Macrolide Resistance in the Aerococcus urinae Complex: Implications for Integrative and Conjugative Elements
Abstract
:1. Introduction
2. Results
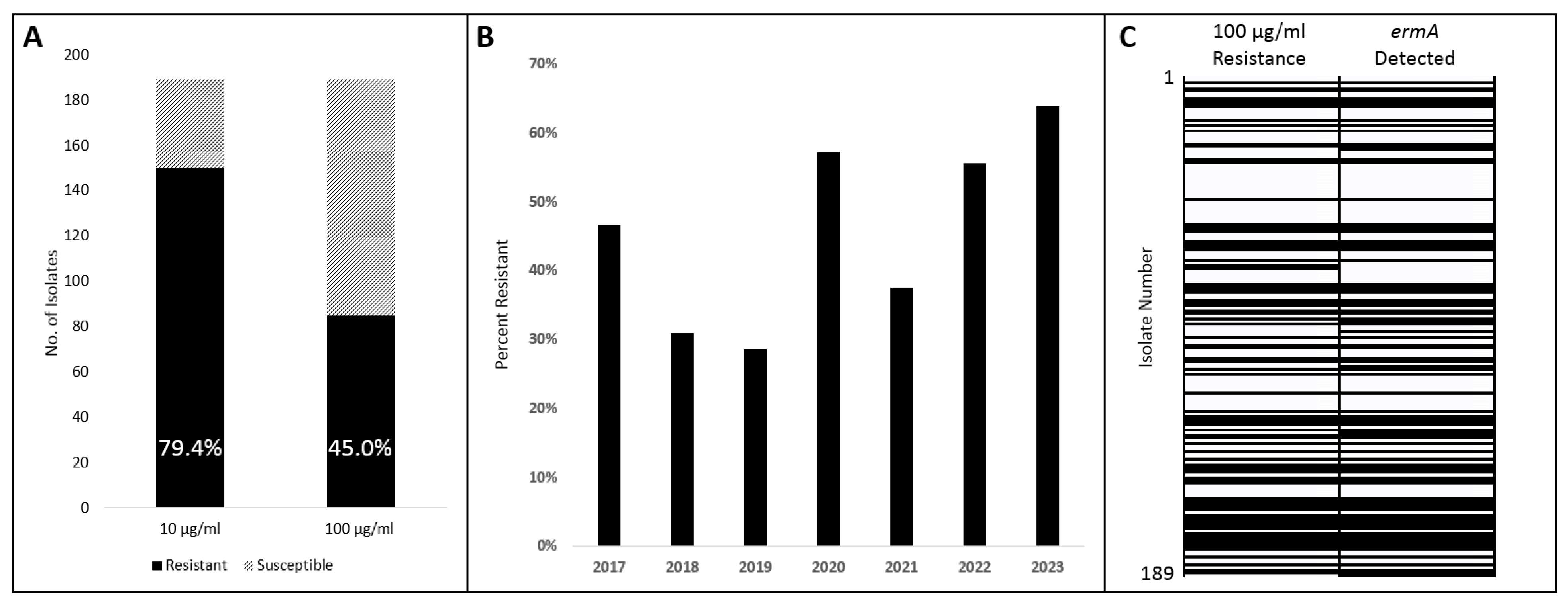
2.1. Survey of Macrolide Susceptibility
2.2. Survey of Macrolide Resistance Gene ermA
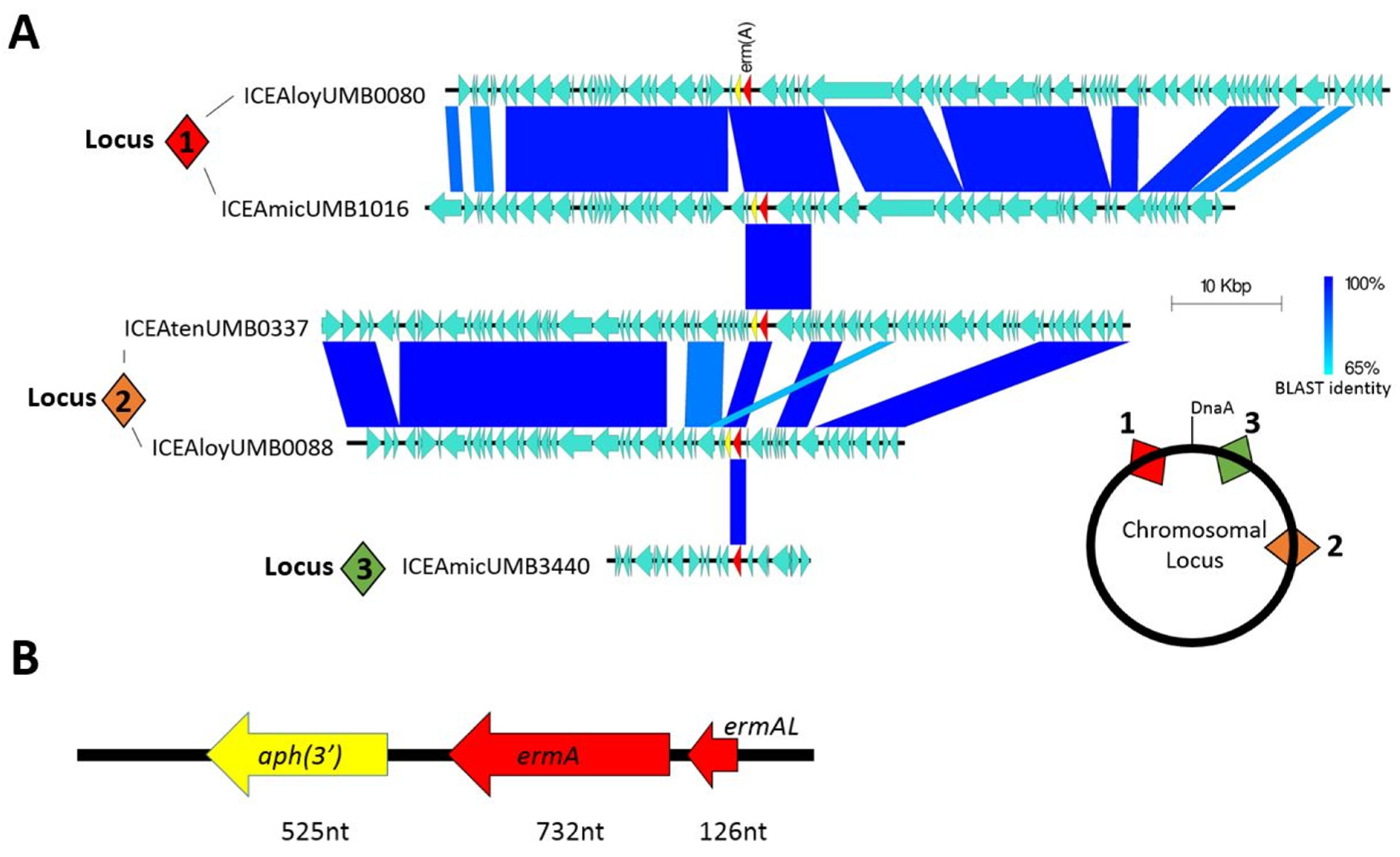
2.3. Macrolide Resistance Harbored within Mobile Genetic Elements
3. Discussion
4. Materials and Methods
4.1. Survey of Publicly Available Genomes for ARGs
4.2. Study Isolates
4.3. Phenotypic Screening of Macrolide Susceptibility
4.4. DNA Extraction
4.5. PCR Survey
4.6. Genome Sequencing
4.7. Integrative and Conjugative Element Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aguirre, M.; Collins, M.D. Phylogenetic Analysis of Some Aerococcus-like Organisms from Urinary Tract Infections: Description of Aerococcus urinae sp. nov. J. Gen. Microbiol. 1992, 138, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Senneby, E.; Petersson, A.C.; Rasmussen, M. Epidemiology and Antibiotic Susceptibility of Aerococci in Urinary Cultures. Diagn. Microbiol. Infect. Dis. 2015, 81, 149–151. [Google Scholar] [CrossRef] [PubMed]
- Narayanasamy, S.; King, K.; Dennison, A.; Spelman, D.W.; Aung, A.K. Clinical Characteristics and Laboratory Identification of Aerococcus Infections: An Australian Tertiary Centre Perspective. Int. J. Microbiol. 2017, 2017, 5684614. [Google Scholar] [CrossRef] [PubMed]
- Sihvonen, R.; Turunen, M.; Lehtola, L.; Pakarinen, L.; Grönroos, J.O.; Rantakokko-Jalava, K.; Pätäri-Sampo, A. Clinical and Microbiological Characterization of Aerococcus urinae Bacteraemias at Helsinki Metropolitan Area, Finland. Eur. J. Clin. Microbiol. Infect. Dis. 2022, 41, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Maezawa, Y.; Nagasaki, K. Aerococcus urinae: An Emerging, Gram-Positive Pathogen Causing Urinary Tract Infection. Am. J. Med. 2024, 137, 89–90. [Google Scholar] [CrossRef] [PubMed]
