Design and Synthesis of Novel Amino and Acetamidoaurones with Antimicrobial Activities
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Biology
4.1.1. Microorganism Strains and Growth Conditions
4.1.2. Antimicrobial Activity Assay
4.1.3. Cytotoxic Assays
4.2. Chemistry
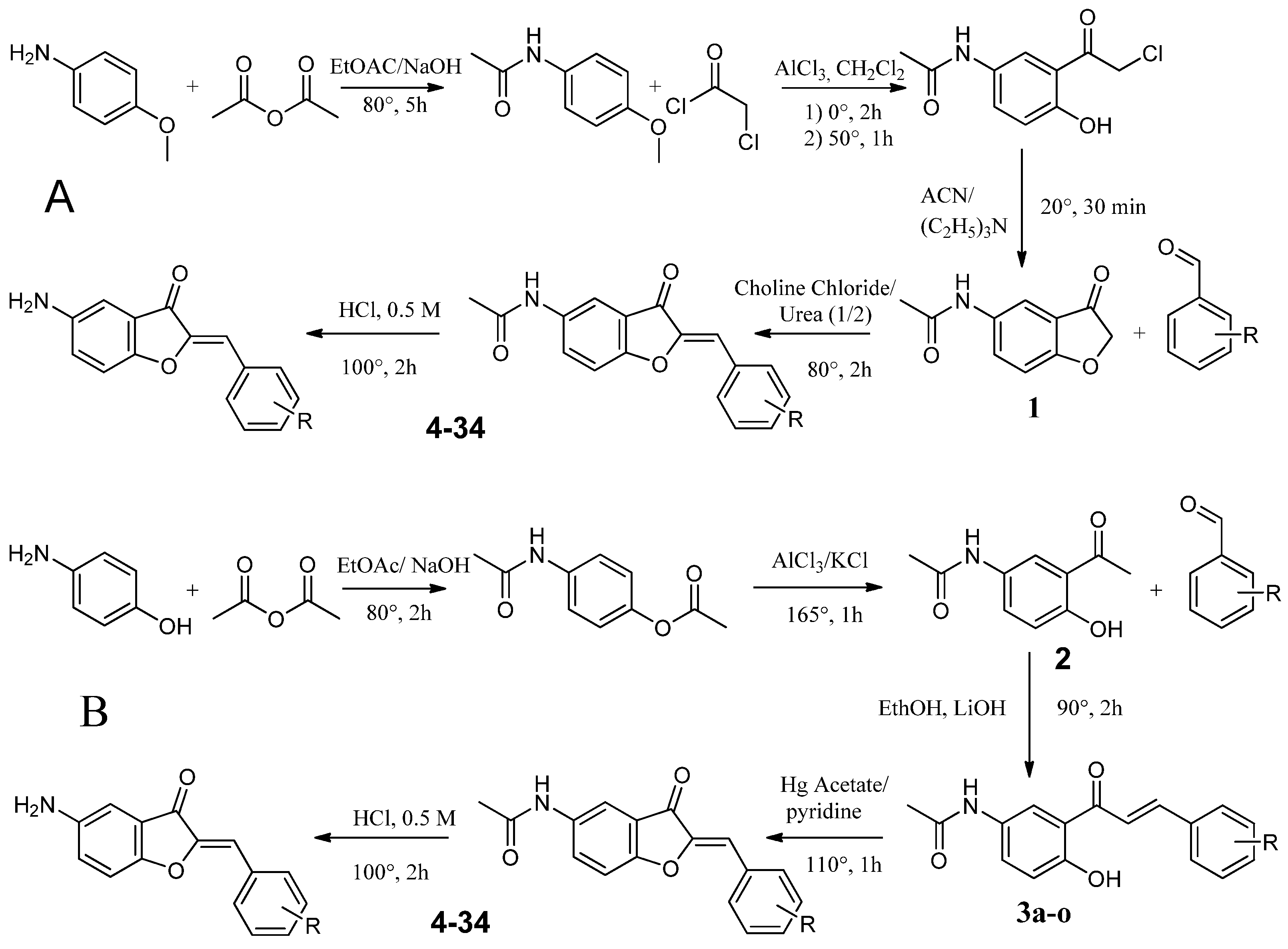
4.2.1. Synthesis Route A
Synthesis of N-(4-methoxyphenyl)acetamide (1a)
Synthesis of 2-Chloro-1-{2-hydroxy-5-[(1-hydroxyethyl)amino]cyclohexyl}ethan-1-one (2a)
Synthesis of N-(3-oxo-2,3-dihydro-1-benzofuran-5-yl)acetamide (3a)
Synthesis of Substituted 5-Acetamidoaurones
Synthesis of Substituted 5-Aminoaurone
4.2.2. Synthesis Route B
Synthesis of 4-Acetamidophenyl Acetate (1b)
Synthesis of N-(3-acetyl-4-hydroxyphenyl)acetamide (2b)
Synthesis of Substituted of 5-Acetamidochalcones (3b)
Synthesis of Substituted 5-Acetamidoaurones
Synthesis of Substituted 5-Aminoaurone
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- O’Neill, A. New Antibacterial Agents for Treating Infections Caused by Multi-Drug Resistant Gram-Negative Bacteria. Expert Opin. Investig. Drugs 2008, 17, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Abdeen, S.; Kunkle, T.; Salim, N.; Ray, A.-M.; Mammadova, N.; Summers, C.; Stevens, M.; Ambrose, A.J.; Park, Y.; Schultz, P.G.; et al. Sulfonamido-2-Arylbenzoxazole GroEL/ES Inhibitors as Potent Antibacterials against Methicillin-Resistant Staphylococcus Aureus (MRSA). J. Med. Chem. 2018, 61, 7345–7357. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Wang, L.; Lü, Y.; Yue, C. Development and Research Progress of Anti-Drug Resistant Fungal Drugs. J. Infect. Public Health 2022, 15, 986–1000. [Google Scholar] [CrossRef]
- Bastos, R.W.; Rossato, L.; Goldman, G.H.; Santos, D.A. Fungicide Effects on Human Fungal Pathogens: Cross-Resistance to Medical Drugs and Beyond. PLoS Pathog. 2021, 17, e1010073. [Google Scholar] [CrossRef] [PubMed]
