Lyophilized Lipid Liquid Crystalline Nanoparticles as an Antimicrobial Delivery System
Abstract
:1. Introduction
2. Materials
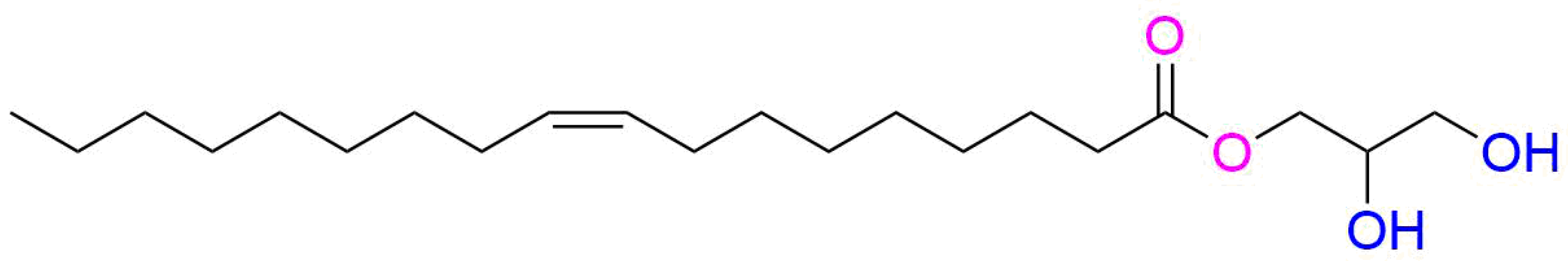
2.1. Fabrication of Liquid Crystalline Nanoparticle Dispersions
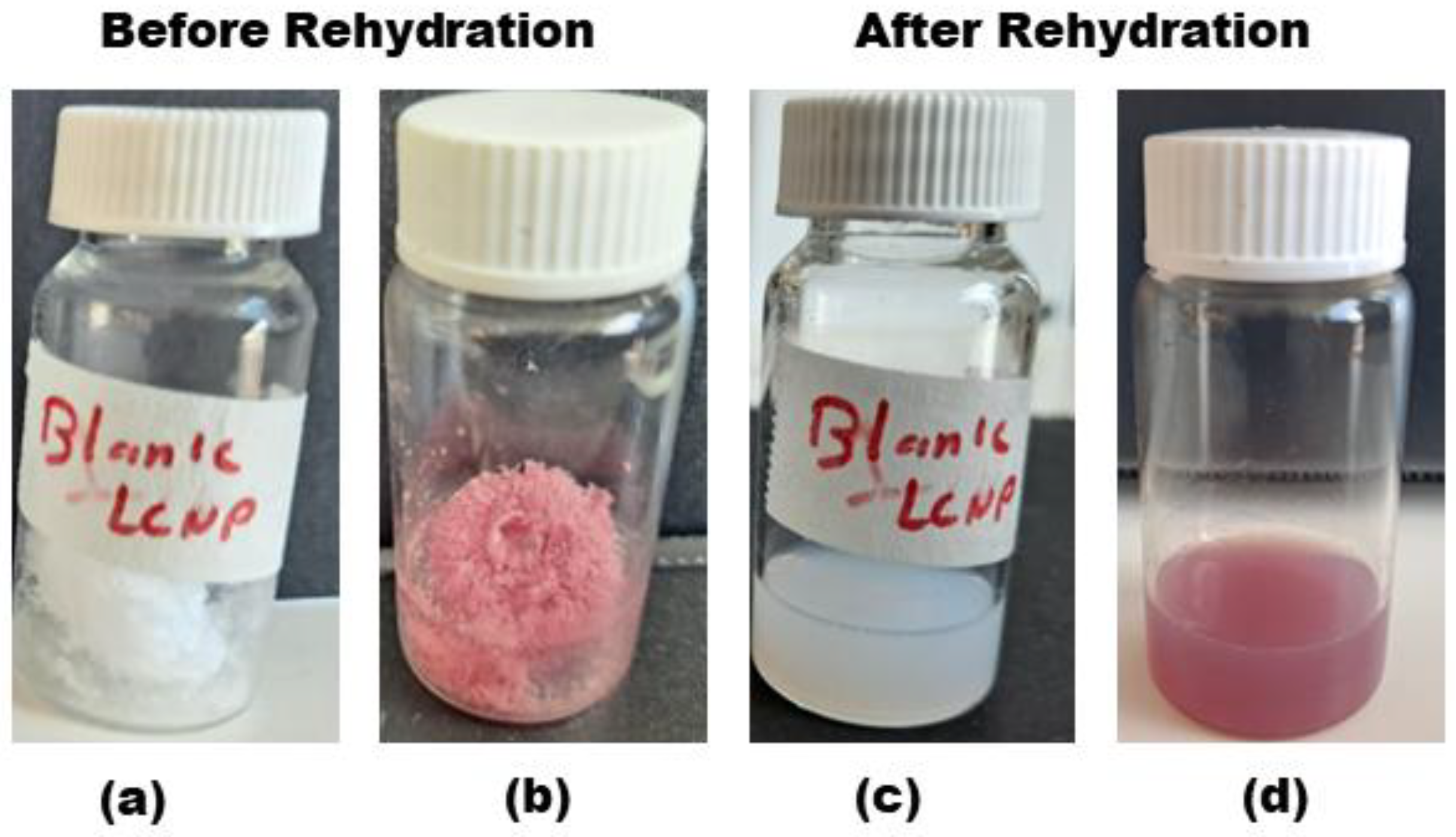
2.2. Freeze-Drying and Redispersion
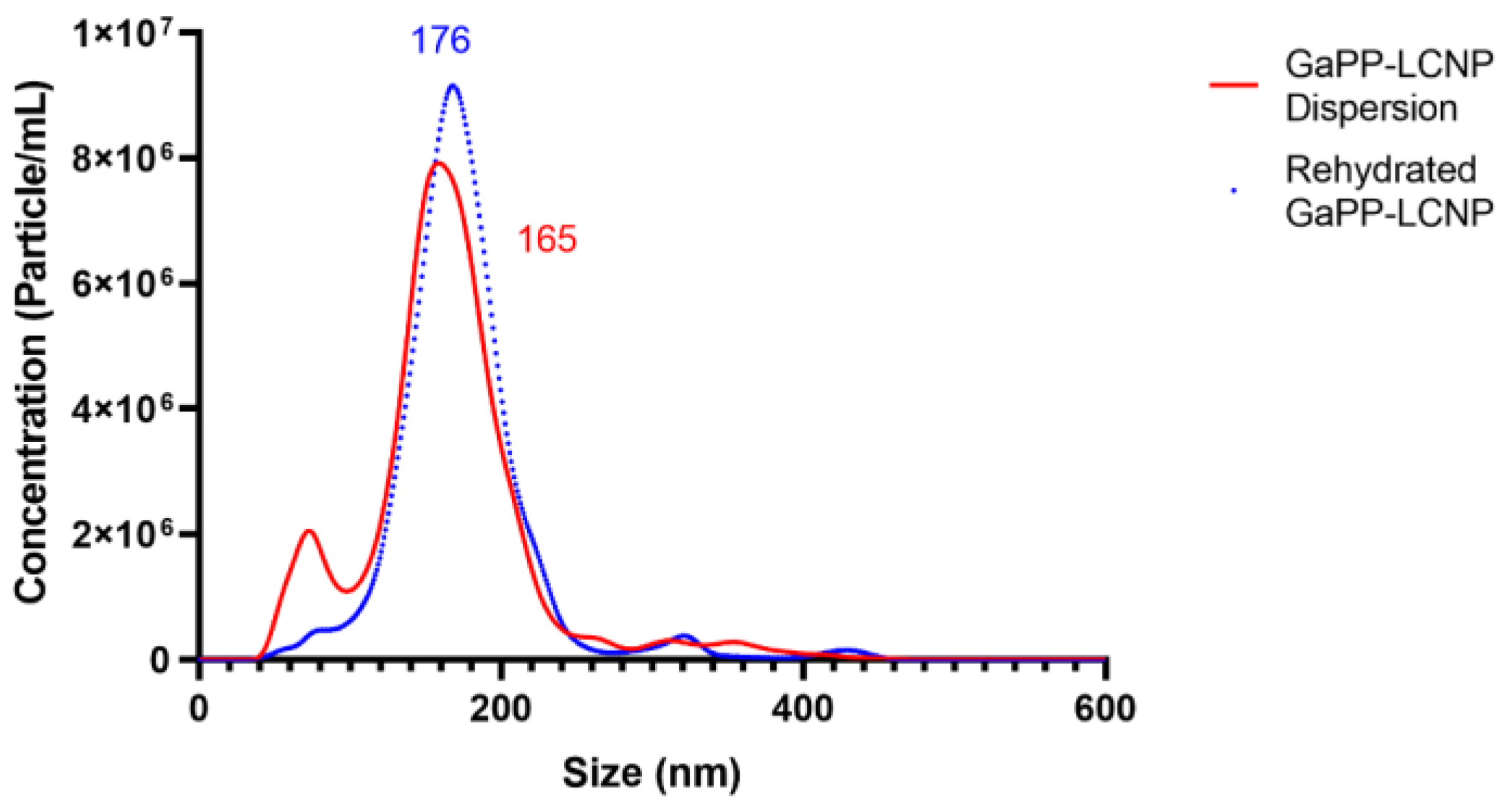
2.3. Determination of Nanoparticle Diameter
2.4. Measuring LCNP Concentration
2.5. Quantifying GaPP in LCNPs
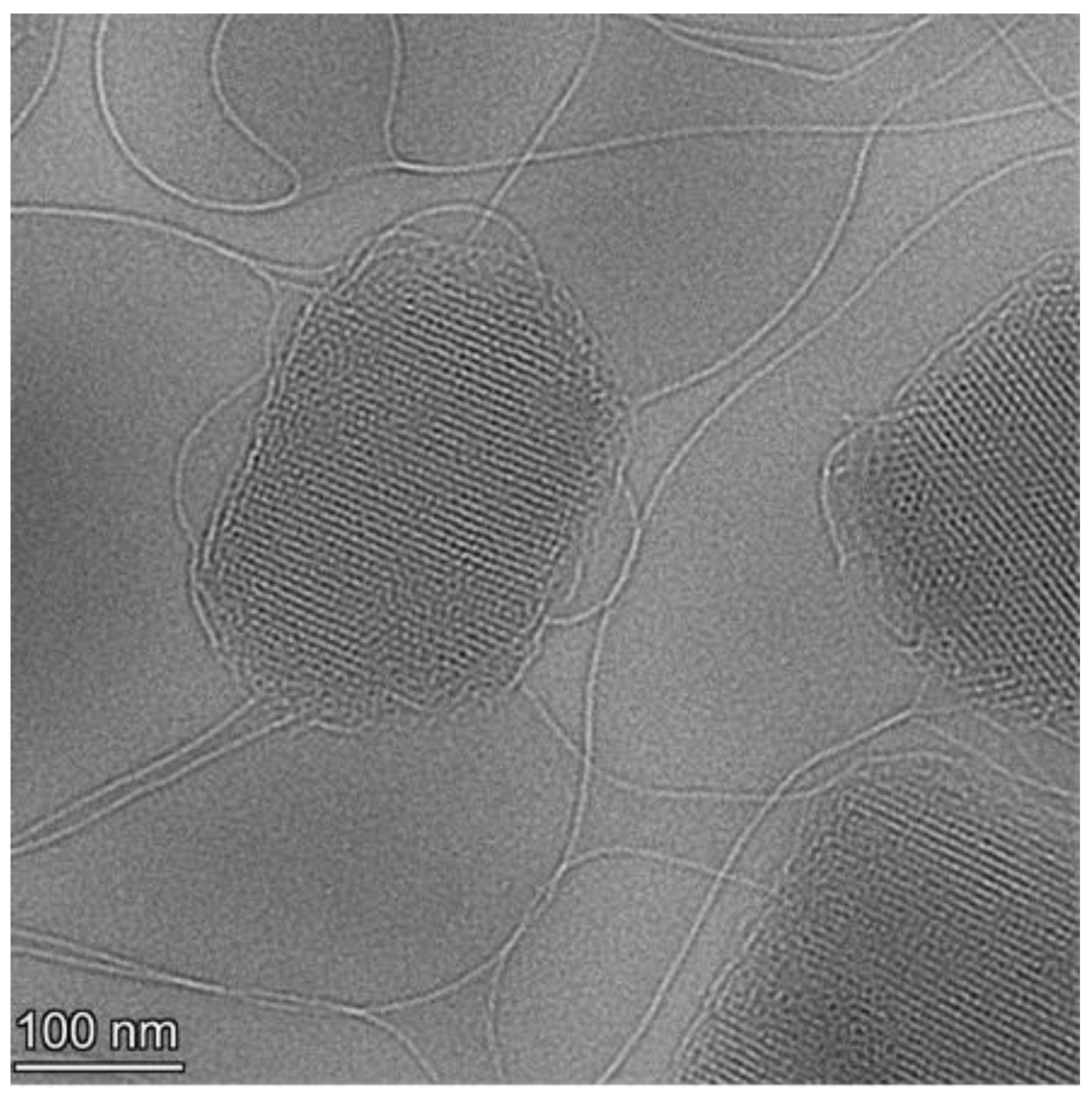
2.6. Cryogenic Transmission Electron Microscopy
2.7. Small-Angle X-ray Scattering (SAXS)
2.8. Illumination Setup
2.9. Determination of Singlet Oxygen Quantum Yield
2.10. Determination of Antibacterial Activity
3. Results and Discussion
3.1. Physicochemical Characteristics of LCNP Dispersion and Rehydrated Powder
3.2. Effect of Freeze-Drying on the Crystalline Structure of LCNPs
3.3. Effect of Lyophilization on the Antimicrobial and Photodynamic Activities of GaPP
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Awad, M.; Thomas, N.; Barnes, T.J.; Prestidge, C.A. Nanomaterials enabling clinical translation of antimicrobial photodynamic therapy. J. Control. Release 2022, 346, 300–316. [Google Scholar] [CrossRef]
- Thorn, C.R.; Thomas, N.; Boyd, B.J.; Prestidge, C.A. Nano-fats for bugs: The benefits of lipid nanoparticles for antimicrobial therapy. Drug Deliv. Transl. Res. 2021, 11, 1598–1624. [Google Scholar] [CrossRef]
- Abourehab, M.A.S.; Ansari, M.J.; Singh, A.; Hassan, A.; Abdelgawad, M.A.; Shrivastav, P.; Abualsoud, B.M.; Amaral, L.S.; Pramanik, S. Cubosomes as an emerging platform for drug delivery: A review of the state of the art. J. Mater. Chem. B 2022, 10, 2781–2819. [Google Scholar] [CrossRef]
