F18:A-:B1 Plasmids Carrying blaCTX-M-55 Are Prevalent among Escherichia coli Isolated from Duck–Fish Polyculture Farms
Abstract
:1. Introduction
2. Results
2.1. Identification of blaCTX-M-55-Positive E. coli Isolates
2.2. Molecular Characterization of blaCTX-M-55-Positive E. coli
2.3. Genomic Analysis of blaCTX-M-55-Positive E. coli
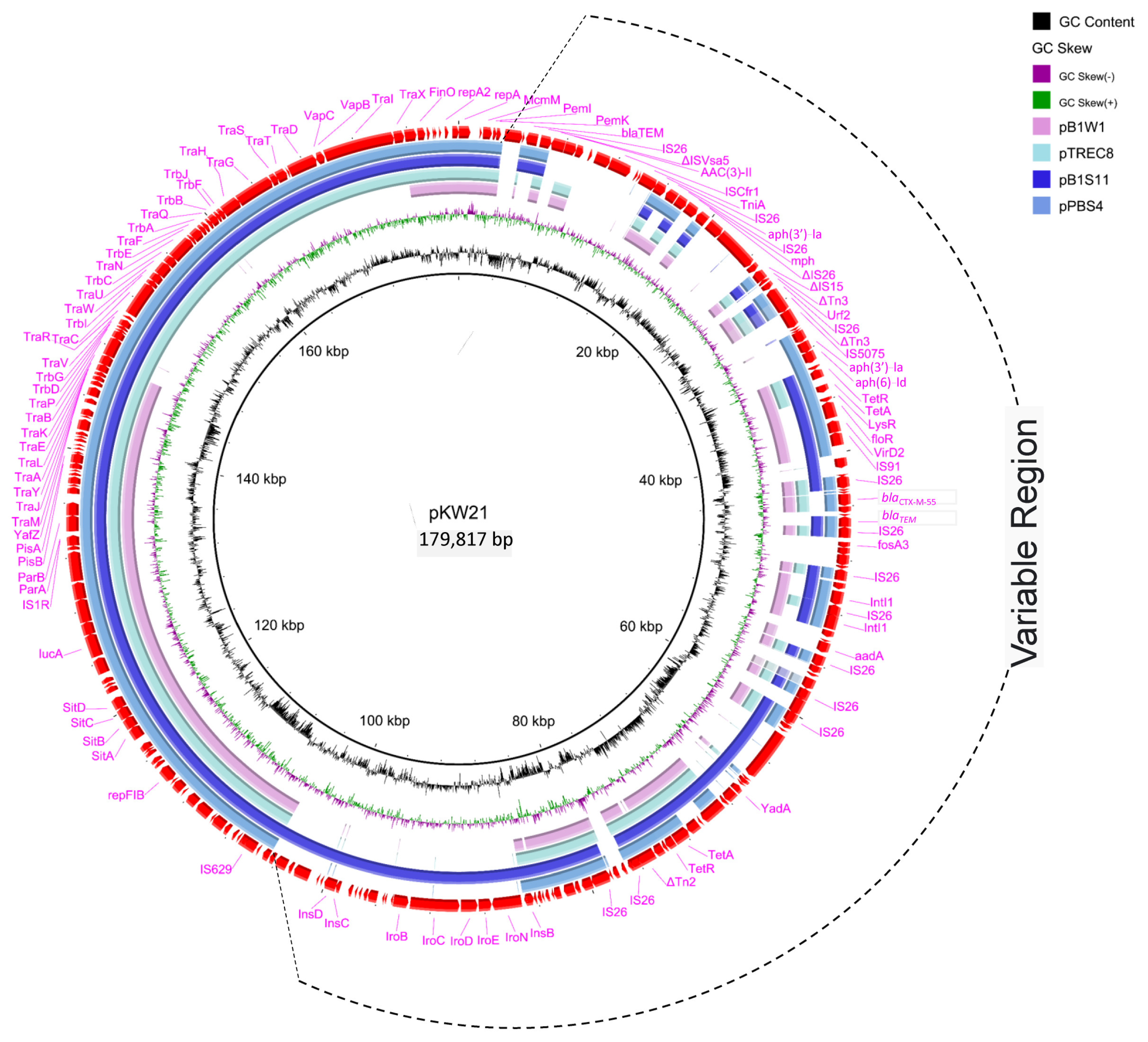
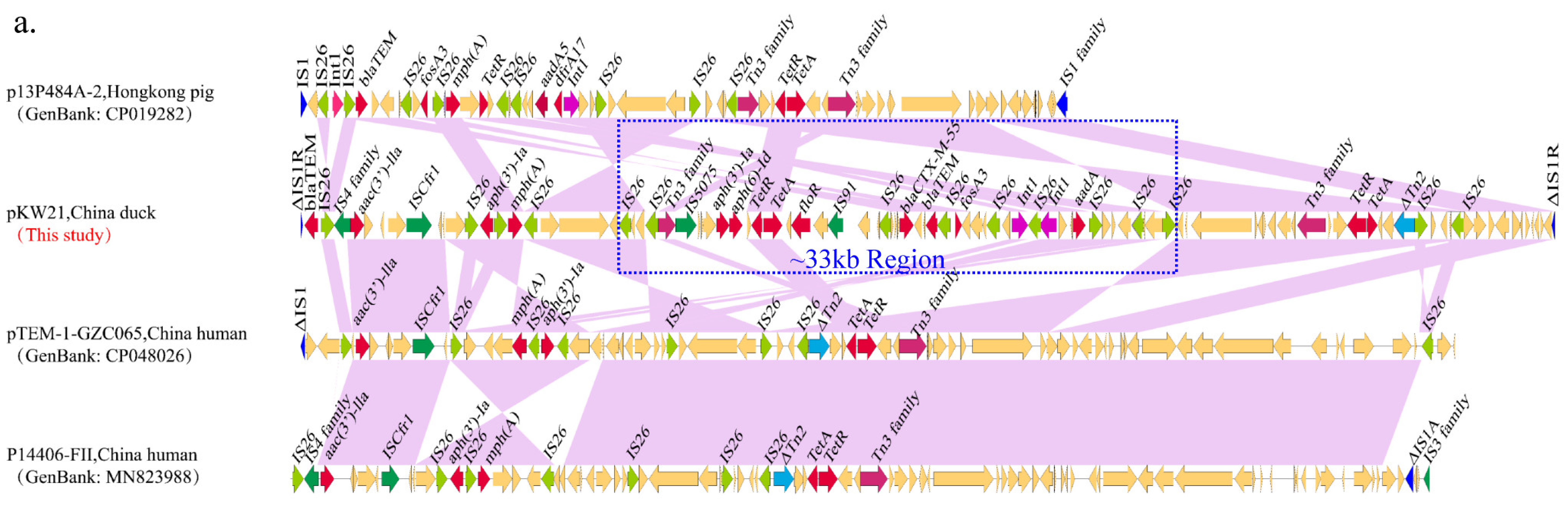
2.4. Complete Sequence Analysis of blaCTX-M-55-Carrying F18:A-:B1 Plasmids
3. Materials and Methods
3.1. Sampling Information, Bacterial Isolation and Identification
3.2. Antimicrobial Susceptibility Testing
3.3. Molecular Typing
3.4. Conjugation Assay and Southern Blotting
3.5. DNA Extraction and Whole-Genome Sequencing
3.6. Nucleotide Sequence Accession Numbers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Laurent, P.; Jean-Yves, M.; Agnese, L.; Anne-Kathrin, S.; Nicolas, K.; Patrice, N.; Stefan, S. Antimicrobial Resistance in Escherichia coli. Microbiol. Spectr. 2018, 6, 0026. [Google Scholar]
- Peirano, G.; Pitout, J. Extended-Spectrum β-Lactamase-Producing Enterobacteriaceae: Update on Molecular Epidemiology and Treatment Options. Drugs 2019, 79, 1529–1541. [Google Scholar] [CrossRef] [PubMed]
- Akya, A.; Ahmadi, M.; Khodamoradi, S.; Rezaei, M.R.; Karani, N.; Elahi, A.; Lorestani, R.C.; Rezaei, M. Prevalence of blaCTX-M, blaCTX-M-2, blaCTX-M-8, blaCTX-M-25 and blaCTX-M-3 Genes in Escherichia coli Isolated from Urinary Tract Infection in Kermanshah City, Iran. J. Clin. Diagn. Res. 2019, 13, 4–7. [Google Scholar] [CrossRef]
- Bevan, E.R.; Jones, A.M.; Hawkey, P.M. Global epidemiology of CTX-M β-lactamases: Temporal and geographical shifts in genotype. J. Antimicrob. Chemother. 2017, 72, 2145–2155. [Google Scholar] [CrossRef] [PubMed]
