossRef] [PubMed]
- Linares, J.F.; Gustafsson, I.; Baquero, F.; Martinez, J.L. Antibiotics as intermicrobial signaling agents instead of weapons. Proc. Natl. Acad. Sci. USA 2006, 103, 19484–19489. [Google Scholar] [CrossRef] [PubMed]
- Wahba, A.H.; Darrell, J.H. The identification of atypical strains of Pseudomonas aeruginosa. J. Gen. Microbiol. 1965, 38, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Stapper, A.P.; Narasimhan, G.; Ohman, D.E.; Barakat, J.; Hentzer, M.; Molin, S.; Kharazmi, A.; Høiby, N.; Mathee, K. Alginate production affects Pseudomonas aeruginosa biofilm development and architecture, but is not essential for biofilm formation. J. Med. Microbiol. 2004, 53, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Leid, J.G.; Willson, C.J.; Shirtliff, M.E.; Hassett, D.J.; Parsek, M.R.; Jeffers, A.K. The exopolysaccharide alginate protects Pseudomonas aeruginosa biofilm bacteria from IFN-gamma-mediated macrophage killing. J. Immunol. 2005, 175, 7512–7518. [Google Scholar] [CrossRef] [PubMed]
- D’Argenio, D.A.; Calfee, M.W.; Rainey, P.B.; Pesci, E.C. Autolysis and autoaggregation in Pseudomonas aeruginosa colony morphology mutants. J. Bacteriol. 2002, 184, 6481–6489. [Google Scholar] [CrossRef] [PubMed]
- Häussler, S.; Ziegler, I.; Löttel, A.; von Götz, F.; Rohde, M.; Wehmhöhner, D.; Saravanamuthu, S.; Tümmler, B.; Steinmetz, I. Highly adherent small-colony variants of Pseudomonas aeruginosa in cystic fibrosis lung infection. J. Med. Microbiol. 2003, 52, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Ikeno, T.; Fukuda, K.; Ogawa, M.; Honda, M.; Tanabe, T.; Taniguchi, H. Small and rough colony Pseudomonas aeruginosa with elevated biofilm formation ability isolated in hospitalized patients. Microbiol. Immunol. 2007, 51, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Loutet, S.A.; Valvano, M.A. Extreme antimicrobial peptide and polymyxin B resistance in the genus Burkholderia. Front. Microbiol. 2011, 2, e159. [Google Scholar] [CrossRef]
- Mahenthiralingam, E.; Vandamme, P.; Campbell, M.E.; Henry, D.A.; Gravelle, A.M.; Wong, L.T.; Davidson, A.G.; Wilcox, P.G.; Nakielna, B.; Speert, D.P. Infection with Burkholderia cepacia complex genomovars in patients with cystic fibrosis: Virulent transmissible strains of genomovar III can replace Burkholderia multivorans. Clin. Infect. Dis. 2001, 33, 1469–1475. [Google Scholar] [CrossRef] [PubMed]
- Hancock, R.E.W.; Sahl, H.G. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 2006, 24, 1551–1557. [Google Scholar] [CrossRef] [PubMed]
- Hilchie, A.L.; Wuerth, K.; Hancock, R.E.W. Immune modulation by multifaceted cationic host defense (antimicrobial) peptides. Nat. Chem. Biol. 2013, 9, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Overhage, J.; Campisano, A.; Bains, M.; Torfs, E.C.; Rehm, B.H.; Hancock, R.E.W. Human host defense peptide LL-37 prevents bacterial biofilm formation. Infect. Immun. 2008, 76, 4176–4182. [Google Scholar] [CrossRef] [PubMed]