- Boge, L.; Hallstensson, K.; Ringstad, L.; Johansson, J.; Andersson, T.; Davoudi, M.; Larsson, P.T.; Mahlapuu, M.; Håkansson, J.; Andersson, M. Cubosomes for topical delivery of the antimicrobial peptide LL-37. Eur. J. Pharm. Biopharm. 2019, 134, 60–67. [Google Scholar] [CrossRef]
- Zhai, J.; Fong, C.; Tran, N.; Drummond, C.J. Non-Lamellar Lyotropic Liquid Crystalline Lipid Nanoparticles for the Next Generation of Nanomedicine. ACS Nano 2019, 13, 6178–6206. [Google Scholar] [CrossRef]
- Awad, M.; Barnes, T.J.; Joyce, P.; Thomas, N.; Prestidge, C.A. Liquid crystalline lipid nanoparticle promotes the photodynamic activity of gallium protoporphyrin against S. aureus biofilms. J. Photochem. Photobiol. B Biol. 2022, 232, 112474. [Google Scholar] [CrossRef]
- Awad, M.; Barnes, T.J.; Thomas, N.; Joyce, P.; Prestidge, C.A. Gallium Protoporphyrin Liquid Crystalline Lipid Nanoparticles: A Third-Generation Photosensitizer against Pseudomonas aeruginosa Biofilms. Pharmaceutics 2022, 14, 2124. [Google Scholar] [CrossRef]
- Awad, M.; Kopecki, Z.; Barnes, T.J.; Wignall, A.; Joyce, P.; Thomas, N.; Prestidge, C.A. Lipid Liquid Crystal Nanoparticles: Promising Photosensitizer Carriers for the Treatment of Infected Cutaneous Wounds. Pharmaceutics 2023, 15, 305. [Google Scholar] [CrossRef]
- Malheiros, B.; Dias de Castro, R.; Lotierzo, M.C.G.; Casadei, B.R.; Barbosa, L.R.S. Design and manufacturing of monodisperse and malleable phytantriol-based cubosomes for drug delivery applications. J. Drug Deliv. Sci. Technol. 2021, 61, 102149. [Google Scholar] [CrossRef]
- Wessman, P.; Edwards, K.; Mahlin, D. Structural effects caused by spray-and freeze-drying of liposomes and bilayer disks. J. Pharm. Sci. 2010, 99, 2032–2048. [Google Scholar] [CrossRef] [PubMed]