- Skalidis, T.; Papaparaskevas, J.; Konstantinou, D.; Kapolou, E.; Falagas, M.E.; Legakis, N. Aerococcus urinae, a Cause of Cystitis with Malodorous Urine in a Child: Clinical and Microbiological Challenges. JMM Case Rep. 2017, 4, e005083. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, M. Aerococcus: An Increasingly Acknowledged Human Pathogen. Clin. Microbiol. Infect. 2016, 22, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Pearce, M.M.; Hilt, E.E.; Rosenfeld, A.B.; Zilliox, M.J.; Thomas-White, K.; Fok, C.; Kliethermes, S.; Schreckenberger, P.C.; Brubaker, L.; Gai, X.; et al. The Female Urinary Microbiome: A Comparison of Women with and without Urgency Urinary Incontinence. mBio 2014, 5, 10-1128. [Google Scholar] [CrossRef] [PubMed]
- Sturm, P.D.; Van Eijk, J.; Veltman, S.; Meuleman, E.; Schülin, T. Urosepsis with Actinobaculum schaalii and Aerococcus urinae. J. Clin. Microbiol. 2006, 44, 652–654. [Google Scholar] [CrossRef]
- Zhang, Q.; Kwoh, C.; Attorri, S.; Clarridge, J.E. 3rd Aerococcus urinae in Urinary Tract Infections. J. Clin. Microbiol. 2000, 38, 1703–1705. [Google Scholar] [CrossRef]
- Ahmed, Y.; Bardia, N.; Judge, C.; Ahmad, S.; Malozzi, C.; Calderon, E. Aerococcus urinae: A Rare Cause of Endocarditis Presenting With Acute Stroke. J. Med. Cases 2021, 12, 65–70. [Google Scholar] [CrossRef]
- Forsvall, A.; Wagenius, M.; Rasmussen, M. Perigenital Necrotizing Soft Tissue Infection Caused by Aerococcus urinae. IDCases 2019, 18, 590. [Google Scholar] [CrossRef] [PubMed]
- Tai, D.B.G.; Go, J.R.; Fida, M.; Saleh, O.A. Management and Treatment of Aerococcus Bacteremia and Endocarditis. Int. J. Infect. Dis. 2021, 102, 584–589. [Google Scholar] [CrossRef] [PubMed]
- Christensen, J.J.; Kilian, M.; Fussing, V.; Andresen, K.; Blom, J.; Korner, B.; Steigerwalt, A.G. Aerococcus urinae: Polyphasic Characterization of the Species. APMIS 2005, 113, 517–525. [Google Scholar] [CrossRef]
