Bacteriophage Therapy in Companion and Farm Animals
Abstract
:1. Introduction
2. Antibiotic Resistance
3. Bacteriophages
4. Phage Therapy in Veterinary Medicine
4.1. Companion Animals
4.1.1. Dogs
4.1.2. Cats
4.1.3. Horses
4.2. Livestock
4.2.1. Ruminants
4.2.2. Pigs
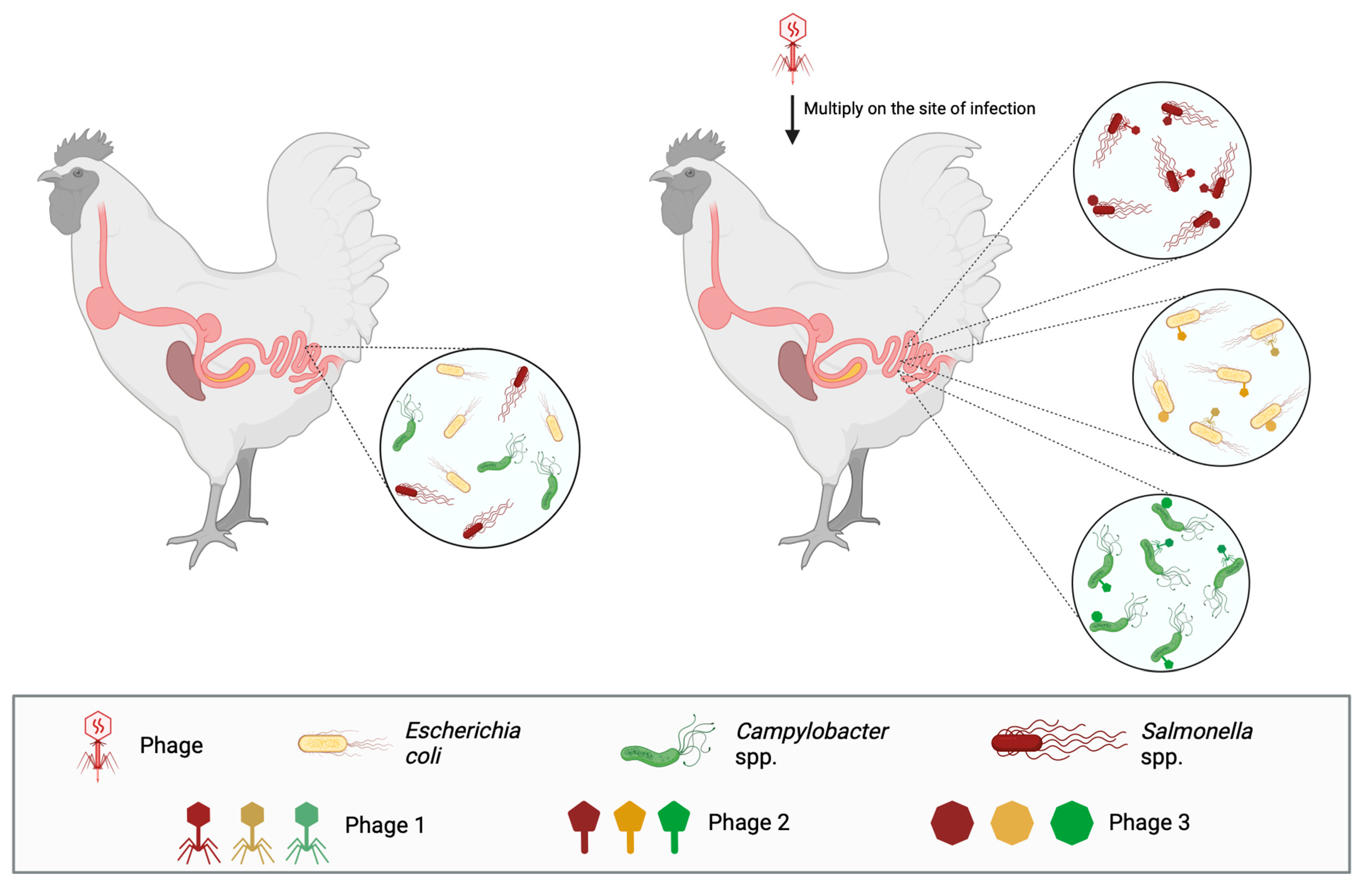
4.2.3. Poultry
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brauner, A.; Fridman, O.; Gefen, O.; Balaban, N.Q. Distinguishing between resistance, tolerance and persistence to antibiotic treatment. Nat. Rev. Microbiol. 2016, 14, 320–330. [Google Scholar] [CrossRef]
- Reygaert, W.C. An overview of the antimicrobial resistance mechanisms of bacteria. AIMS Microbiol. 2018, 4, 482–501. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, Q.; Wang, T.; Xu, N.; Lu, T.; Hong, W.; Penuelas, J.; Gillings, M.; Wang, M.; Gao, W.; et al. Assessment of global health risk of antibiotic resistance genes. Nat. Commun. 2022, 13, 1553. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, J. Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations. In Review on Antimicrobial Resistance; Her Majesty’s Government: London, UK, 2014. Available online: https://amr-review.org/sites/default/files/AMR%20Review%20Paper%20-%20Tackling%20a%20crisis%20for%20the%20health%20and%20wealth%20of%20nations_1.pdf (accessed on 25 February 2024).