- Abdelwahed, W.; Degobert, G.; Stainmesse, S.; Fessi, H. Freeze-drying of nanoparticles: Formulation, process and storage considerations. Adv. Drug Deliv. Rev. 2006, 58, 1688–1713. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-L.; Hanafy, M.S.; Xu, H.; Leal, J.; Zhai, Y.; Ghosh, D.; Williams, R.O., III; Smyth, H.D.C.; Cui, Z. Aerosolizable siRNA-encapsulated solid lipid nanoparticles prepared by thin-film freeze-drying for potential pulmonary delivery. Int. J. Pharm. 2021, 596, 120215. [Google Scholar] [CrossRef]
- Salminen, H.; Ankenbrand, J.; Zeeb, B.; Badolato Bönisch, G.; Schäfer, C.; Kohlus, R.; Weiss, J. Influence of spray drying on the stability of food-grade solid lipid nanoparticles. Food Res. Int. 2019, 119, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.E.; Lamprecht, A. Spray freeze drying as an alternative technique for lyophilization of polymeric and lipid-based nanoparticles. Int. J. Pharm. 2017, 516, 170–177. [Google Scholar] [CrossRef]
- Glavas-Dodov, M.; Fredro-Kumbaradzi, E.; Goracinova, K.; Simonoska, M.; Calis, S.; Trajkovic-Jolevska, S.; Hincal, A.A. The effects of lyophilization on the stability of liposomes containing 5-FU. Int. J. Pharm. 2005, 291, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Christensen, D.; Foged, C.; Rosenkrands, I.; Nielsen, H.M.; Andersen, P.; Agger, E.M. Trehalose preserves DDA/TDB liposomes and their adjuvant effect during freeze-drying. Biochim. Biophys. Acta (BBA)-Biomembr. 2007, 1768, 2120–2129. [Google Scholar] [CrossRef]
- Ingvarsson, P.T.; Yang, M.; Nielsen, H.M.; Rantanen, J.; Foged, C. Stabilization of liposomes during drying. Expert Opin. Drug Deliv. 2011, 8, 375–388. [Google Scholar] [CrossRef]
- Dou, M.; Lu, C.; Sun, Z.; Rao, W. Natural cryoprotectants combinations of l-proline and trehalose for red blood cells cryopreservation. Cryobiology 2019, 91, 23–29. [Google Scholar] [CrossRef]