- Carkaci, D.; Højholt, K.; Nielsen, X.C.; Dargis, R.; Rasmussen, S.; Skovgaard, O.; Fuursted, K.; Andersen, P.S.; Stegger, M.; Christensen, J.J. Genomic Characterization, Phylogenetic Analysis, and Identification of Virulence Factors in Aerococcus sanguinicola and Aerococcus urinae Strains Isolated from Infection Episodes. Microb. Pathog. 2017, 112, 327–340. [Google Scholar] [CrossRef]
- Choi, B.I.; Ene, A.; Du, J.; Johnson, G.; Putonti, C.; Schouw, C.H.; Dargis, R.; Senneby, E.; Christensen, J.J.; Wolfe, A.J. Taxonomic Considerations on Aerococcus urinae with Proposal of Subdivision into Aerococcus urinae, Aerococcus tenax sp. nov., Aerococcus mictus Sp. Nov., and Aerococcus loyolae sp. nov. Int. J. Syst. Evol. Microbiol. 2023, 73, 006066. [Google Scholar] [CrossRef]
- Moreland, R.B.; Choi, B.; Geaman, W.; Álvarez, C.J.; Hochstedler-Kramer, B.R.; John, J.; Kaindl, J.; Kesav, N.; Lamichhane, J.; Lucio, L.; et al. Beyond the Usual Suspects: Emerging Uropathogens in the Microbiome Age. Front. Urol. 2023, 3, 1212590. [Google Scholar] [CrossRef]
- Ahmadzada, A.; Fuchs, F.; Hamprecht, A. Susceptibility of Aerococcus urinae and Aerococcus sanguinicola to Standard Antibiotics and to Nitroxoline. Microbiol. Spectr. 2023, 11, e0276322. [Google Scholar] [CrossRef]
- Krishnan, A.; Nadeau, L. 1468. Determination of Antibiotic Susceptibilities in Aerococcus urinae Urinary Isolates. Open Forum Infect. Dis. 2019, 6, S536. [Google Scholar] [CrossRef]
- Humphries, R.M.; Hindler, J.A. In Vitro Antimicrobial Susceptibility of Aerococcus urinae. J. Clin. Microbiol. 2014, 52, 2177–2180. [Google Scholar] [CrossRef]
- Humphries, R.M.; Lee, C.; Hindler, J.A. Aerococcus urinae and Trimethoprim-Sulfamethoxazole. J. Clin. Microbiol. 2011, 49, 3934–3935. [Google Scholar] [CrossRef]
- Saad, A.; Hailes, J.; Jacobs, M.R.; Navas, M.E. Antimicrobial Susceptibility Profile of Aerococcus urinae: Recommendations for Empirical Therapy. Infect. Dis. Clin. Pract. 2023, 31, 1176. [Google Scholar] [CrossRef]
- CDC. COVID-19: U.S. Impact on Antimicrobial Resistance, Special Report 2022; CDC: Atlanta, GA, USA, 2022. [CrossRef]