- Perron, G.G.; Lee, A.E.; Wang, Y.; Huang, W.E.; Barraclough, T.G. Bacterial recombination promotes the evolution of multi-drug-resistance in functionally diverse populations. Proc. Biol. Sci. 2012, 279, 1477–1484. [Google Scholar] [CrossRef]
- Sim, W.J.; Lee, J.W.; Lee, E.S.; Shin, S.K.; Hwang, S.R.; Oh, J.E. Occurrence and distribution of pharmaceuticals in wastewater from households, livestock farms, hospitals and pharmaceutical manufactures. Chemosphere 2011, 82, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Joakim Larsson, D.G.; Flach, C. Antibiotic resistance in the environment. Nat. Rev. Microbiol. 2022, 20, 257–269. [Google Scholar] [CrossRef]
- Forsberg, K.J.; Reyes, A.; Wang, B.; Selleck, E.M.; Sommer, M.O.A.; Dantas, G. The shared antibiotic resistome of soil bacteria and human pathogens. Science 2012, 337, 1107–1111. [Google Scholar] [CrossRef]
- Twort, F.W. An investigation on the nature of ultra-microscopic viruses. Lancet 1915, 186, 1241–1243. [Google Scholar] [CrossRef]
- D’Herelle, M.F. On an invisible microbe antagonistic to dysentery bacilli. Note by M.F. D’Herelle, presented by M. Roux. Comptes Rendus Acad. Des Sci. 1917, 165, 373–375. [Google Scholar]
- Harper, D.R.; Kutter, E. Bacteriophage: Therapeutic uses. In The Encyclopedia of Life Sciences; John Wiley & Sons: Chichester, UK, 2008. [Google Scholar]
- Hatfull, G.F.; Hendrix, R.W. Bacteriophages and their genomes. Curr. Opin. Virol. 2011, 1, 298–303. [Google Scholar] [CrossRef]
- Thierauf, A.; Perez, G.; Maloy, A.S. Generalized transduction. In Bacteriophages; Methods in Molecular Biology; Clokie, M.R., Kropinski, A.M., Eds.; Humana Press: Totowa, NJ, USA, 2009; Volume 501, pp. 267–286. [Google Scholar]
- Monteiro, R.; Pires, D.P.; Costa, A.R.; Azeredo, J. Phage therapy: Going temperate? Trends Microbiol. 2019, 27, 368–378. [Google Scholar] [CrossRef]
- Dedrick, R.M.; Jacobs-Sera, D.; Bustamante, C.A.; Garlena, R.A.; Mavrich, T.N.; Pope, W.H.; Reyes, J.C.; Russell, D.A.; Adair, T.; Alvey, R.; et al. Prophage-mediated defence against viral attack and viral counter-defence. Nat. Microbiol. 2017, 2, 16251. [Google Scholar] [CrossRef] [PubMed]
- Kilcher, S.; Studer, P.; Muessner, C.; Klumpp, J.; Loessner, M.J. Cross-genus rebooting of custom-made, synthetic bacteriophage genomes in L-form bacteria. Proc. Natl. Acad. Sci. USA 2018, 115, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Luong, T.; Salabarria, A.C.; Roach, D.R. Phage therapy in the resistance era: Where do we stand and where are we going? Clin. Ther. 2020, 42, 1659–1680. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.K.; Abedon, S.T.; Loc-Carrillo, C. Phage cocktails and the future of phage therapy. Future Microbiol. 2013, 8, 769–783. [Google Scholar] [CrossRef]
- Marza, J.A.; Soothill, J.S.; Boydell, P.; Collins, T.A. Multiplication of therapeutically administered bacteriophages in Pseudomonas aeruginosa infected patients. Burns 2006, 32, 644–646. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, C.; Harper, D.; Burch, D.; Anggard, E.; Soothill, J. Topical treatment of Pseudomonas aeruginosa otitis of dogs with a bacteriophage mixture: A before/after clinical trial. Vet. Microbiol. 2010, 146, 309–313. [Google Scholar] [CrossRef]
- Antoine, G.; Laforet, F.; Blasdel, B.; Glonti, T.; Kutter, E.; Pirnay, J.P.; Mainil, J.; Delcenserie, V.; Thiry, D. Efficacy assessment of PEV2 phage on Galleria mellonella larvae infected with Pseudomonas aeruginosa dog otitis isolate. Res. Vet. Sci. 2020, 136, 598–601. [Google Scholar] [CrossRef]
- Furusawa, T.; Iwano, H.; Higuchi, H.; Yokota, H.; Usui, M.; Iwasaki, T.; Tamura, Y. Bacteriophage can lyse antibiotic-resistant Pseudomonas aeruginosa isolated from canine diseases. J. Vet. Med. Sci. 2016, 78, 1035–1038. [Google Scholar] [CrossRef]
- Santos, T.M.A.; Ledbetter, E.C.; Caixeta, L.S.; Bicalho, M.L.; Bicalho, R.C. Isolation and characterization of two bacteriophages with strong in vitro antimicrobial activity against Pseudomonas aeruginosa isolated from dogs with ocular infections. Am. J. Vet. Res. 2011, 72, 1079–1086. [Google Scholar] [CrossRef]