- Kiratisin, P.; Apisarnthanarak, A.; Saifon, P.; Laesripa, C.; Kitphati, R.; Mundy, L.M. The emergence of a novel ceftazidime-resistant CTX-M extended-spectrum β-lactamase, CTX-M-55, in both community-onset and hospital-acquired infections in Thailand. Diagn. Microbiol. Infect. Dis. 2007, 58, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zeng, L.; Doi, Y.; Lv, L.; Liu, J.-H. Extended-spectrum β-lactamase-producing Escherichia coli. Lancet Infect. Dis. 2020, 20, 404–405. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Liu, W.; Liu, Y.; Wang, J.; Lv, L.; Chen, X.; He, D.; Yang, T.; Hou, J.; Tan, Y. F33:A-:B-, IncHI2/ST3, and IncI1/ST71 plasmids drive the dissemination of fosA3 and blaCTX-M-55/-14/-65 in Escherichia coli from chickens in China. Front. Microbiol. 2014, 5, 688. [Google Scholar] [CrossRef]
- Wang, J.; Zeng, Z.L.; Huang, X.Y.; Ma, Z.B.; Guo, Z.W. Evolution and Comparative Genomics of F33:A-:B- Plasmids Carrying blaCTX-M-55 or blaCTX-M-65 in Escherichia coli and Klebsiella pneumoniae Isolated from Animals, Food Products, and Humans in China. mSphere 2018, 3, e00137-18. [Google Scholar] [CrossRef]
- Agnese, L.; Estelle, S.; Jean-Yves, M.; Marisa, H. Emergence of blaCTX-M-55 associated with fosA, rmtB and mcr gene variants in Escherichia coli from various animal species in France. J. Antimicrob. Chemother. 2018, 73, 867–872. [Google Scholar]
- Liu, F.; Tian, A.; Wang, J.; Zhu, Y.; Xie, Z.; Zhang, R.; Jiang, S. Occurrence and molecular epidemiology of fosA3-bearing Escherichia coli from ducks in Shandong province of China. Poult. Sci. 2022, 101, 101620. [Google Scholar] [CrossRef]