- Stark, B.; Pabst, G.; Prassl, R. Long-term stability of sterically stabilized liposomes by freezing and freeze-drying: Effects of cryoprotectants on structure. Eur. J. Pharm. Sci. 2010, 41, 546–555. [Google Scholar] [CrossRef]
- Pereira, C.S.; Hünenberger, P.H. Interaction of the sugars trehalose, maltose and glucose with a phospholipid bilayer: A comparative molecular dynamics study. J. Phys. Chem. B 2006, 110, 15572–15581. [Google Scholar] [CrossRef]
- Abdelsalam, A.M.; Somaida, A.; Ambreen, G.; Ayoub, A.M.; Tariq, I.; Engelhardt, K.; Garidel, P.; Fawaz, I.; Amin, M.U.; Wojcik, M.; et al. Surface tailored zein as a novel delivery system for hypericin: Application in photodynamic therapy. Mater. Sci. Eng. C 2021, 129, 112420. [Google Scholar] [CrossRef]
- Boge, L.; Västberg, A.; Umerska, A.; Bysell, H.; Eriksson, J.; Edwards, K.; Millqvist-Fureby, A.; Andersson, M. Freeze-dried and re-hydrated liquid crystalline nanoparticles stabilized with disaccharides for drug-delivery of the plectasin derivative AP114 antimicrobial peptide. J. Colloid Interface Sci. 2018, 522, 126–135. [Google Scholar] [CrossRef]
- Avachat, A.M.; Parpani, S.S. Formulation and development of bicontinuous nanostructured liquid crystalline particles of efavirenz. Colloids Surf. B Biointerfaces 2015, 126, 87–97. [Google Scholar] [CrossRef]
- Gerola, A.P.; Semensato, J.; Pellosi, D.S.; Batistela, V.R.; Rabello, B.R.; Hioka, N.; Caetano, W. Chemical determination of singlet oxygen from photosensitizers illuminated with LED: New calculation methodology considering the influence of photobleaching. J. Photochem. Photobiol. A Chem. 2012, 232, 14–21. [Google Scholar] [CrossRef]
- Ludvíková, L.; Friš, P.; Heger, D.; Šebej, P.; Wirz, J.; Klán, P. Photochemistry of rose bengal in water and acetonitrile: A comprehensive kinetic analysis. Phys. Chem. Chem. Phys. 2016, 18, 16266–16273. [Google Scholar] [CrossRef]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef]