- Sati, H.; Alastruey-Izquierdo, A.; Perfect, J.; Govender, N.P.; Harrison, T.S.; Chiller, T.; Sorrell, T.C.; Bongomin, F.; Oladele, R.; Chakrabarti, A.; et al. HIV and Fungal Priority Pathogens. Lancet HIV 2023, 10, e750–e754. [Google Scholar] [CrossRef] [PubMed]
- Bongomin, F.; Gago, S.; Oladele, R.O.; Denning, D.W. Global and Multi-National Prevalence of Fungal Diseases—Estimate Precision. J. Fungi 2017, 3, 57. [Google Scholar] [CrossRef] [PubMed]
- Haudecoeur, R.; Boumendjel, A. Recent Advances in the Medicinal Chemistry of Aurones. Curr. Med. Chem. 2012, 19, 2861–2875. [Google Scholar] [CrossRef] [PubMed]
- Boumendjel, A. Aurones: A Subclass of Flavones with Promising Biological Potential. Curr. Med. Chem. 2003, 10, 2621–2630. [Google Scholar] [CrossRef]
- Lazinski, L.M.; Royal, G.; Robin, M.; Maresca, M.; Haudecoeur, R. Bioactive Aurones, Indanones, and Other Hemiindigoid Scaffolds: Medicinal Chemistry and Photopharmacology Perspectives. J. Med. Chem. 2022, 65, 12594–12625. [Google Scholar] [CrossRef]
- Boucherle, B.; Peuchmaur, M.; Boumendjel, A.; Haudecoeur, R. Occurrences, Biosynthesis and Properties of Aurones as High-End Evolutionary Products. Phytochemistry 2017, 142, 92–111. [Google Scholar] [CrossRef]
- Pare, P.W.; Dmitrieva, N.; Mabry, T.J. Phytoalexin Aurone Induced in Cephalocereus Senilis Liquid Suspension Culture. Phytochemistry 1991, 30, 1133–1135. [Google Scholar] [CrossRef]
- Paré, P.W.; Mischke, C.F.; Edwards, R.; Dixon, R.A.; Norman, H.A.; Mabry, T.J. Induction of Phenylpropanoid Pathway Enzymes in Elicitor-Treated Cultures ofCephalocereus Senilis. Phytochemistry 1992, 31, 149–153. [Google Scholar] [CrossRef]
- Farag, M.A.; Deavours, B.E.; de Fátima, A.; Naoumkina, M.; Dixon, R.A.; Sumner, L.W. Integrated Metabolite and Transcript Profiling Identify a Biosynthetic Mechanism for Hispidol in Medicago Truncatula Cell Cultures. Plant Physiol. 2009, 151, 1096–1113. [Google Scholar] [CrossRef] [PubMed]