- Wang, M.G.; Yu, Y.; Wang, D.; Yang, R.S.; Liao, X.P. The Emergence and Molecular Characteristics of New Delhi Metallo β-Lactamase-Producing Escherichia coli From Ducks in Guangdong, China. Front. Microbiol. 2021, 12, 677633. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.R.; Lian, X.L.; Su, T.T.; Long, T.F.; Sun, J. Duck wastes as a potential reservoir of novel antibiotic resistance genes. Sci. Total. Environ. 2021, 771, 144828. [Google Scholar] [CrossRef]
- Xu, C.; Lv, Z.; Shen, Y.; Liu, D.; Shen, J. Metagenomic insights into differences in environmental resistome profiles between integrated and monoculture aquaculture farms in China. Environ. Int. 2020, 144, 106005. [Google Scholar] [CrossRef]
- Zhou, M.; Xu, Y.; Ouyang, P.; Ling, J.; Zheng, L. Spread of resistance genes from duck manure to fish intestine in simulated fish-duck pond and the promotion of cefotaxime and As. Sci. Total. Environ. 2020, 731, 138693. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.; Partridge, S.R.; He, L.; Zeng, Z.; He, D.; Ye, J.; Liu, J.H. Genetic characterization of IncI2 plasmids carrying blaCTX-M-55 spreading in both pets and food animals in China. Antimicrob. Agents Chemother. 2013, 57, 2824–2827. [Google Scholar] [CrossRef]
- Carattoli, A.; Garcia-Fernandez, A.; Varesi, P.; Fortini, D.; Gerardi, S. Molecular Epidemiology of Escherichia coli Producing Extended-Spectrum -Lactamases Isolated in Rome, Italy. J. Clin. Microbiol. 2008, 46, 103–108. [Google Scholar] [CrossRef]
- M100; Performance Standards for Antimicrobial Susceptibility Testing, 29th Edition. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2019.
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters, version 9.0. 2019. Available online: http://www.eucast.org (accessed on 1 January 2020).