- Elgindy, N.A.; Mehanna, M.M.; Mohyeldin, S.M. Self-assembled nano-architecture liquid crystalline particles as a promising carrier for progesterone transdermal delivery. Int. J. Pharm. 2016, 501, 167–179. [Google Scholar] [CrossRef]
- Gustafsson, J.; Ljusberg-Wahren, H.; Almgren, M.; Larsson, K. Submicron particles of reversed lipid phases in water stabilized by a nonionic amphiphilic polymer. Langmuir 1997, 13, 6964–6971. [Google Scholar] [CrossRef]
- Nakano, M.; Sugita, A.; Matsuoka, H.; Handa, T. Small-angle X-ray scattering and 13C NMR investigation on the internal structure of “cubosomes”. Langmuir 2001, 17, 3917–3922. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Hanley, T.; Porter, C.J.H.; Larson, I.; Boyd, B.J. Phytantriol and glyceryl monooleate cubic liquid crystalline phases as sustained-release oral drug delivery systems for poorly water soluble drugs I. Phase behaviour in physiologically-relevant media. J. Pharm. Pharmacol. 2010, 62, 844–855. [Google Scholar] [CrossRef]
- Muller, F.; Salonen, A.; Glatter, O. Phase behavior of Phytantriol/water bicontinuous cubic Pn3m cubosomes stabilized by Laponite disc-like particles. J. Colloid Interface Sci. 2010, 342, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Mohanraj, V.J. Nanoparticle Coated Liposomes for Encapsulation and Delivery of Proteins. Doctoral Thesis, University of South Australia, Mawson Lakes, Australia, 2010. [Google Scholar]