- Olleik, H.; Yahiaoui, S.; Roulier, B.; Courvoisier-Dezord, E.; Perrier, J.; Pérès, B.; Hijazi, A.; Baydoun, E.; Raymond, J.; Boumendjel, A.; et al. Aurone Derivatives as Promising Antibacterial Agents against Resistant Gram-Positive Pathogens. Eur. J. Med. Chem. 2019, 165, 133–141. [Google Scholar] [CrossRef]
- Tiwari, K.N.; Monserrat, J.-P.; Hequet, A.; Ganem-Elbaz, C.; Cresteil, T.; Jaouen, G.; Vessières, A.; Hillard, E.A.; Jolivalt, C. In Vitro Inhibitory Properties of Ferrocene-Substituted Chalcones and Aurones on Bacterial and Human Cell Cultures. Dalton Trans. 2012, 41, 6451–6457. [Google Scholar] [CrossRef] [PubMed]
- Hadj-esfandiari, N.; Navidpour, L.; Shadnia, H.; Amini, M.; Samadi, N.; Faramarzi, M.A.; Shafiee, A. Synthesis, Antibacterial Activity, and Quantitative Structure–Activity Relationships of New (Z)-2-(Nitroimidazolylmethylene)-3(2H)-Benzofuranone Derivatives. Bioorganic Med. Chem. Lett. 2007, 17, 6354–6363. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Lathwal, E.; Saroha, B.; Kumar, S.; Kumar, S.; Chauhan, N.S.; Kumar, T. Synthesis and Biological Evaluation of Quinoline-Based Novel Aurones. ChemistrySelect 2020, 5, 3539–3543. [Google Scholar] [CrossRef]
- Olleik, H.; Nicoletti, C.; Lafond, M.; Courvoisier-Dezord, E.; Xue, P.; Hijazi, A.; Baydoun, E.; Perrier, J.; Maresca, M. Comparative Structure-Activity Analysis of the Antimicrobial Activity, Cytotoxicity, and Mechanism of Action of the Fungal Cyclohexadepsipeptides Enniatins and Beauvericin. Toxins 2019, 11, 514. [Google Scholar] [CrossRef]
- Berthier, M.; Fauchère, J.-L.; Perrin, J.; Grignon, B.; Oriot, D. Fulminant Meningitis Due to Bacillus Anthracis in 11-Year-Old Girl during Ramadan. Lancet 1996, 347, 828. [Google Scholar] [CrossRef]