- Carattoli, A.; Bertini, A.; Villa, L.; Falbo, V.; Hopkins, K.L.; Threlfall, E.J. Identification of plasmids by PCR-based replicon typing. J. Microbiol. Methods 2005, 63, 219–228. [Google Scholar] [CrossRef]
- Laura, V.; Aurora, G.F.; Daniela, F.; Alessandra, C. Replicon sequence typing of IncF plasmids carrying virulence and resistance determinants. J. Antimicrob. Chemother. 2010, 65, 2518–2529. [Google Scholar]
- Jiang, H.X.; Song, L.; Liu, J.; Zhang, X.H.; Ren, Y.N.; Zhang, W.H.; Zhang, J.Y.; Liu, Y.H.; Webber, M.A.; Ogbolu, D.O. Multiple transmissible genes encoding fluoroquinolone and third-generation cephalosporin resistance co-located in non-typhoidal Salmonella isolated from food-producing animals in China. Int. J. Antimicrob. Agents 2014, 43, 242–247. [Google Scholar] [CrossRef]
- Zhang, L.J.; Gu, X.X.; Zhang, J.; Yang, L.; Lu, Y.W.; Fang, L.X.; Jiang, H.X. Characterization of a fosA3 Carrying IncC-IncN Plasmid From a Multidrug-Resistant ST17 Salmonella Indiana Isolate. Front. Microbiol. 2020, 11, 1582. [Google Scholar] [CrossRef]
- Lv, L.C.; Lu, Y.Y.; Gao, X.; He, W.Y.; Gao, M.Y.; Mo, K.B.; Liu, J.H. Characterization of NDM-5-producing Enterobacteriaceae isolates from retail grass carp (Ctenopharyngodon idella) and evidence of blaNDM-5-bearing IncHI2 plasmid transfer between ducks and fish. Zool. Res. 2022, 43, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Wang, M.; Zhong, X.; Liu, P.; Xie, X.; Wangxiao, J.; Sun, Y. Dissemination of resistance genes in duck/fish polyculture ponds in Guangdong Province: Correlations between Cu and Zn and antibiotic resistance genes. Env. Sci. Pollut. Res. Int. 2019, 26, 8182–8193. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Xu, Y.B.; Xu, J.X.; Ling, J.Y.; Chen, J.L.; Zhou, J.L.; Zheng, L.; Du, Q.P. Antibiotic resistance genes (ARGs) in duck and fish production ponds with integrated or non-integrated mode. Chemosphere 2017, 168, 1107–1114. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Peng, S.; Xu, J.; Li, Y.; Pu, L.; Han, X.; Feng, Y. Genetic context diversity of plasmid-borne blaCTX-M-55 in Escherichia coli isolated from waterfowl. J. Glob. Antimicrob. Resist. 2022, 28, 185–194. [Google Scholar]
- Na, S.H.; Dong, C.M.; Choi, M.J.; Oh, S.J.; Lim, S.K. Antimicrobial Resistance and Molecular Characterization of Extended-Spectrum β-Lactamase-Producing Escherichia coli Isolated from Ducks in South Korea. Foodborne Pathog. Dis. 2019, 16, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Day, M.J.; Hopkins, K.L.; Wareham, D.W.; Toleman, M.A.; Elviss, N.; Randall, L.; Teale, C.; Cleary, P.; Wiuff, C.; Doumith, M.; et al. Extended-spectrum β-lactamase-producing Escherichia coli in human-derived and foodchain-derived samples from England, Wales, and Scotland: An epidemiological surveillance and typing study. Lancet Infect. Dis. 2019, 19, 1325–1335. [Google Scholar] [CrossRef] [PubMed]
- Milner, K.A.; Bay, D.C.; Alexander, D.; Walkty, A.; Zhanel, G.G. Identification and Characterization of a Novel FosA7 Member from Fosfomycin-Resistant Escherichia coli Clinical Isolates from Canadian Hospitals. Antimicrob. Agents Chemother. 2020, 65, e00865-20. [Google Scholar] [CrossRef]
- Nadimpalli, M.L.; de Lauzanne, A.; Phe, T.; Borand, L.; Jacobs, J.; Fabre, L.; Naas, T.; Le Hello, S.; Stegger, M. Escherichia coli ST410 among humans and the environment in Southeast Asia. Int. J. Antimicrob. Agents 2019, 54, 228–232. [Google Scholar]