- Man, D.; Słota, R.; Broda, M.A.; Mele, G.; Li, J. Metalloporphyrin intercalation in liposome membranes: ESR study. J. Biol. Inorg. Chem. 2011, 16, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Morales-de-Echegaray, A.V.; Maltais, T.R.; Lin, L.; Younis, W.; Kadasala, N.R.; Seleem, M.N.; Wei, A. Rapid Uptake and Photodynamic Inactivation of Staphylococci by Ga (III)-Protoporphyrin IX. ACS Infect. Dis. 2018, 4, 1564–1573. [Google Scholar] [CrossRef] [PubMed]
- Thorn, C.R.; de Souza Carvalho-Wodarz, C.; Horstmann, J.C.; Lehr, C.-M.; Prestidge, C.A.; Thomas, N. Tobramycin Liquid Crystal Nanoparticles Eradicate Cystic Fibrosis-Related Pseudomonas aeruginosa Biofilms. Small 2021, 17, 2100531. [Google Scholar] [CrossRef] [PubMed]


| Original LCNPs | Reconstituted LCNPs | |||
|---|---|---|---|---|
| Sample | Mean Diameter | Concentration of Nanoparticles/mL | Mean Diameter | Concentration of Nanoparticles/mL |
| Blank LCNPs | 154 ± 3.8 | 7.8 × 108 ± 1.1 × 108 | 180 ± 5.1 | 7.5 × 108 ± 3.6 × 107 |
| GaPP-LCNPs | 165 ± 4.2 | 6.8 × 108 ± 4.9 × 107 | 176 ± 4.3 | 6.5 × 108 ± 2.1 × 107 |
| Formulation | Unformulated GaPP | GaPP-LCNP (Original) | GaPP-LCNP (2% w/v Trehalose) Before Freeze-Drying | GaPP-LCNP (2% w/v Trehalose) After Rehydration |
|---|---|---|---|---|
| MIC (µg/mL) | 0.5 | 0.125 | 0.125 | 0.125 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Awad, M.; Barnes, T.J.; Prestidge, C.A. Lyophilized Lipid Liquid Crystalline Nanoparticles as an Antimicrobial Delivery System. Antibiotics 2023, 12, 1405. https://doi.org/10.3390/antibiotics12091405
Awad M, Barnes TJ, Prestidge CA. Lyophilized Lipid Liquid Crystalline Nanoparticles as an Antimicrobial Delivery System. Antibiotics. 2023; 12(9):1405. https://doi.org/10.3390/antibiotics12091405
Chicago/Turabian StyleAwad, Muhammed, Timothy J. Barnes, and Clive A. Prestidge. 2023. "Lyophilized Lipid Liquid Crystalline Nanoparticles as an Antimicrobial Delivery System" Antibiotics 12, no. 9: 1405. https://doi.org/10.3390/antibiotics12091405