- Doll, J.M.; Zeitz, P.S.; Ettestad, P.; Bucholtz, A.L.; Davis, T.; Gage, K. Cat-Transmitted Fatal Pneumonic Plague in a Person Who Traveled from Colorado to Arizona. Am. J. Trop. Med. Hyg. 1994, 51, 109–114. [Google Scholar] [CrossRef]
- Olleik, H.; Yacoub, T.; Hoffer, L.; Gnansounou, S.M.; Benhaiem-Henry, K.; Nicoletti, C.; Mekhalfi, M.; Pique, V.; Perrier, J.; Hijazi, A.; et al. Synthesis and Evaluation of the Antibacterial Activities of 13-Substituted Berberine Derivatives. Antibiotics 2020, 9, 381. [Google Scholar] [CrossRef] [PubMed]
- Casanova, M.; Olleik, H.; Hdiouech, S.; Roblin, C.; Cavalier, J.-F.; Point, V.; Jeannot, K.; Caron, B.; Perrier, J.; Charriau, S.; et al. Evaluation of the Efficiency of Random and Diblock Methacrylate-Based Amphiphilic Cationic Polymers against Major Bacterial Pathogens Associated with Cystic Fibrosis. Antibiotics 2023, 12, 120. [Google Scholar] [CrossRef] [PubMed]
- Loupias, P.; Laumaillé, P.; Morandat, S.; Mondange, L.; Guillier, S.; El Kirat, K.; Da Nascimento, S.; Biot, F.; Taudon, N.; Dassonville-Klimpt, A.; et al. Synthesis and Study of New Siderophore Analog-Ciprofloxacin Conjugates with Antibiotic Activities against Pseudomonas aeruginosa and Burkholderia spp. Eur. J. Med. Chem. 2023, 245, 114921. [Google Scholar] [CrossRef]
- Broncano-Lavado, A.; Senhaji-Kacha, A.; Santamaría-Corral, G.; Esteban, J.; García-Quintanilla, M. Alternatives to Antibiotics against Mycobacterium Abscessus. Antibiotics 2022, 11, 1322. [Google Scholar] [CrossRef] [PubMed]
- Madani, A.; Ridenour, J.N.; Martin, B.P.; Paudel, R.R.; Abdul Basir, A.; Le Moigne, V.; Herrmann, J.-L.; Audebert, S.; Camoin, L.; Kremer, L.; et al. Cyclipostins and Cyclophostin Analogues as Multitarget Inhibitors That Impair Growth of Mycobacterium Abscessus. ACS Infect. Dis. 2019, 5, 1597–1608. [Google Scholar] [CrossRef]
