New Strategies to Kill Metabolically-Dormant Cells Directly Bypassing the Need for Active Cellular Processes
Abstract
:1. Introduction
2. Methods
3. Bacterial Cell Envelope
4. Bacterial Cell Wall Hydrolases
4.1. Phage-Derived Peptidoglycan Hydrolases
4.1.1. Efficacy of Phage-Derived PGHs against Persistent Bacteria and Biofilm
4.1.2. Lack of Effectiveness of Phage-Derived PGHs against Cell Wall-Deficient Bacteria
5. Phage-Derived Polysaccharide Depolymerases
Efficacy of Phage-Derived Polysaccharide Depolymerases against Persistent Bacteria and Biofilm
6. Antimicrobial Peptides and Peptidomimetics
Efficacy of AMPs against Persistent Bacteria and Biofilm
7. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report 2022; World Health Organization: Geneva, Switzerland, 2022; ISBN 9789240062702.
- Gollan, B.; Grabe, G.; Michaux, C.; Helaine, S.; ARjatscls, H. Bacterial Persisters and Infection: Past, Present, and Progressing. Annu. Rev. Microbiol. 2019, 73, 359–385. [Google Scholar] [CrossRef] [PubMed]
- Michaux, C.; Ronneau, S.; Giorgio, R.T.; Helaine, S. Antibiotic Tolerance and Persistence Have Distinct Fitness Trade-Offs. PLoS Pathog. 2022, 18, e1010963. [Google Scholar] [CrossRef]
- Brauner, A.; Fridman, O.; Gefen, O.; Balaban, N.Q. Distinguishing between Resistance, Tolerance and Persistence to Antibiotic Treatment. Nat. Rev. Microbiol. 2016, 14, 320–330. [Google Scholar] [CrossRef]
- Balaban, N.Q.; Merrin, J.; Chait, R.; Kowalik, L.; Leibler, S. Bacterial Persistence as a Phenotypic Switch. Science 2004, 305, 1622–1625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elowitz, M.B.; Levine, A.J.; Siggia, E.D.; Swain, P.S. Stochastic Gene Expression in a Single Cell. Science 2002, 297, 1183–1186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manuse, S.; Shan, Y.; Canas-Duarte, S.J.; Bakshi, S.; Sun, W.S.; Mori, H.; Paulsson, J.; Lewis, K. Bacterial Persisters Are a Stochastically Formed Subpopulation of Low-Energy Cells. PLoS Biol. 2021, 19, e3001194. [Google Scholar] [CrossRef] [PubMed]
- Veening, J.W.; Smits, W.K.; Kuipers, O.P. Bistability, Epigenetics, and Bet-Hedging in Bacteria. Annu. Rev. Microbiol. 2008, 62, 193–210. [Google Scholar] [CrossRef] [Green Version]
- Leung, V.; Lévesque, C.M. A Stress-Inducible Quorum-Sensing Peptide Mediates the Formation of Persister Cells with Noninherited Multidrug Tolerance. J. Bacteriol. 2012, 194, 2265–2274. [Google Scholar] [CrossRef] [Green Version]
- Vega, N.M.; Allison, K.R.; Khalil, A.S.; Collins, J.J. Signaling-Mediated Bacterial Persister Formation. Nat. Chem. Biol. 2012, 8, 431–433. [Google Scholar] [CrossRef]
- Wu, Y.; Vulić, M.; Keren, I.; Lewis, K. Role of Oxidative Stress in Persister Tolerance. Antimicrob. Agents Chemother. 2012, 56, 4922–4926. [Google Scholar] [CrossRef] [Green Version]
- Pacios, O.; Blasco, L.; Bleriot, I.; Fernandez-Garcia, L.; Ambroa, A.; López, M.; Bou, G.; Cantón, R.; Garcia-Contreras, R.; Wood, T.K.; et al. (P)PpGpp and Its Role in Bacterial Persistence: New Challenges. Antimicrob. Agents Chemother. 2020, 64, e01283-20. [Google Scholar] [CrossRef]