- Abdel Gawad, A.M.; Ashry, W.M.O.; El-Ghannam, S.; Hussein, M.; Yousef, A. Antibiotic Resistance Profile of Common Uropathogens during COVID-19 Pandemic: Hospital Based Epidemiologic Study. BMC Microbiol. 2023, 23, 28. [Google Scholar] [CrossRef]
- Mareș, C.; Petca, R.-C.; Petca, A.; Popescu, R.-I.; Jinga, V. Does the COVID Pandemic Modify the Antibiotic Resistance of Uropathogens in Female Patients? A New Storm? Antibiotics 2022, 11, 376. [Google Scholar] [CrossRef]
- Thänert, R.; Reske, K.A.; Hink, T.; Wallace, M.A.; Wang, B.; Schwartz, D.J.; Seiler, S.; Cass, C.; Burnham, C.-A.D.; Dubberke, E.R.; et al. Comparative Genomics of Antibiotic-Resistant Uropathogens Implicates Three Routes for Recurrence of Urinary Tract Infections. mBio 2019, 10, e01977-19. [Google Scholar] [CrossRef]
- Harris, M.; Fasolino, T.; Ivankovic, D.; Davis, N.J.; Brownlee, N. Genetic Factors That Contribute to Antibiotic Resistance through Intrinsic and Acquired Bacterial Genes in Urinary Tract Infections. Microorganisms 2023, 11, 1407. [Google Scholar] [CrossRef]
- Khan, U.B.; Portal, E.A.R.; Sands, K.; Lo, S.; Chalker, V.J.; Elita, J.; Spiller, O.B. Genomic Analysis Reveals New Integrative Conjugal Elements and Transposons in GBS Conferring Antimicrobial Resistance. Antibiotics 2023, 12, 544. [Google Scholar] [CrossRef]
- Choi, B.I.; Fontes Noronha, M.; Kaindl, J.; Wolfe, A.J. Complete Genome Sequences of Aerococcus loyolae ATCC TSD-300T, Aerococcus mictus ATCC TSD-301T, and Aerococcus tenax ATCC TSD-302T. Microbiol. Resour. Announc. 2024. Advance online publication. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Methods for Antimicrobial Dilution and Disk Susceptibility Testing of Infrequently Isolated or Fastidious Bacteria, M45, 3rd ed.; Clinical and Laboratory Standards Institute: Berwyn, PA, USA, 2016; Available online: https://clsi.org/standards/products/microbiology/documents/m45/ (accessed on 19 December 2023).