- Benkhaled, B.T.; Hadiouch, S.; Olleik, H.; Perrier, J.; Ysacco, C.; Guillaneuf, Y.; Gigmes, D.; Maresca, M.; Lefay, C. Elaboration of Antimicrobial Polymeric Materials by Dispersion of Well-Defined Amphiphilic Methacrylic SG1-Based Copolymers. Polym. Chem. 2018, 9, 3127–3141. [Google Scholar] [CrossRef]
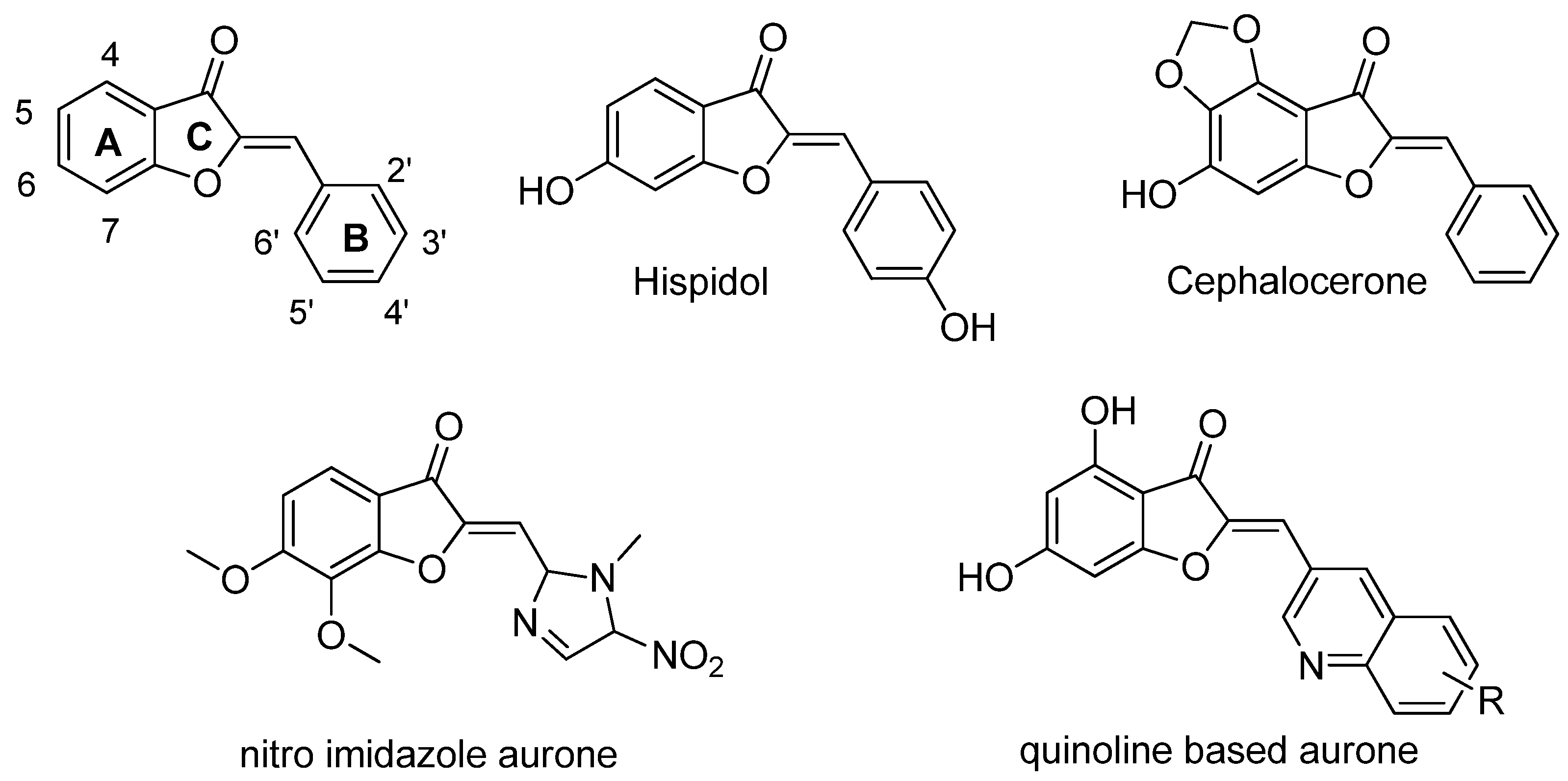

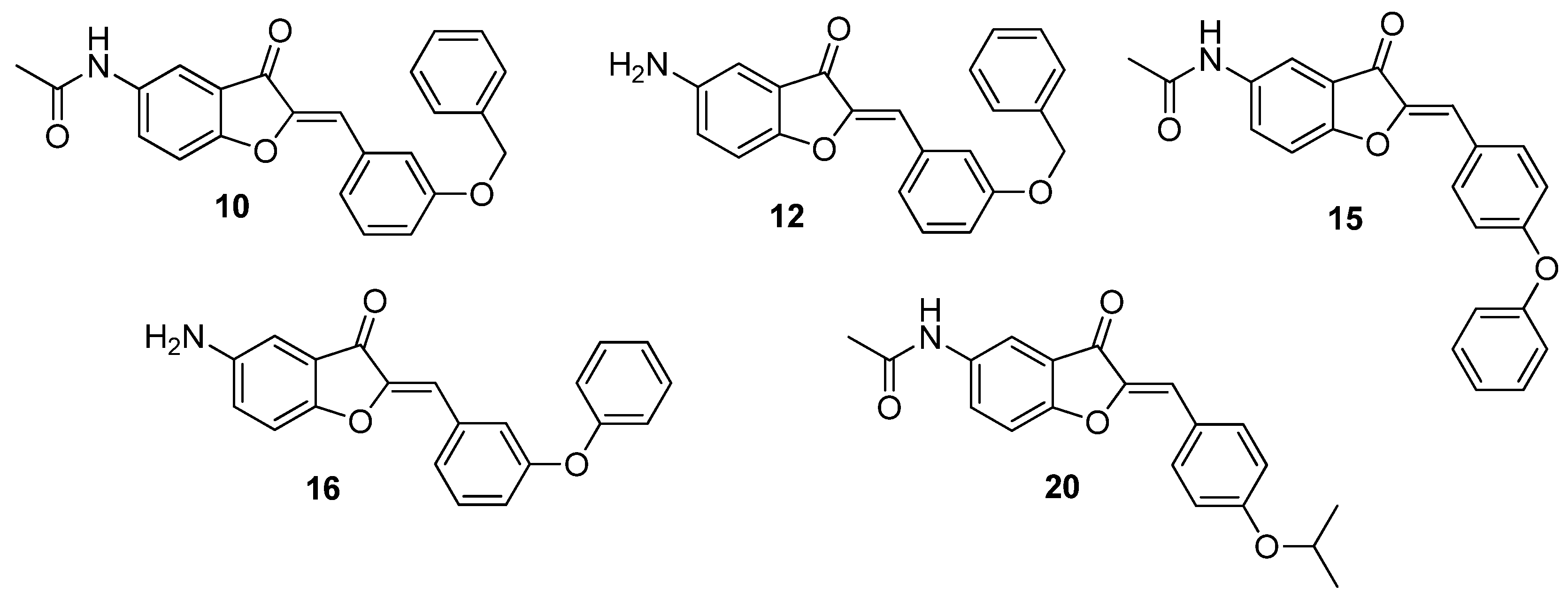

| Compound | 5 | 2′ | 3′ | 4′ |
|---|---|---|---|---|
| 4 | NHCOCH3 | OCH3 | H | H |
| 5 | NHCOCH3 | H | OCH3 | H |
| 6 | NHCOCH3 | H | H | OCH3 |
| 7 | NH2 | OCH3 | H | H |
| 8 | NH2 | H | OCH3 | H |
| 9 | NH2 | H | H | OCH3 |
| 10 | NHCOCH3 | H | OBn | H |
| 11 | NHCOCH3 | H | H | OBn |
| 12 | NH2 | H | OBn | H |
| 13 | NH2 | H | H | OBn |
| 14 | NHCOCH3 | H | OPh | H |
| 15 | NHCOCH3 | H | H | OPh |
| 16 | NH2 | H | OPh | H |
| 17 | NH2 | H | H | OPh |
| 18 | NHCOCH3 | OiPr | H | H |
| 19 | NHCOCH3 | H | OiPr | H |
| 20 | NHCOCH3 | H | H | OiPr |
| 21 | NH2 | OiPr | H | H |
| 22 | NH2 | H | OiPr | H |
| 23 | NH2 | H | H | OiPr |
| 24 | NHCOCH3 | F | H | H |
| 25 | NHCOCH3 | H | F | H |
| 26 | NH2 | F | H | H |
| 27 | NH2 | H | F | H |
| 28 | NH2 | H | H | F |
| 29 | NHCOCH3 | H | CF3 | H |
| 30 | NH2 | H | CF3 | H |
| 31 | NHCOCH3 | H | H | COOH |
| 32 | NHCOCH3 | H | OH | H |
| 33 | NH2 | H | OH | H |
| 34 | NHCOCH3 | H | H | H |
| Gram-Positive | Gram-Negative | Mycobacteria | Fungi | |||
|---|---|---|---|---|---|---|
| B. subtilis ATCC6633 | S. aureus ATCC6538P | E. coli ATCC8739 | P. aeruginosa ATCC9027 | M. smegmatis ATCC700084 | C. albicans DSM10697 | |
| Gemifloxacin | 0.03 | 0.06 | 0.06 | 0.25 | 2 | - |
| Amphotericin B | - | - | - | - | - | 0.62 |
| 4 | 50 | 100 | >100 | >100 | >100 | >100 |
| 5 | >100 | 100 | >100 | >100 | 100 | >100 |
| 6 | >100 | >100 | >100 | >100 | >100 | >100 |
| 7 | >100 | >100 | >100 | >100 | >100 | >100 |