- Zou, J.; Peng, B.; Qu, J.; Zheng, J. Are Bacterial Persisters Dormant Cells Only? Front. Microbiol. 2022, 12, 708580. [Google Scholar] [CrossRef]
- Dörr, T.; Vulić, M.; Lewis, K. Ciprofloxacin Causes Persister Formation by Inducing the TisB Toxin in Escherichia Coli. PLoS Biol. 2010, 8, e1000317. [Google Scholar] [CrossRef] [Green Version]
- Leszczynska, D.; Matuszewska, E.; Kuczynska-Wisnik, D.; Furmanek-Blaszk, B.; Laskowska, E. The Formation of Persister Cells in Stationary-Phase Cultures of Escherichia Coli Is Associated with the Aggregation of Endogenous Proteins. PLoS ONE 2013, 8, e54737. [Google Scholar] [CrossRef] [Green Version]
- Goode, O.; Smith, A.; Łapińska, U.; Bamford, R.; Kahveci, Z.; Glover, G.; Attrill, E.; Carr, A.; Metz, J.; Pagliara, S. Heterologous Protein Expression Favors the Formation of Protein Aggregates in Persister and Viable but Nonculturable Bacteria. ACS Infect. Dis. 2021, 7, 1848–1858. [Google Scholar] [CrossRef]
- Pu, Y.; Li, Y.; Jin, X.; Tian, T.; Ma, Q.; Zhao, Z.; Lin, S.-Y.; Chen, Z.; Li, B.; Yao, G.; et al. ATP-Dependent Dynamic Protein Aggregation Regulates Bacterial Dormancy Depth Critical for Antibiotic Tolerance. Mol. Cell 2019, 73, 143–156.e4. [Google Scholar] [CrossRef] [Green Version]
- Wilmaerts, D.; Bayoumi, M.; Dewachter, L.; Knapen, W.; Mika, J.T.; Hofkens, J.; Dedecker, P.; Maglia, G.; Verstraeten, N.; Michiels, J. The Persistence-Inducing Toxin HokB Forms Dynamic Pores That Cause ATP Leakage. mBio 2018, 9, e00744-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, S.; Wood, T.K. PpGpp Ribosome Dimerization Model for Bacterial Persister Formation and Resuscitation. Biochem. Biophys. Res. Commun. 2020, 523, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Ueno, H.; Kato, Y.; Tabata, K.V.; Noji, H. Revealing the Metabolic Activity of Persisters in Mycobacteria by Single-Cell D2O Raman Imaging Spectroscopy. Anal. Chem. 2019, 91, 15171–15178. [Google Scholar] [CrossRef]
- Goormaghtigh, F.; Melderen, L. Van Single-Cell Imaging and Characterization of Escherichia Coli Persister Cells to Ofloxacin in Exponential Cultures. Sci. Adv. 2019, 5, eaav9462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eisenreich, W.; Rudel, T.; Heesemann, J.; Goebel, W. Persistence of Intracellular Bacterial Pathogens—With a Focus on the Metabolic Perspective. Front. Cell. Infect. Microbiol. 2021, 10, 615450. [Google Scholar] [CrossRef]
- Verstraete, L.; Van den Bergh, B.; Verstraeten, N.; Michiels, J. Ecology and Evolution of Antibiotic Persistence. Trends Microbiol. 2022, 30, 466–479. [Google Scholar] [CrossRef] [PubMed]
- Urbaniec, J.; Xu, Y.; Hu, Y.; Hingley-Wilson, S.; McFadden, J. Phenotypic Heterogeneity in Persisters: A Novel “hunker” Theory of Persistence. FEMS Microbiol. Rev. 2021, 46, fuab042. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y. Persisters, Persistent Infections and the Yin-Yang Model. Emerg. Microbes Infect. 2014, 3, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jõers, A.; Kaldalu, N.; Tenson, T. The Frequency of Persisters in Escherichia Coli Reflects the Kinetics of Awakening from Dormancy. Bacteriol. 2010, 192, 3379–3384. [Google Scholar] [CrossRef] [Green Version]