- Johnson, C.M.; Grossman, A.D. Integrative and Conjugative Elements (ICEs): What They Do and How They Work. Annu. Rev. Genet. 2015, 49, 577–601. [Google Scholar] [CrossRef]
- Li, X.; Fan, H.; Zi, H.; Hu, H.; Li, B.; Huang, J.; Luo, P.; Zeng, X. Global and Regional Burden of Bacterial Antimicrobial Resistance in Urinary Tract Infections in 2019. J. Clin. Med. 2022, 11, 2817. [Google Scholar] [CrossRef] [PubMed]
- Wildfeuer, A.; Laufen, H.; Leitold, M.; Zimmermann, T. Comparison of the pharmacokinetics of three-day and five-day regimens of azithromycin in plasma and urine. J. Antimicrob. Chemother. 1993, 31, 51–56. [Google Scholar] [CrossRef]
- Kim, M.; Welch, T. Update on Azithromycin and Cardiac Side Effects. Southwest Respir. Crit. Care Chron. 2014, 2, 48–51. [Google Scholar] [CrossRef]
- Gallacher, D.J.; Zhang, L.; Aboklaish, A.F.; Mitchell, E.; Wach, R.; Marchesi, J.R.; Kotecha, S. Baseline Azithromycin Resistance in the Gut Microbiota of Preterm Born Infants. Pediatr. Res. 2024, 95, 205–212. [Google Scholar] [CrossRef]
- Lipszyc, A.; Szuplewska, M.; Bartosik, D. How Do Transposable Elements Activate Expression of Transcriptionally Silent Antibiotic Resistance Genes? Int. J. Mol. Sci. 2022, 23, 8063. [Google Scholar] [CrossRef]
- Jeong, H.; Arif, B.; Caetano-Anollés, G.; Kim, K.M.; Nasir, A. Horizontal Gene Transfer in Human-associated Microorganisms Inferred by Phylogenetic Reconstruction and Reconciliation. Sci. Rep. 2019, 9, 5953. [Google Scholar] [CrossRef]
- Smillie, C.S.; Smith, M.B.; Friedman, J.; Cordero, O.X.; David, L.A.; Alm, E.J. Ecology Drives a Global Network of Gene Exchange Connecting the Human Microbiome. Nature 2011, 480, 241–244. [Google Scholar] [CrossRef]
- Davies, M.G.; Shera, J.; Van Domselaar, G.H.; Sriprakash, K.S.; McMillan, D.J. A Novel Integrative Conjugative Element Mediates Genetic Transfer from Group G Streptococcus to Other β-Hemolytic Streptococci. J. Bacteriol. 2009, 191, 2257–2265. [Google Scholar] [CrossRef]
- Brenciani, A.; Tiberi, E.; Bacciaglia, A.; Petrelli, D.; Varaldo, P.E.; Giovanetti, E. Two Distinct Genetic Elements Are Responsible Forerm(TR)-Mediated Erythromycin Resistance in Tetracycline-Susceptible and Tetracycline-Resistant Strains of Streptococcus Pyogenes. Antimicrob. Agents Chemother. 2011, 55, 2106–2112. [Google Scholar] [CrossRef]
- Wang, H.; Zhuang, H.; Ji, S.; Sun, L.; Zhao, F.; Wu, D.; Shen, P.; Jiang, Y.; Yu, Y.; Chen, Y. Distribution of Erm Genes among MRSA Isolates with Resistance to Clindamycin in a Chinese Teaching Hospital. Infect. Genet. Evol. 2021, 96, 105127. [Google Scholar] [CrossRef]
- Giovanetti, E. Conjugative Transfer of the Erm(A) Gene from Erythromycin-Resistant Streptococcus Pyogenes to Macrolide-Susceptible, S. Pyogenes, Enterococcus Faecalis and Listeria Innocua. J. Antimicrob. Chemother. 2002, 50, 249–252. [Google Scholar] [CrossRef]
- Wolfe, A.J.; Toh, E.; Shibata, N.; Rong, R.; Kenton, K.; FitzGerald, M.; Mueller, E.R.; Schreckenberger, P.; Dong, Q.; Nelson, D.E.; et al. Evidence of Uncultivated Bacteria in the Adult Female Bladder. J. Clin. Microbiol. 2012, 50, 1376–1383. [Google Scholar] [CrossRef]