- Lynch, S.A.; Helbig, K.J. The complex diseases of Staphylococcus pseudintermedius in canines: Where to next? Vet. Sci. 2021, 8, 11. [Google Scholar] [CrossRef]
- Zeman, M.; Bardy, P.; Vrbovska, V.; Roudnicky, P.; Zdrahal, Z.; Ruzickova, V.; Doskar, J.; Pantucek, R. New genus Fibralongavirus in Siphoviridae phages of Staphylococcus pseudintermedius. Viruses 2019, 11, 1143. [Google Scholar] [CrossRef]
- Moodley, A.; Kot, W.; Naigard, S.; Jakociune, D.; Neve, H.; Hansen, L.H.; Guardabassi, L.; Vogensen, F.K. Isolation and characterization of bacteriophages active against methicillin-resistant Staphylococcus pseudintermedius. Res. Vet. Sci. 2019, 122, 81–85. [Google Scholar] [CrossRef]
- Azam, A.H.; Kadoi, K.; Miyanaga, K.; Usui, M.; Tamura, Y.; Cui, L.; Tanji, Y. Analysis host-recognition mechanism of staphylococcal kayvirus φSA039 reveals a novel strategy that protects Staphylococcus aureus against infection by Staphylococcus pseudintermedius Siphoviridae phages. Appl. Microbiol. Biotechnol. 2019, 103, 6809–6823. [Google Scholar] [CrossRef]
- Nakamura, T.; Kitana, J.; Fujiki, J.; Takase, M.; Iyori, K.; Simoike, K.; Iwano, H. Lytic activity of polyvalent Staphylococcal bacteriophage PhiSA012 and its endolysin Lys-PhiSA012 against antibiotic-resistant Staphylococcal clinical isolates from canine skin infection sites. Front. Med. 2020, 7, 234. [Google Scholar] [CrossRef]
- Junjappa, R.P.; Desai, S.N.; Roy, P.; Narasimhaswamy, N.; Raj, J.R.; Durgaiah, M.; Vipra, A.; Bhat, U.R.; Satyanarayana, S.K.; Shankara, N.; et al. Efficacy of anti-staphylococcal protein P128 for the treatment of canine pyoferma: Potential applications. Vet. Res. Commun. 2013, 37, 217–228. [Google Scholar] [CrossRef]
- Marques, C.; Gama, L.T.; Belas, A.; Bergström, K.; Beurlet, S.; Briend-Marchal, A.; Broens, E.M.; Costa, M.; Criel, D.; Damborg, P.; et al. European multicenter study on antimicrobial resistance in bacteria isolated from companion animal urinary tract infections. BMC Vet. Res. 2016, 12, 213. [Google Scholar] [CrossRef] [PubMed]
- Zechner, V.; Sofka, D.; Paulsen, P.; Hilbert, F. Antimicrobial resistance in Escherichia coli and resistance genes in coliphages from a small animal clinic and in a patient dog with chronic urinary tract infection. Antibiotics 2020, 9, 652. [Google Scholar] [CrossRef] [PubMed]
- LeCuyer, T.E.; Byrne, B.A.; Daniels, J.B.; Diaz-Campos, D.V.; Hammac, G.K.; Miller, C.B.; Besser, T.E.; Davis, M.A. Population structure and antimicrobial resistance of canine uropathogenic Escherichia coli. J. Clin. Microbiol. 2018, 56, e00788-18. [Google Scholar] [CrossRef] [PubMed]
- Freitag, T.; Squires, R.A.; Schmid, J. Naturally occurring bacteriophages lyse a large proportion of canine and feline uropathogenic Escherichia coli isolates in vitro. Res. Vet. Sci. 2008, 85, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Marshall, K.; Marsella, R. Topical bacteriophage therapy for Staphylococcal superficial pyoderma in horses: A double-blind, placebo-controlled pilot study. Pathogens 2023, 12, 828. [Google Scholar] [CrossRef] [PubMed]
- Chiers, K.; Decostere, A.; Devriese, L.A.; Haesebrouck, F. Bacteriological and mycological findings, and in vitro antibiotic sensitivity of pathogenic staphylococci in equine skin infections. Vet. Rec. 2003, 152, 138–141. [Google Scholar] [CrossRef]
- Ewers, C.; Stamm, I.; Pfeifer, Y.; Wieler, L.H.; Kopp, P.A.; Schønning, K.; Prenger-Berninghoff, E.; Scheufen, S.; Stolle, I.; Günther, S.; et al. Clonal spread of highly successful ST15-CTX-M-15 Klebsiella pneumoniae in companion animals and horses. J. Antimicrob. Chemother. 2014, 69, 2676–2680. [Google Scholar] [CrossRef] [PubMed]
- Venturini, C.; Bowring, B.; Patridge, S.R.; Ben Zakour, N.L.; Fajardo-Lubian, A.; Lopez Ayala, A.; Qin, J.; Totsika, M.; van Galen, G.; Norris, J.; et al. Co-occurrence of multidrug resistant Klebsiella pneumoniae pathogenic clones of human relevance in an equine pneumonia case. Microbiol. Spectr. 2022, 10, e0215821. [Google Scholar] [CrossRef] [PubMed]