- Ayrapetyan, M.; Williams, T.C.; Oliver, J.D. Bridging the Gap between Viable but Non-Culturable and Antibiotic Persistent Bacteria. Trends Microbiol. 2015, 23, 7–13. [Google Scholar] [CrossRef]
- Ayrapetyan, M.; Williams, T.; Oliver, J.D. Relationship between the Viable but Nonculturable State and Antibiotic Persister Cells. J. Bacteriol. 2018, 200, e00249-18. [Google Scholar] [CrossRef] [Green Version]
- Nyström, T. Bacterial Senescence, Programmed Death, and Premeditated Sterility. ASM News 2005, 71, 363–369. [Google Scholar]
- Kim, J.S.; Chowdhury, N.; Yamasaki, R.; Wood, T.K. Viable but Non-Culturable and Persistence Describe the Same Bacterial Stress State. Environ. Microbiol. 2018, 20, 2038–2048. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Yamasaki, R.; Song, S.; Zhang, W.; Wood, T.K. Single Cell Observations Show Persister Cells Wake Based on Ribosome Content. Environ. Microbiol. 2018, 20, 2085–2098. [Google Scholar] [CrossRef] [PubMed]
- Bruhn-Olszewska, B.; Szczepaniak, P.; Matuszewska, E.; Kuczyńska-Wiśnik, D.; Stojowska-Swędrzyńska, K.; Moruno Algara, M.; Laskowska, E. Physiologically Distinct Subpopulations Formed in Escherichia Coli Cultures in Response to Heat Shock. Microbiol. Res. 2018, 209, 33–42. [Google Scholar] [CrossRef]
- Römling, U. Is Biofilm Formation Intrinsic to the Origin of Life? Environ. Microbiol. 2022, 25, 26–39. [Google Scholar] [CrossRef]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial Biofilms: A Common Cause of Persistent Infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [Green Version]
- Flemming, H.C.; Wingender, J. The Biofilm Matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef]
- Karygianni, L.; Ren, Z.; Koo, H.; Thurnheer, T. Biofilm Matrixome: Extracellular Components in Structured Microbial Communities. Trends Microbiol. 2020, 28, 668–681. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An Emergent Form of Bacterial Life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Van Acker, H.; Van Dijck, P.; Coenye, T. Molecular Mechanisms of Antimicrobial Tolerance and Resistance in Bacterial and Fungal Biofilms. Trends Microbiol. 2014, 22, 326–333. [Google Scholar] [CrossRef]
- Stewart, P.S. Antimicrobial Tolerance in Biofilms. Microbiol. Spectr. 2015, 3, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stewart, P.S.; Franklin, M.J. Physiological Heterogeneity in Biofilms. Nat. Rev. Microbiol. 2008, 6, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Levin-Reisman, I.; Ronin, I.; Gefen, O.; Braniss, I.; Shoresh, N.; Balaban, N.Q. Antibiotic Tolerance Facilitates the Evolution of Resistance. Science 2017, 355, 826–830. [Google Scholar] [CrossRef]
- Windels, E.M.; Michiels, J.E.; Fauvart, M.; Wenseleers, T.; Van den Bergh, B.; Michiels, J. Bacterial Persistence Promotes the Evolution of Antibiotic Resistance by Increasing Survival and Mutation Rates. ISME J. 2019, 13, 1239–1251. [Google Scholar] [CrossRef]