- Hilt, E.E.; McKinley, K.; Pearce, M.M.; Rosenfeld, A.B.; Zilliox, M.J.; Mueller, E.R.; Brubaker, L.; Gai, X.; Wolfe, A.J.; Schreckenberger, P.C. Urine Is Not Sterile: Use of Enhanced Urine Culture Techniques to Detect Resident Bacterial Flora in the Adult Female Bladder. J. Clin. Microbiol. 2013, 52, 871–876. [Google Scholar] [CrossRef]
- Vollstedt, A.; Baunoch, D.; Wolfe, A.; Luke, N.; Wojno, K.J.; Cline, K.; Belkoff, L.; Milbank, A.; Sherman, N.; Haverkorn, R.; et al. Bacterial Interactions as Detected by Pooled Antibiotic Susceptibility Testing (P-AST) in Polymicrobial Urine Specimens. J. Surg. Urol. 2020, 1, 101. [Google Scholar]
- Zhou, X.; Cuasquer, G.J.P.; Li, Z.; Mang, H.P.; Lv, Y. Occurrence of Typical Antibiotics, Representative Antibiotic-resistant Bacteria, and Genes in Fresh and Stored Source-separated Human Urine. Environ. Int. 2021, 146, 106280. [Google Scholar] [CrossRef]
- Wistrand-Yuen, E.; Knopp, M.; Hjort, K.; Koskiniemi, S.; Berg, O.G.; Andersson, D.I. Evolution of High-level Resistance during Low-level Antibiotic Exposure. Nat. Commun. 2018, 9, 1599. [Google Scholar] [CrossRef]
- Ding, M.; Ye, Z.; Liu, L.; Wang, W.; Chen, Q.; Zhang, F.; Wang, Y.; Sjöling, Å.; Martín-Rodríguez, A.J.; Hu, R.; et al. Subinhibitory Antibiotic Concentrations Promote the Horizontal Transfer of Plasmid-borne Resistance Genes from Klebsiellae pneumoniae to Escherichia coli. Front. Microbiol. 2022, 13, 1017092. [Google Scholar] [CrossRef]
- Morley, V.J.; Woods, R.J.; Read, A.F. Bystander Selection for Antimicrobial Resistance: Implications for Patient Health. Trends Microbiol. 2019, 27, 864–877. [Google Scholar] [CrossRef]
- Chua, K.P.; Fischer, M.A.; Rahman, M.; Linder, J.A. Changes in the Appropriateness of US Outpatient Antibiotic Prescribing After the Coronavirus Disease 2019 Outbreak: An Interrupted Time Series Analysis of 2016-2021 Data. Clin. Infect. Dis. 2024, ciae135. [Google Scholar] [CrossRef]
- Da Cunha, V.; Davies, M.R.; Douarre, P.-E.; Rosinski-Chupin, I.; Margarit, I.; Spinali, S.; Perkins, T.; Lechat, P.; Dmytruk, N.; Sauvage, E.; et al. Streptococcus agalactiae Clones Infecting Humans Were Selected and Fixed through the Extensive Use of Tetracycline. Nat. Commun. 2014, 5, 4544. [Google Scholar] [CrossRef]
- Alcock, B.P.; Raphenya, A.R.; Lau, T.T.Y.; Tsang, K.K.; Bouchard, M.; Edalatmand, A.; Huynh, W.; Nguyen, A.-L.V.; Cheng, A.A.; Liu, S.; et al. CARD 2020: Antibiotic Resistome Surveillance with the Comprehensive Antibiotic Resistance Database. Nucleic Acids Res. 2020, 48, D517–D525. [Google Scholar] [CrossRef]
- Antipov, D.; Korobeynikov, A.; McLean, J.S.; Pevzner, P.A. HybridSPAdes: An Algorithm for Hybrid Assembly of Short and Long Reads. Bioinformatics 2015, 32, 1009–1015. [Google Scholar] [CrossRef]
- Lin, Y.; Yuan, J.; Kolmogorov, M.; Shen, M.W.; Chaisson, M.; Pevzner, P.A. Assembly of Long Error-Prone Reads Using de Bruijn Graphs. Proc. Natl. Acad. Sci. USA 2016, 113, E8396–E8405. [Google Scholar] [CrossRef]