- Roer, L.; Overballe-Petersen, S.; Hansen, A.F.; Schønning, K.; Wang, C.M.; Hinkle, M.; Whitman, T.; Lesho, E.; Schaecher, K.E. Escherichia coli Sequence Type 410 Is Causing New International High-Risk Clones. Msphere 2018, 3, e00337-18. [Google Scholar] [CrossRef]
- Li, R.; Mohsin, M.; Lu, X.; Abdullah, S.; Munir, A.; Wang, Z. Emergence of Plasmid-Mediated Resistance Genes tet(X) and mcr-1 in Escherichia coli Clinical Isolates from Pakistan. mSphere 2021, 6, e0069521. [Google Scholar] [CrossRef]
- McGann, P.; Snesrud, E.; Maybank, R.; Corey, B.; Ana, C.; Clifford, R. Escherichia coli Harboring mcr-1 and blaCTX-M on a Novel IncF Plasmid: First Report of mcr-1 in the United States. Antimicrob. Agents Chemother. 2016, 60, 4420–4421. [Google Scholar] [CrossRef]
- Zheng, Z.; Li, R.; Ye, L.; Chan, W.C.; Xia, X.; Chen, S. Genetic Characterization of blaCTX–M–55-Bearing Plasmids Harbored by Food-Borne Cephalosporin-Resistant Vibrio parahaemolyticus Strains in China. Front. Microbiol. 2019, 10, 1338. [Google Scholar] [CrossRef]
- Xia, L.; Liu, Y.; Xia, S.; Kudinha, T.; Xiao, S.N.; Zhong, N.S.; Ren, G.S.; Zhuo, C. Prevalence of ST1193 clone and IncI1/ST16 plasmid in E. coli isolates carrying blaCTX-M-55 gene from urinary tract infections patients in China. Sci. Rep. 2017, 7, 44866. [Google Scholar] [CrossRef]
- He, W.Y.; Zhang, X.X.; Gao, G.L.; Gao, M.Y.; Zhong, F.G.; Lv, L.C.; Cai, Z.P.; Si, X.F.; Yang, J.; Liu, J.H. Clonal spread of Escherichia coli O101:H9-ST10 and O101:H9-ST167 strains carrying fosA3 and blaCTX-M-14 among diarrheal calves in a Chinese farm, with Australian Chroicocephalus as the possible origin of E. coli O101:H9-ST10. Zool. Res. 2021, 42, 8. [Google Scholar] [CrossRef] [PubMed]
- BenSallem, R.; BenSlama, K.; Rojo-Bezares, B.; Porres-Osante, N.; Jouini, A.; Klibi, N.; Boudabous, A.; Sáenz, Y.; Torres, C. IncI1 plasmids carrying blaCTX-M-1 or blaCMY-2 genes in Escherichia coli from healthy humans and animals in Tunisia. Microb. 2014, 20, 495–500. [Google Scholar]
- Jiang, W.; Men, S.; Kong, L.; Ma, S.; Yang, Y.; Wang, Y.; Yuan, Q.; Cheng, G.; Zou, W.; Wang, H. Prevalence of Plasmid-Mediated Fosfomycin Resistance Gene fosA3 Among CTX-M-Producing Escherichia coli Isolates from Chickens in China. Foodborne Pathog. Dis. 2017, 14, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Harmer, C.J.; Hall, R.M. Targeted Conservative Cointegrate Formation Mediated by IS26 Family Members Requires Sequence Identity at the Reacting End. mSphere 2021, 6, e01321-20. [Google Scholar] [CrossRef] [PubMed]
- Partridge, S.R.; Kwong, S.M.; Neville, F.; Jensen, S.O. Mobile Genetic Elements Associated with Antimicrobial Resistance. Clin. Microbiol. Rev. 2018, 31, e00088-17. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.-J.; Yang, J.-T.; Chen, H.-X.; Liu, W.-Z.; Ding, Y.-L.; Chen, R.-A.; Zhang, R.-M.; Jiang, H.-X. F18:A-:B1 Plasmids Carrying blaCTX-M-55 Are Prevalent among Escherichia coli Isolated from Duck–Fish Polyculture Farms. Antibiotics 2023, 12, 961. https://doi.org/10.3390/antibiotics12060961
Zhang L-J, Yang J-T, Chen H-X, Liu W-Z, Ding Y-L, Chen R-A, Zhang R-M, Jiang H-X. F18:A-:B1 Plasmids Carrying blaCTX-M-55 Are Prevalent among Escherichia coli Isolated from Duck–Fish Polyculture Farms. Antibiotics. 2023; 12(6):961. https://doi.org/10.3390/antibiotics12060961
Chicago/Turabian StyleZhang, Li-Juan, Jin-Tao Yang, Hai-Xin Chen, Wen-Zi Liu, Yi-Li Ding, Rui-Ai Chen, Rong-Min Zhang, and Hong-Xia Jiang. 2023. "F18:A-:B1 Plasmids Carrying blaCTX-M-55 Are Prevalent among Escherichia coli Isolated from Duck–Fish Polyculture Farms" Antibiotics 12, no. 6: 961. https://doi.org/10.3390/antibiotics12060961