- Van Den Bergh, B.; Michiels, J.E.; Wenseleers, T.; Windels, E.M.; Vanden Boer, P.; Kestemont, D.; De Meester, L.; Verstrepen, K.J.; Verstraeten, N.; Fauvart, M.; et al. Frequency of Antibiotic Application Drives Rapid Evolutionary Adaptation of Escherichia Coli Persistence. Nat. Microbiol. 2016, 1, 16020. [Google Scholar] [CrossRef] [Green Version]
- Fridman, O.; Goldberg, A.; Ronin, I.; Shoresh, N.; Balaban, N.Q. Optimization of Lag Time Underlies Antibiotic Tolerance in Evolved Bacterial Populations. Nature 2014, 513, 418–421. [Google Scholar] [CrossRef]
- Silhavy, T.J.; Kahne, D.; Walker, S. The Bacterial Cell Envelope. Cold Spring Harb. Perspect. Biol. 2010, 2, a000414. [Google Scholar] [CrossRef] [PubMed]
- Zerbib, D. Bacterial Cell Envelopes: Composition, Architecture, and Origin. In Handbook of Electroporation; Springer: Cham, Switzerland, 2017; Volume 1, pp. 417–436. [Google Scholar] [CrossRef]
- Davies, J.S.; Currie, M.J.; Wright, J.D.; Newton-Vesty, M.C.; North, R.A.; Mace, P.D.; Allison, J.R.; Dobson, R.C.J. Selective Nutrient Transport in Bacteria: Multicomponent Transporter Systems Reign Supreme. Front. Mol. Biosci. 2021, 8. [Google Scholar] [CrossRef] [PubMed]
- Vollmer, W.; Blanot, D.; De Pedro, M.A. Peptidoglycan Structure and Architecture. FEMS Microbiol. Rev. 2008, 32, 149–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vollmer, W. Structural Variation in the Glycan Strands of Bacterial Peptidoglycan. FEMS Microbiol. Rev. 2008, 32, 287–306. [Google Scholar] [CrossRef] [PubMed]
- Schleifer, K.H.; Kandler, O. PeptidoglycanTypes of Bacterial Cell Walls and Their Taxonomic Implications. Bacteriol. Rev. 1972, 36, 407–477. [Google Scholar] [CrossRef]
- Huang, K.C.; Mukhopadhyay, R.; Wen, B.; Gitai, Z.; Wingreen, N.S.; Fisher, M.E. Cell Shape and Cell-Wall Organization in Gram-Negative Bacteria. Proc. Natl. Acad. Sci. USA 2008, 105, 19282–19287. [Google Scholar] [CrossRef] [Green Version]
- Allan, E.J.; Hoischen, C.; Gumpert, J. Bacterial L-Forms. Adv. Appl. Microbiol. 2009, 68, 1–39. [Google Scholar] [CrossRef]
- Sun, Y.; Sun, T.L.; Huang, H.W. Physical Properties of Escherichia Coli Spheroplast Membranes. Biophys. J. 2014, 107, 2082–2090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajagopal, M.; Walker, S. Envelope Structures of Gram-Positive Bacteria. In Current Topics in Microbiology and Immunology; Springer: Cham, Switzerland, 2017; Volume 404. [Google Scholar] [CrossRef] [Green Version]
- Xia, G.; Kohler, T.; Peschel, A. The Wall Teichoic Acid and Lipoteichoic Acid Polymers of Staphylococcus Aureus. Int. J. Med Microbiol. 2010, 300, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.; Santa Maria, J.P.; Walker, S. Wall Teichoic Acids of Gram-Positive Bacteria. Annu. Rev. Microbiol. 2013, 67, 313–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swoboda, J.G.; Campbell, J.; Meredith, T.C.; Walker, S. Wall Teichoic Acid Function, Biosynthesis, and Inhibition. ChemBioChem 2010, 11, 35–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eugster, M.R.; Loessner, M.J. Wall Teichoic Acids Restrict Access of Bacteriophage Endolysin Ply118, Ply511, and Plyp40 Cell Wall Binding Domains to the Listeria Monocytogenes Peptidoglycan. J. Bacteriol. 2012, 194, 6498–6506. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Rutherford, S.T.; Silhavy, T.J.; Huang, K.C. Physical Properties of the Bacterial Outer Membrane. Nat. Rev. Microbiol. 2022, 20, 236–248. [Google Scholar] [CrossRef]