- Amer, L.S.; Bishop, B.M.; van Hoek, M.L. Antimicrobial and antibiofilm activity of cathelicidins and short, synthetic peptides against Francisella. Biochem. Biophys. Res. Commun. 2010, 396, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Pompilio, A.; Scocchi, M.; Pomponio, S.; Guida, F.; di Primio, A.; Fiscarelli, E.; Gennaro, R.; di Bonaventura, G. Antibacterial and anti-biofilm effects of cathelicidin peptides against pathogens isolated from cystic fibrosis patients. Peptides 2011, 32, 1807–1814. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente-Núñez, C.; Korolik, V.; Bains, M.; Nguyen, U.; Breidenstein, E.B.; Horsman, S.; Lewenza, S.; Burrows, L.; Hancock, R.E.W. Inhibition of bacterial biofilm formation and swarming motility by a small synthetic cationic peptide. Antimicrob. Agents Chemother. 2012, 56, 2696–2704. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente-Núñez, C.; Reffuveille, F.; Haney, E.F.; Straus, S.K.; Hancock, R.E.W. Broad-spectrum anti-biofilm peptide that targets a cellular stress response. PLoS Pathog. 2014, 10, e1004152. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Sambanthamoorthy, K.; Palys, T.; Paranavitana, C. The human antimicrobial peptide LL-37 and its fragments possess both antimicrobial and antibiofilm activities against multidrug-resistant Acinetobacter baumannii. Peptides 2013, 49, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Hirt, H.; Gorr, S.U. Antimicrobial peptide GL13K is effective in reducing biofilms of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2013, 57, 4903–4910. [Google Scholar] [CrossRef] [PubMed]
- Zaïri, A.; Ferrières, L.; Latour-Lambert, P.; Beloin, C.; Tangy, F.; Ghigo, J.M.; Hani, K. In vitro activities of dermaseptins K4S4 and K4K20S4 against Escherichia coli, Staphylococcus aureus, and Pseudomonas aeruginosa planktonic growth and biofilm formation. Antimicrob Agents Chemother. 2014, 58, 2221–2228. [Google Scholar] [CrossRef] [PubMed]
- Gopal, R.; Kim, Y.G.; Lee, J.H.; Lee, S.K.; Chae, J.D.; Son, B.K.; Seo, C.H.; Park, Y. Synergistic effects and antibiofilm properties of chimeric peptides against multidrug-resistant Acinetobacter. baumannii strains. Antimicrob. Agents Chemother. 2014, 58, 1622–1629. [Google Scholar] [CrossRef] [PubMed]
- Reffuveille, F.; de la Fuente-Núñez, C.; Mansour, S.; Hancock, R.E.W. A broad-spectrum anti-biofilm peptide enhances antibiotic action against bacterial biofilms. Antimicrob. Agents Chemother. 2014, 58, 5363–5371. [Google Scholar] [CrossRef] [PubMed]
- Wieczorek, M.; Jenssen, H.; Kindrachuk, J.; Scott, W.R.; Elliott, M.; Hilpert, K.; Cheng, J.T.; Hancock, R.E.W.; Straus, S.K. Structural studies of a peptide with immune modulating and direct antimicrobial activity. Chem. Biol. 2010, 17, 970–980. [Google Scholar] [CrossRef] [PubMed]
- Achtman, A.H.; Pilat, S.; Law, C.W.; Lynn, D.J.; Janot, L.; Mayer, M.L.; Ma, S.; Kindrachuk, J.; Finlay, B.B.; Brinkman, F.S.; et al. Effective adjunctive therapy by an innate defense regulatory peptide in a pre-clinical model of severe malaria. Sci. Transl. Med. 2012, 4. 135ra64. [Google Scholar] [PubMed]
- Rivas-Santiago, B.; Castañeda-Delgado, J.E.; Rivas Santiago, C.E.; Waldbrook, M.; González-Curiel, I.; León-Contreras, J.C.; Enciso-Moreno, J.A.; del Villar, V.; Mendez-Ramos, J.; Hancock, R.E.W.; et al. Ability of innate defence regulator peptides IDR-1002, IDR-HH2 and IDR-1018 to protect against Mycobacterium tuberculosis infections in animal models. PLoS One 2013, 8, e59119. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Grigg, J. New insights into pulmonary inflammation in cystic fibrosis. Arch. Dis. Child. 2006, 91, 786–788. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.A.; Hancock, R.E.W. Involvement of outer membrane of Pseudomonas cepacia in aminoglycoside and polymyxin resistance. Antimicrob. Agents Chemother. 1986, 30, 923–926. [Google Scholar] [CrossRef] [PubMed]