- Gomes, F.; Henriques, M. Control of bovine mastitis: Old and recent therapeutic approaches. Curr. Microbiol. 2016, 72, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Gill, J.J.; Pacan, J.C.; Carson, M.E.; Leslie, K.E.; Griffiths, M.W.; Sabour, P.M. Efficacy and pharmacokinetics of bacteriophage therapy in treatment of subclinical Staphylococcus aureus mastitis in lactating dairy cattle. Antimicrob. Agents Chemother. 2006, 50, 2912–2918. [Google Scholar] [CrossRef] [PubMed]
- Ngassam-Tchamba, C.; Duprez, J.N.; Fergestad, M.; De Visscher, A.; L’Abee-Lund, T.; De Vliegher, S.; Wasteson, Y.; Touzain, F.; Blanchard, Y.; Lavigne, R.; et al. In vitro and in vivo assessment of phage therapy against Staphylococcus aureus causing bovine mastitis. J. Glob. Antimicrob. Resist. 2020, 22, 762–770. [Google Scholar] [CrossRef]
- Kwiatek, M.; Parasion, S.; Mizak, L.; Gryko, R.; Bartoszcze, M.; Kocik, J. Characterization of a bacteriophage, isolated from a cow with mastitis, that is lytic against Staphylococcus aureus strains. Arch. Virol. 2012, 157, 225–234. [Google Scholar] [CrossRef]
- Dias, R.S.; Eller, M.R.; Duarte, V.S.; Pereira, Â.L.; Silva, C.C.; Mantovani, H.C.; Oliveira, L.L.; Silva Ede, A.; De Paula, S.O. Use of phages against antibiotic-resistant Staphylococcus aureus isolated from bovine mastitis. J. Anim. Sci. 2013, 91, 3930–3939. [Google Scholar] [CrossRef]
- Titze, I.; Krömker, V. Antimicrobial activity of a phage mixture and a lactic acid bacterium against Staphylococcusaureus from bovine mastitis. Vet. Sci. 2020, 7, 31. [Google Scholar] [CrossRef]
- Mao, J.; Schmelcher, M.; Harty, W.J.; Foster-Frey, J.; Donovan, D.M. Chimeric Ply187 endolysin kills Staphylococcus aureus more effectively than the parental enzyme. FEMS Microbiol. Lett. 2013, 342, 30–36. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, H.; Bao, H.; Wang, X.; Wang, R. The lytic activity of recombinant phage lysin LysKΔamidase against staphylococcal strains associated with bovine and human infections in the Jiangsu province of China. Res. Vet. Sci. 2017, 111, 113–119. [Google Scholar] [CrossRef]
- Guo, M.; Gao, Y.; Xue, Y.; Liu, Y.; Zeng, X.; Cheng, Y.; Ma, J.; Wang, H.; Sun, J.; Wang, Z.; et al. Bacteriophage cocktails protect dairy cows against mastitis caused by drug resistant Escherichia coli infection. Front. Cell. Infect. Microbiol. 2021, 11, 690377. [Google Scholar] [CrossRef]
- Alomari, M.M.M.; Dec, M.; Nowaczek, A.; Puchalski, A.; Wernicki, A.; Kowalski, C.; Urban-Chmiel, R. Therapeutic and prophylactic effect of the experimental bacteriophage treatment to control diarrhea caused by E. coli in newborn calves. ACS Infect. Dis. 2021, 7, 2093–2101. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Qu, K.; Li, X.; Cao, Z.; Wang, X.; Li, Z.; Song, Y.; Xu, Y. Use of bacteriophages to control Escherichia coli O157:H7 in domestic ruminants, meat products, and fruits and vegetables. Foodborne Pathog. Dis. 2017, 14, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.W.; Huggins, M.B. Effectiveness of phages in treating experimental Escherichia coli diarrhoea in calves, piglets and lambs. J. Gen. Microbiol. 1983, 129, 2659–2675. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.W.; Huggins, M.B.; Shaw, K.M. The control of experimental Escherichia coli diarrhoea in calves by means of bacteriophages. J. Gen. Microbiol. 1987, 133, 1111–1126. [Google Scholar] [CrossRef] [PubMed]
- Barrow, P.; Lovell, M.; Berchieri, A., Jr. Use of lytic bacteriophage for control of experimental Escherichia coli septicemia and meningitis in chickens and calves. Clin. Diagn. Lab. Immunol. 1998, 5, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Rivas, L.; Coffey, B.; McAuliffe, O.; McDonnell, M.J.; Burgess, C.M.; Coffey, A.; Ross, R.P.; Duffy, G. In vivo and ex vivo evaluations of bacteriophages e11/2 and e4/1c for use in the control of Escherichia coli O157:H7. Appl. Environ. Microbiol. 2010, 76, 7210–7216. [Google Scholar] [CrossRef]