| 8 | >100 | >100 | >100 | >100 | >100 | >100 |
| 9 | >100 | >100 | >100 | >100 | >100 | >100 |
| 10 | 3.12 | 12.5 | 12.5 | 25 | 50 | 50 |
| 11 | >100 | >100 | >100 | >100 | >100 | >100 |
| 12 | 25 | 100 | >100 | >100 | 50 | >100 |
| 13 | 100 | >100 | >100 | >100 | >100 | >100 |
| 14 | >100 | >100 | >100 | >100 | >100 | >100 |
| 15 | 50 | 100 | >100 | >100 | 50 | >100 |
| 16 | 25 | 25 | 25 | >100 | 50 | >100 |
| 17 | >100 | >100 | >100 | >100 | >100 | >100 |
| 18 | >100 | >100 | >100 | >100 | >100 | >100 |
| 19 | >100 | >100 | >100 | 25 | >100 | >100 |
| 20 | 25 | 12.5 | 25 | >100 | 50 | 50 |
| 21 | >100 | >100 | >100 | >100 | >100 | >100 |
| 22 | >100 | >100 | >100 | >100 | >100 | >100 |
| 23 | >100 | >100 | >100 | >100 | >100 | >100 |
| 24 | >100 | >100 | >100 | >100 | >100 | >100 |
| 25 | 50 | >100 | >100 | >100 | 100 | >100 |
| 26 | 50 | >100 | >100 | >100 | >100 | >100 |
| 27 | >100 | >100 | >100 | >100 | >100 | >100 |
| 28 | >100 | >100 | >100 | >100 | >100 | >100 |
| 29 | >100 | >100 | >100 | >100 | >100 | >100 |
| 30 | >100 | >100 | >100 | >100 | >100 | >100 |
| 31 | 50 | 100 | >100 | >100 | 100 | >100 |
| 32 | >100 | >100 | >100 | >100 | >100 | >100 |
| 33 | >100 | >100 | >100 | >100 | >100 | >100 |
| 34 | 50 | >100 | >100 | >100 | >100 | >100 |
| Compound | 10 | 12 | 15 | 16 | 20 |
|---|---|---|---|---|---|
| A498 | 398.2 +/− 164.6 | 152.4 +/− 31.4 | 145.7 +/− 17.3 | 452.3 +/− 147.9 | 453.0 +/− 46.4 |
| BEAS-2B | 169.0 +/− 28.3 | 74.6 +/− 12.1 | 109.5 +/− 17.4 | 129.6 +/− 18.3 | 125.9 +/− 17.2 |
| Caco-2 | 199.6 +/− 33.5 | 111.7 +/− 15.0 | 136.8 +/− 7.2 | 131.7 +/− 18.0 | 186.5 +/− 27.8 |
| HaCaT | 268.5 +/− 51.6 | 51.3 +/− 9.5 | >500 | 80.4 +/− 17.8 | 322.9 +/− 73.2 |
| HepG2 | 472.4 +/− 145.9 | 343.4 +/− 68.0 | >500 | 397.8 +/− 94.1 | >500 |
| IMR-90 | 421.4 +/− 119.3 | 42.4 +/− 9.6 | >500 | 116.5 +/− 29.4 | 437.6 +/− 128 |