- Cherkasov, A.; Hilpert, K.; Jenssen, H.; Fjell, C.D.; Waldbrook, M.; Mullaly, S.C.; Volkmer, R.; Hancock, R.E.W. Use of artificial intelligence in the design of small peptide antibiotics effective against a broad spectrum of highly antibiotic-resistant superbugs. ACS Chem. Biol. 2009, 4, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Qian, W.; Chung, C.; Olsen, I.; Haapasalo, M. Evaluation of the effect of two chlorhexidine preparations on biofilm bacteria in vitro: A three-dimensional quantitative analysis. J. Endod. 2009, 35, 981–985. [Google Scholar] [CrossRef] [PubMed]
- Bowdish, D.M.; Davidson, D.J.; Scott, M.G.; Hancock, R.E.W. Immunomodulatory activities of small host defense peptides. Antimicrob. Agents Chemother. 2005, 49, 1727–1732. [Google Scholar] [PubMed]
- Benoit, M.R.; Conant, C.G.; Ionescu-Zanetti, C.; Schwartz, M.; Matin, A. New device for high-throughput viability screening of flow biofilms. Appl. Environ. Microbiol. 2010, 76, 4136–4142. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Stojicic, S.; Haapasalo, M. Antimicrobial efficacy of chlorhexidine against bacteria in biofilms at different stages of development. J. Endod. 2011, 37, 657–661. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Stojicic, S.; Haapasalo, M. Bacterial viability in starved and revitalized biofilms: Comparison of viability staining and direct culture. J. Endod. 2010, 36, 1820–1823. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De la Fuente-Núñez, C.; Mansour, S.C.; Wang, Z.; Jiang, L.; Breidenstein, E.B.M.; Elliott, M.; Reffuveille, F.; Speert, D.P.; Reckseidler-Zenteno, S.L.; Shen, Y.; et al. Anti-Biofilm and Immunomodulatory Activities of Peptides That Inhibit Biofilms Formed by Pathogens Isolated from Cystic Fibrosis Patients. Antibiotics 2014, 3, 509-526. https://doi.org/10.3390/antibiotics3040509
De la Fuente-Núñez C, Mansour SC, Wang Z, Jiang L, Breidenstein EBM, Elliott M, Reffuveille F, Speert DP, Reckseidler-Zenteno SL, Shen Y, et al. Anti-Biofilm and Immunomodulatory Activities of Peptides That Inhibit Biofilms Formed by Pathogens Isolated from Cystic Fibrosis Patients. Antibiotics. 2014; 3(4):509-526. https://doi.org/10.3390/antibiotics3040509
Chicago/Turabian StyleDe la Fuente-Núñez, César, Sarah C. Mansour, Zhejun Wang, Lucy Jiang, Elena B.M. Breidenstein, Melissa Elliott, Fany Reffuveille, David P. Speert, Shauna L. Reckseidler-Zenteno, Ya Shen, and et al. 2014. "Anti-Biofilm and Immunomodulatory Activities of Peptides That Inhibit Biofilms Formed by Pathogens Isolated from Cystic Fibrosis Patients" Antibiotics 3, no. 4: 509-526. https://doi.org/10.3390/antibiotics3040509
APA StyleDe la Fuente-Núñez, C., Mansour, S. C., Wang, Z., Jiang, L., Breidenstein, E. B. M., Elliott, M., Reffuveille, F., Speert, D. P., Reckseidler-Zenteno, S. L., Shen, Y., Haapasalo, M., & Hancock, R. E. W. (2014). Anti-Biofilm and Immunomodulatory Activities of Peptides That Inhibit Biofilms Formed by Pathogens Isolated from Cystic Fibrosis Patients. Antibiotics, 3(4), 509-526. https://doi.org/10.3390/antibiotics3040509