- Niu, Y.D.; Xu, Y.; McAllister, T.A.; Rozema, E.A.; Stephens, T.P.; Bach, S.J.; Johnson, R.P.; Stanford, K. Comparison of fecal versus rectoanal mucosal swab sampling for detecting Escherichia coli O157:H7 in experimentally inoculated cattle used in assessing bacteriophage as a mitigation strategy. J. Food Prot. 2008, 71, 691–698. [Google Scholar] [CrossRef]
- Rozema, E.A.; Stephens, T.P.; Bach, S.J.; Okine, E.K.; Johnson, R.P.; Stanford, K.; McAllister, T.A. Oral and rectal administration of bacteriophages for control of Escherichia coli O157:H7 in feedlot cattle. J. Food Prot. 2009, 72, 241–250. [Google Scholar] [CrossRef]
- Sheng, H.; Knecht, H.J.; Kudva, I.T.; Hovde, C.J. Application of bacteriophages to control intestinal Escherichia coli O157:H7 levels in ruminants. Appl. Environ. Microbiol. 2006, 72, 5359–5366. [Google Scholar] [CrossRef] [PubMed]
- Bach, S.J.; McAllister, T.A.; Veira, D.M.; Gannon, V.P.J.; Holley, R.A. Effect of bacteriophage DC22 on Escherichia coli O157: H7 in an artificial rumen system (Rusitec) and inoculated sheep. Anim. Res. 2003, 52, 89–101. [Google Scholar] [CrossRef]
- Raya, R.R.; Varey, P.; Oot, R.A.; Dyen, M.R.; Callaway, T.R.; Edrington, T.S.; Kutter, E.M.; Brabban, A.D. Isolation and characterization of a new T-even bacteriophage, CEV1, and determination of its potential to reduce Escherichia coli O157: H7 levels in sheep. Appl. Environ. Microbiol 2006, 72, 6405–6410. [Google Scholar] [CrossRef] [PubMed]
- Raya, R.R.; Oot, R.A.; Moore-Maley, B.; Wieland, S.; Callaway, T.R.; Kutter, E.M.; Brabban, A.D. Naturally resident and exogenously applied T4-like and T5-like bacteriophages can reduce Escherichia coli O157: H7 levels in sheep guts. Bacteriophage 2011, 1, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Stanford, K.; McAllister, T.A.; Niu, Y.D.; Stephens, T.P.; Mazzocco, A.; Waddell, T.E.; Johnson, R.P. Oral delivery systems for encapsulated bacteriophages targeted at Escherichia coli O157:H7 in feedlot cattle. J. Food Prot. 2010, 73, 1304–1312. [Google Scholar] [CrossRef]
- Elsayed, M.M.; Elkenany, R.M.; Zakari, A.I.; Badawy, B.M. Isolation and characterization of bacteriophages for combating multidrug-resistant Listeria monocytogenes from dairy cattle farms in conjugation with silver nanoparticles. BMC Microbiol. 2023, 23, 146. [Google Scholar] [CrossRef] [PubMed]
- Rossi, L.; Dell’Anno, M.; Turin, L.; Reggi, S.; Lombardi, A.; Alborali, G.L.; Filipe, J.; Riva, F.; Riccaboni, P.; Scanziani, E.; et al. Tobacco seed-based oral vaccination against verocytotoxic O138 Escherichia coli as alternative approach to antibiotics in weaned piglets. Antibiotics 2023, 12, 715. [Google Scholar] [CrossRef]
- Jamalludeen, N.; Johnson, R.P.; Friendship, R.; Kropinski, A.M.; Lingohr, E.J.; Gyles, C.L. Isolation and characterization of nine bacteriophages that lyse O149 enterotoxigenic Escherichia coli. Vet. Microbiol. 2007, 124, 47–57. [Google Scholar] [CrossRef]
- Jamalludeen, N.; Johnson, R.P.; Shewen, P.E.; Gyles, C.L. Evaluation of bacteriophages for prevention and treatment of diarrhea due to experimental enterotoxigenic Escherichia coli O149 infection of pigs. Vet. Microbiol. 2009, 136, 135–141. [Google Scholar] [CrossRef]
- Mao, X.; Wu, Y.; Ma, R.; Li, L.; Wang, L.; Tan, Y.; Li, Z.; Liu, H.; Han, K.; Cao, Y.; et al. Oral phage therapy with microencapsulated phage A221 against Escherichia coli infections in weaned piglets. BMC Vet. Res. 2023, 19, 165. [Google Scholar] [CrossRef]
- Callaway, T.R.; Edrington, T.S.; Brabban, A.; Kutter, B.; Karriker, L.; Stahl, C.; Wagstrom, E.; Anderson, R.; Poole, T.L.; Genovese, K.; et al. Evaluation of phage treatment as a strategy to reduce Salmonella populations in growing swine. Foodborne. Pathog. Dis. 2011, 8, 261–266. [Google Scholar] [CrossRef]
- Sriprasong, P.; Imklin, N.; Nasanit, R. Selection and characterization of bacteriophages specific to Salmonella Choleraesuis in swine. Vet. World 2022, 15, 2856–2869. [Google Scholar] [CrossRef]