| Mean CC50 | 321.5 | 129.3 | 130.6 | 218.0 | 305.1 |
| Compound | 10 | 12 | 15 | 16 | 20 |
|---|---|---|---|---|---|
| MIC on S. aureus | 12.5 | 100 | 100 | 25 | 12.5 |
| Lowest CC50 | 169.0 | 42.4 | 109.5 | 80.4 | 125.9 |
| Highest CC50 | 472.4 | 343.4 | >500 | 452.3 | >500 |
| Lowest TI | 13.5 | 0.4 | 1.0 | 3.2 | 10.0 |
| Highest TI | 37.7 | 3.4 | >5 | 18.0 | >40 |
| 10 | 20 | |
|---|---|---|
| Gram-positive | ||
| Bacillus anthracis (CNR-charbon_04022) | 12.5 | 6.25 |
| Bacillus cereus (DSM31) | 12.5 | 25 |
| Bacillus subtilis (ATCC6633) | 3.12 | 25 |
| Clostridium botulinum (DSM1985) | 0.78 | 3.12 |
| Clostridium difficile (DSM1296) | 12.5 | 3.12 |
| Clostridium perfringens (ATCC13124) | >100 | >100 |
| Enterococcus faecalis (DSM2570) | 50 | 100 |
| Enterococcus faecium (DSM20477) | 100 | 25 |
| Listeria monocytogenes (DSM20600) | 3.12 | 6.25 |
| Propionibacterium acnes (ATCC6919) | 100 | >100 |
| Staphylococcus aureus (ATCC6538P) | 12.5 | 12.5 |
| MRSA (ATCCBAA-1717) | 12.5 | 25 |
| Streptococcus pyogenes (DSM20565) | 50 | 50 |
| Gram-negative | ||
| Acinetobacter baumannii (DSM30007) | 12.5 | 25 |
| Brucella melitensis (NR-256) | 25 | 12.5 |
| Enterobacter cloacae (DSM30054) | >100 | >100 |
| Escherichia coli (ATCC8739) | 12.5 | 25 |
| Francisella tularensis (NR-643) | 50 | 12.5 |
| Helicobacter pylori (ATCC43504) | 12.5 | 12.5 |
| Klebsiella pneumonia (DSM26371) | >100 | >100 |
| Pseudomonas aeruginosa (ATCC9027) | 25 | >100 |
| Salmonella enterica (CIP80.39) | 25 | 50 |
| Shigella flexneri (ATCC12022) | 25 | 50 |
| Vibrio alginolyticus (DSM2171) | 25 | 50 |
| Yersinia pestis (NR-641) | 12.5 | 12.5 |
| Mycobacteria | ||
| Mycobacterium abscessus S (CIP 104536T) | >100 | >100 |
| Mycobacterium abscessus R(CIP 104536T) | >100 | >100 |
| Mycobacterium smegmatis (ATCC700084) | 50 | 50 |
| Mycobacterium tuberculosis H37Rv (mc26230) | >100 | >100 |