- Koren, S.; Walenz, B.P.; Berlin, K.; Miller, J.R.; Bergman, N.H.; Phillippy, A.M. Canu: Scalable and Accurate Long-Read Assembly via Adaptivek-Mer Weighting and Repeat Separation. Genome Res. 2017, 27, 722–736. [Google Scholar] [CrossRef]
- Walker, B.J.; Abeel, T.; Shea, T.; Priest, M.; Abouelliel, A.; Sakthikumar, S.; Cuomo, C.A.; Zeng, Q.; Wortman, J.; Young, S.K.; et al. Pilon: An Integrated Tool for Comprehensive Microbial Variant Detection and Genome Assembly Improvement. PLoS ONE 2014, 9, e112963. [Google Scholar] [CrossRef]
- Gurevich, A.; Saveliev, V.; Vyahhi, N.; Tesler, G. QUAST: Quality Assessment Tool for Genome Assemblies. Bioinformatics 2013, 29, 1072–1075. [Google Scholar] [CrossRef]
- Hunt, M.; Silva, N.D.; Otto, T.D.; Parkhill, J.; Keane, J.A.; Harris, S.R. Circlator: Automated Circularization of Genome Assemblies Using Long Sequencing Reads. Genome Biol. 2015, 16, 294. [Google Scholar] [CrossRef]
- Tatusova, T.; Di Cuccio, M.; Badretdin, A.; Chetvernin, V.; Nawrocki, E.P.; Zaslavsky, L.; Lomsadze, A.; Pruitt, K.D.; Borodovsky, M.; Ostell, J. NCBI Prokaryotic Genome Annotation Pipeline. Nucleic Acids Res. 2016, 44, 6614–6624. [Google Scholar] [CrossRef]
- Lao, J.; Guédon, G.; Lacroix, T.; Charron-Bourgoin, F.; Libante, V.; Loux, V.; Chiapello, H.; Payot, S.; Leblond-Bourget, N. Abundance, Diversity and Role of ICEs and IMEs in the Adaptation of Streptococcus salivarius to the Environment. Genes 2020, 11, 999. [Google Scholar] [CrossRef]
- Ambroset, C.; Coluzzi, C.; Guédon, G.; Devignes, M.; Loux, V.; Lacroix, T.; Payot, S.; Leblond-Bourget, N. New Insights into the Classification and Integration Specificity of Streptococcus Integrative Conjugative Elements through Extensive Genome Exploration. Front. Microbiol. 2016, 6, 1483. [Google Scholar] [CrossRef]
- Wang, M.; Goh, Y.-X.; Tai, C.; Wang, H.; Deng, Z.; Ou, H.-Y. VRprofile2: Detection of Antibiotic Resistance-Associated Mobilome in Bacterial Pathogens. Nucleic Acids Res. 2022, 50, W768–W773. [Google Scholar] [CrossRef]
- Sullivan, M.J.; Petty, N.K.; Beatson, S.A. Easyfig: A Genome Comparison Visualizer. Bioinformatics 2011, 27, 1009–1010. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lamichhane, J.; Choi, B.I.; Stegman, N.; Fontes Noronha, M.; Wolfe, A.J. Macrolide Resistance in the Aerococcus urinae Complex: Implications for Integrative and Conjugative Elements. Antibiotics 2024, 13, 433. https://doi.org/10.3390/antibiotics13050433
Lamichhane J, Choi BI, Stegman N, Fontes Noronha M, Wolfe AJ. Macrolide Resistance in the Aerococcus urinae Complex: Implications for Integrative and Conjugative Elements. Antibiotics. 2024; 13(5):433. https://doi.org/10.3390/antibiotics13050433
Chicago/Turabian StyleLamichhane, Jyoti, Brian I. Choi, Natalie Stegman, Melline Fontes Noronha, and Alan J. Wolfe. 2024. "Macrolide Resistance in the Aerococcus urinae Complex: Implications for Integrative and Conjugative Elements" Antibiotics 13, no. 5: 433. https://doi.org/10.3390/antibiotics13050433