- Thanki, A.M.; Mignard, G.; Atterbury, R.J.; Barrow, P.; Millard, A.D.; Clokie, M.R.J. Prophylactic delivery of a bacteriophage cocktail in feed significantly reduces salmonella colonization in pigs. Microbiol. Spectr. 2022, 10, e0042222. [Google Scholar] [CrossRef] [PubMed]
- Verstappen, K.M.; Tulinski, P.; Duim, B.; Fluit, A.C.; Carney, J.; van Nes, A.; Wagenaar, J.A. The effectiveness of bacteriophages against methicillin-resistant Staphylococcus aureus ST398 nasal colonization in pigs. PLoS ONE 2016, 11, e0160242. [Google Scholar] [CrossRef]
- Meng, X.; Shi, Y.; Ji, W.; Meng, X.; Zhang, J.; Wang, H.; Lu, C.; Sun, J.; Yan, Y. Application of a bacteriophage lysin to disrupt biofilms formed by the animal pathogen Streptococcus suis. Appl. Environ. Microbiol. 2011, 77, 8272–8279. [Google Scholar] [CrossRef]
- Ji, W.; Huang, Q.; Sun, L.; Wang, H.; Yan, Y.; Sun, J. A novel endolysin disrupts Streptococcus suis with high efficiency. FEMS Microbiol. Lett. 2015, 362, fnv205. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, C.; Wang, H.; Yan, Y.X.; Sun, J. A novel prophage lysin Ply5218 with extended lytic activity and stability against Streptococcus suis infection. FEMS Microbiol. Lett. 2016, 363, fnw186. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, J.; Wang, J.; Yang, D.; Kong, L.; Fu, Q.; Cheng, Y.; Wang, H.; Yan, Y.; Sun, J. Application of the phage Lysin Ply5218 in the treatment of Streptococcus suis infection in piglets. Viruses 2019, 11, 715. [Google Scholar] [CrossRef] [PubMed]
- Atterbury, R.J.; Van Bergen, M.A.; Ortiz, F.; Lovell, M.A.; Harris, J.A.; De Boer, A.; Wagenaar, J.A.; Allen, V.M.; Barrow, P.A. Bacteriophage therapy to reduce salmonella colonization of broiler chickens. Appl. Environ. Microbiol. 2007, 73, 4543–4549. [Google Scholar] [CrossRef]
- Andreatti Filho, R.L.; Higgins, J.P.; Higgins, S.E.; Gaona, G.; Wolfenden, A.D.; Tellez, G.; Hargis, B.M. Ability of bacteriophages isolated from different sources to reduce Salmonella enterica serovar enteritidis in vitro and in vivo. Poult. Sci. 2007, 86, 1904e1909. [Google Scholar] [CrossRef] [PubMed]
- Bardina, C.; Spricigo, D.A.; Cortes, P.; Llagostera, M. Significance of the bacteriophage treatment schedule in reducing Salmonella colonization of poultry. Appl. Environ. Microbiol. 2012, 78, 6600e6607. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo-Rebenaque, L.; Malik, D.J.; Catalá-Gregori, P.; Marin, C.; Sevilla-Navarro, S. In vitro and in vivo gastrointestinal survival of non-encapsulated and microencapsulated Salmonella bacteriophages: Implications for bacteriophage therapy in poultry. Pharmaceuticals 2021, 14, 434. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wang, Y.; Wang, F.; Zhang, Y.; Hao, H.; Lv, X.; Hao, L.; Shi, Y. Microencapsulated phage composites with increased gastrointestinal stability for the oral treatment of salmonella colonization in chicken. Front. Vet. Sci. 2023, 9, 1101872. [Google Scholar] [CrossRef] [PubMed]
- Thanki, A.M.; Hooton, S.; Whenham, N.; Salter, M.G.; Bedford, M.R.; O’Neill, H.V.M.; Clokie, M.R.J. A bacteriophage cocktail delivered in feed significantly reduced salmonella olonization in challenged broiler chickens. Emerg. Microbes Infect. 2023, 12, 2217947. [Google Scholar] [CrossRef] [PubMed]
- Sklar, I.B.; Joerger, R.D. Attempts to utilize bacteriophage to combat Salmonella enterica serovar Enteritidis infection in chickens. J. Food Saf. 2001, 21, 15–29. [Google Scholar] [CrossRef]
- Fiorentin, L.; Vieira, N.D.; Barioni, J.R.W. Oral treatment with bacteriophages reduces the concentration of Salmonella Enteritidis PT4 in caecal contents of broilers. Avian Pathol. 2005, 34, 258–263. [Google Scholar] [CrossRef]
- Hao, G.; Li, P.; Huang, J.; Cui, K.; Liang, L.; Lin, F.; Lu, Z.; Sun, S. Research Note: Therapeutic effect of a Salmonella phage combination on chicks infected with Salmonella Typhimurium. Poult. Sci. 2023, 102, 102715. [Google Scholar] [CrossRef]