| Filamentous fungi | ||
| Aspergillus fumigatus (DSM819) | >100 | >100 |
| Fusarium oxysporum (DSM62316) | 25 | 25 |
| Yeasts | ||
| Candida albicans (DSM10697) | 50 | 50 |
| Candida auris (DSM21092) | 50 | 12.5 |
| Candida glabrata (DSM11226) | 50 | 50 |
| Candida tropicalis (DSM9419) | 100 | 100 |
| Cryptococcus neoformans (DSM11959) | 25 | 25 |
| A498 | BEAS-2B | Caco-2 | HaCaT | HepG-2 | IMR-90 | |
|---|---|---|---|---|---|---|
| CC50 (µM) | 398.2 | 169.0 | 199.6 | 268.5 | 472.4 | 421.4 |
| TI with MIC of 0.78 µM | 510.5 | 216.6 | 255.8 | 344.2 | 605.6 | 540.2 |
| A498 | BEAS-2B | Caco-2 | HaCaT | HepG-2 | IMR-90 | |
|---|---|---|---|---|---|---|
| IC50 (µM) | 453.0 | 125.9 | 186.5 | 322.9 | >500 | 437.6 |
| TI with MIC of 3.12 µM | 145.1 | 40.3 | 59.7 | 103.4 | >160.2 | 140.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Maio, A.; Olleik, H.; Courvoisier-Dezord, E.; Guillier, S.; Neulat-Ripoll, F.; Haudecoeur, R.; Bolla, J.-M.; Casanova, M.; Cavalier, J.-F.; Canaan, S.; et al. Design and Synthesis of Novel Amino and Acetamidoaurones with Antimicrobial Activities. Antibiotics 2024, 13, 300. https://doi.org/10.3390/antibiotics13040300
Di Maio A, Olleik H, Courvoisier-Dezord E, Guillier S, Neulat-Ripoll F, Haudecoeur R, Bolla J-M, Casanova M, Cavalier J-F, Canaan S, et al. Design and Synthesis of Novel Amino and Acetamidoaurones with Antimicrobial Activities. Antibiotics. 2024; 13(4):300. https://doi.org/10.3390/antibiotics13040300
Chicago/Turabian StyleDi Maio, Attilio, Hamza Olleik, Elise Courvoisier-Dezord, Sophie Guillier, Fabienne Neulat-Ripoll, Romain Haudecoeur, Jean-Michel Bolla, Magali Casanova, Jean-François Cavalier, Stéphane Canaan, and et al. 2024. "Design and Synthesis of Novel Amino and Acetamidoaurones with Antimicrobial Activities" Antibiotics 13, no. 4: 300. https://doi.org/10.3390/antibiotics13040300