- Pelyuntha, W.; Ngasaman, R.; Yingkajorn, M.; Chukiatsiri, K.; Guyonnet, V.; Vongkamjan, K. Phage cocktail administration to reduce Salmonella load in broilers. Res. Vet. Sci. 2024, 169, 105163. [Google Scholar] [CrossRef]
- Hong, S.S.; Jeong, J.; Lee, J.; Kim, S.; Min, W.; Myung, H. Therapeutic effects of bacteriophages against Salmonella gallinarum infection in chickens. J. Microbiol. Biotechnol. 2013, 23, 1478–1483. [Google Scholar] [CrossRef]
- Cui, K.; Li, P.; Huang, J.; Lin, F.; Li, R.; Cao, D.; Hao, G.; Sun, S. Salmonella phage CKT1 effectively controls the vertical transmission of Salmonella Pullorum in adult broiler breeders. Biology 2023, 12, 312. [Google Scholar] [CrossRef] [PubMed]
- Sadekuzzaman, M.; Mizan, M.F.R.; Yang, S.; Kim, H.S.; Ha, S.D. Application of bacteriophages for the inactivation of Salmonella spp. in biofilms. Food Sci. Technol. Int. 2018, 24, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, N.N.; Pottker, E.S.; Webber, B.; Borges, K.A.; Duarte, S.C.; Levandowski, R.; Ruschel, L.R.; Rodrigues, L.B. Effect of two lytic bacteriophages against multidrug-resistant and biofilm-forming Salmonella gallinarum from poultry. Br. Poult. Sci. 2020, 61, 640–645. [Google Scholar] [CrossRef]
- El-Shibiny, A.; Scott, A.; Timms, A.; Metawea, Y.; Connerton, P.; Connerton, I. Application of a group II Campylobacter bacteriophage to reduce strains of Campylobacter jejuni and Campylobacter coli colonizing broiler chickens. J. Food Prot. 2009, 72, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, C.M.; Gannon, B.W.; Halfhide, D.E.; Santos, S.B.; Hayes, C.M.; Roe, J.M.; Azeredo, J. The in vivo efficacy of two administration routes of phage cocktail to reduce numbers of Campylobacter coli and Campylobacter jejuni in chickens. BMC Microbiol. 2010, 10, 232. [Google Scholar] [CrossRef] [PubMed]
- Wagenaar, J.A.; Van Bergen, M.A.; Mueller, M.A.; Wassenaar, T.M.; Carlton, R.M. Phage therapy reduces Campylobacter jejuni colonization in broilers. Vet. Microbiol. 2005, 9, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Kittler, S.; Fischer, S.; Abdulmawjood, A.; Glunder, G.; Klein, G. Effect of bacteriophage application on Campylobacter jejuni loads in commercial broiler flocks. Appl. Environ. Microbiol. 2013, 79, 7525e7533. [Google Scholar] [CrossRef]
- Peh, E.; Szott, V.; Reichelt, B.; Friese, A.; Rösler, U.; Plötz, M.; Kittler, S. Bacteriophage cocktail application for Campylobacter mitigation—From in vitro to in vivo. BMC Microbiol. 2023, 23, 209. [Google Scholar] [CrossRef]
- Loc Carrillo, C.; Atterbury, R.J.; el-Shibiny, A.; Connerton, P.L.; Dillon, E.; Scott, A.; Connerton, I.F. Bacteriophage therapy to reduce Campylobacter jejuni colonization of broiler chickens. Appl. Environ. Microbiol. 2005, 71, 6554–6563. [Google Scholar] [CrossRef]
- Mosimann, S.; Desiree, K.; Ebner, P. Efficacy of phage therapy in poultry: A systematic review and meta-analysis. Poult. Sci. 2021, 100, 101472. [Google Scholar] [CrossRef]
- D’Angelantonio, D.; Scattolini, S.; Boni, A.; Neri, D.; Di Serafino, G.; Connerton, P.; Connerton, I.; Pomilio, F.; Di Giannatale, E.; Migliorati, G.; et al. Bacteriophage therapy to reduce colonization of Campylobacter jejuni in broiler chickens before slaughter. Viruses 2021, 13, 1428. [Google Scholar] [CrossRef]
- Huff, W.E.; Huff, G.R.; Rath, N.C.; Balog, J.M.; Xie, H.; Moore, P.A., Jr.; Donoghue, A.M. Prevention of Escherichia coli respiratory infection in broiler chickens with bacteriophage (SPR02). Poult. Sci. 2002, 81, 437–441. [Google Scholar] [CrossRef]
- Huff, W.E.; Huff, G.R.; Rath, N.C.; Balog, J.M.; Donoghue, A.M. Prevention of Escherichia coli infection in broiler chickens with a bacteriophage aerosol spray. Poult. Sci. 2002, 81, 1486–1491. [Google Scholar] [CrossRef]
- Huff, W.E.; Huff, G.R.; Rath, N.C.; Balog, J.M.; Donoghue, A.M. Evaluation of aerosol spray and intramuscular injection of bacteriophage to treat an Escherichia coli respiratory infection. Poult. Sci. 2003, 82, 1108–1112. [Google Scholar] [CrossRef]
- Huff, W.E.; Huff, G.R.; Rath, N.C.; Balog, J.M.; Donoghue, A.M. Therapeutic efficacy of bacteriophage and Baytril (enrofloxacin) individually and in combination to treat colibacillosis in broilers. Poult. Sci. 2004, 83, 1944–1947. [Google Scholar] [CrossRef]
- Oliveira, A.; Sereno, R.; Azeredo, J. In vivo efficiency evaluation of a phage cocktail in controlling severe colibacillosis in confined conditions and experimental poultry houses. Vet. Microbiol. 2010, 146, 303–308. [Google Scholar] [CrossRef]
- Jhandai, P.; Mittal, D.; Gupta, R.; Kumar, M.; Khurana, R. Therapeutics and prophylactic efficacy of novel lytic Escherichia phage vB_EcoS_PJ16 against multidrug-resistant avian pathogenic E. coli using in vivo study. Int. Microbiol. 2023. [Google Scholar] [CrossRef]
- Tang, Z.; Tang, N.; Wang, X.; Ren, H.; Zhang, C.; Zou, L.; Han, L.; Guo, L.; Liu, W. Characterization of a lytic Escherichia coli phage CE1 and its potential use in therapy against avian pathogenic Escherichia coli infections. Front. Microbiol. 2023, 14, 1091442. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Zhuang, X.; Kong, J.; Ma, G.; Zhang, H. Bacteriophage Esc-A is an efficient therapy for Escherichia coli 3-1 caused diarrhea in chickens. J. Gen. Appl. Microbiol. 2005, 51, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, M.; Trotereau, A.; Culot, A.; Moodley, A.; Atterbury, R.; Wagemans, J.; Lavigne, R.; Velge, P.; Schouler, C. Isolation and characterization of a novel phage collection against avian-pathogenic Escherichia coli. Microbiol. Spectr. 2023, 11, e0429622. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, M.; Faurie, A.; Girault, M.; Lavillatte, S.; Menanteau, P.; Chaumeil, T.; Riou, M.; Velge, P.; Schouler, C. In ovo administration of a phage cocktail partially prevents colibacillosis in chicks. Poult. Sci. 2023, 102, 102967. [Google Scholar] [CrossRef] [PubMed]
- Bielke, L.; Higgins, S.; Donoghue, A.; Donoghue, D.; Hargis, B.M. Salmonella host range of bacteriophages that infect multiple genera. Poult. Sci. 2007, 86, 2536–2540. [Google Scholar] [CrossRef] [PubMed]
- Russo, S.; Turin, L.; Zanella, A.; Ponti, W.; Poli, G. What’s going on in vaccine technology? Med. Res. Rev. 1997, 17, 277–301. [Google Scholar] [CrossRef]

| Animal | Target Bacteria | Target Disease | Reference |
|---|---|---|---|
| dog | Pseudomonas aeruginosa | chronic otitis externa | [19,20,21] |
| horse | Staphylococcus aureus | superficial pyoderma | [34] |
| cattle | Staphylococcus aureus | mastitis | [39] |
| heifer | Escherichia coli | mastitis | [46] |
| calf/steer | Escherichia coli | diarrhea 1 | [47,49,50,52,53,54,55,59] |
| calf | Escherichia coli | septicemia | [51] |
| sheep/lamb | Escherichia coli | diarrhea 1 | [49,55,56,57,58] |
| pig/piglet | Escherichia coli | diarrhea 1 | [49,63,64] |
| pig/piglet | Salmonella enterica | salmonellosis 1 | [65,67] |
| piglet | Staphylococcus aureus | nasal infection 1 | [68] |
| chicken | Salmonella enterica Enteritidis and Typhimurium | salmonellosis 1 | [73,74,75,76,77,78,79,80,81,82] |
| chicken | Salmonella enterica Gallinarum | fowl typhoid | [83,84] |
| chicken | Campylobacter jejuni and C. coli | campylobacteriosis 1 | [87,88,89,90,91,92,94] |
| chicken | Escherichia coli | septicemia and meningitis | [51] |
| chicken | Escherichia coli | airsacculitis and colibacillosis 1 | [95,96,97,98,99,100,101,102,103,104] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bianchessi, L.; De Bernardi, G.; Vigorelli, M.; Dall’Ara, P.; Turin, L. Bacteriophage Therapy in Companion and Farm Animals. Antibiotics 2024, 13, 294. https://doi.org/10.3390/antibiotics13040294
Bianchessi L, De Bernardi G, Vigorelli M, Dall’Ara P, Turin L. Bacteriophage Therapy in Companion and Farm Animals. Antibiotics. 2024; 13(4):294. https://doi.org/10.3390/antibiotics13040294
Chicago/Turabian StyleBianchessi, Laura, Giulia De Bernardi, Martina Vigorelli, Paola Dall’Ara, and Lauretta Turin. 2024. "Bacteriophage Therapy in Companion and Farm Animals" Antibiotics 13, no. 4: 294. https://doi.org/10.3390/antibiotics13040294