- Lai, W.C.B.; Chen, X.; Ho, M.K.Y.; Xia, J.; Leung, S.S.Y. Bacteriophage-Derived Endolysins to Target Gram-Negative Bacteria. Int. J. Pharm. 2020, 589, 119833. [Google Scholar] [CrossRef]
- Mesnage, S.; Tosi-couture, E.; Gounon, P.; Mock, L.; Fouet, S. The Capsule and S-Layer: Two Independent and Yet Compatible Macromolecular Structures in Bacillus Anthracis. J. Bacteriol. 1998, 180, 52–58. [Google Scholar] [CrossRef] [Green Version]
- Corbett, D.; Roberts, I.S. The Role of Microbial Polysaccharides in Host-Pathogen Interaction. F1000 Biol. Rep. 2009, 1, 30. [Google Scholar] [CrossRef]
- Limoli, D.H.; Jones, C.J.; Wozniak, D.J. Bacterial Extracellular Polysaccharides in Biofilm Formation and Function. Microbiol. Spectr. 2015, 3, 10.1128. [Google Scholar] [CrossRef] [Green Version]
- Elias, S.; Banin, E. Multi-Species Biofilms: Living with Friendly Neighbors. FEMS Microbiol. Rev. 2012, 36, 990–1004. [Google Scholar] [CrossRef]
- Jung, J.H.; Choi, N.Y.; Lee, S.Y. Biofilm Formation and Exopolysaccharide (EPS) Production by Cronobacter Sakazakii Depending on Environmental Conditions. Food Microbiol. 2013, 34, 70–80. [Google Scholar] [CrossRef]
- Payne, K.M.; Hatfull, G.F. Mycobacteriophage Endolysins: Diverse and Modular Enzymes with Multiple Catalytic Activities. PLoS ONE 2012, 7, e34052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knecht, L.E.; Veljkovic, M.; Fieseler, L. Diversity and Function of Phage Encoded Depolymerases. Front. Microbiol. 2020, 10, 2949. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.I.; Prenner, E.J.; Vogel, H.J. Tryptophan- and Arginine-Rich Antimicrobial Peptides: Structures and Mechanisms of Action. Biochim. Biophys. Acta Biomembr. 2006, 1758, 1184–1202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Defraine, V.; Schuermans, J.; Grymonprez, B.; Govers, S.K.; Aertsen, A.; Fauvart, M.; Michiels, J.; Lavigne, R.; Briers, Y. Efficacy of Artilysin Art-175 against Resistant and Persistent Acinetobacter Baumannii. Antimicrob. Agents Chemother. 2016, 60, 3480–3488. [Google Scholar] [CrossRef] [Green Version]
- Masschalck, B.; Michiels, C.W. Antimicrobial Properties of Lysozyme in Relation to Foodborne Vegetative Bacteria. Crit. Rev. Microbiol. 2003, 29, 191–214. [Google Scholar] [CrossRef]
- Yazawa, R.; Hirono, I.; Aoki, T. Transgenic Zebrafish Expressing Chicken Lysozyme Show Resistance against Bacterial Diseases. Transgenic. Res. 2006, 15, 385–391. [Google Scholar] [CrossRef]
- Rahman, M.U.; Wang, W.; Sun, Q.; Shah, J.A.; Li, C.; Sun, Y.; Li, Y.; Zhang, B.; Chen, W.; Wang, S. Endolysin, a Promising Solution against Antimicrobial Resistance. Antibiotics 2021, 10, 1277. [Google Scholar] [CrossRef]
- Callewaert, L.; Michiels, C.W. Lysozymes in the Animal Kingdom. J. Biosci. 2010, 35, 127–160. [Google Scholar] [CrossRef]
- Bastos, M.D.C.D.F.; Coutinho, B.G.; Coelho, M.L.V. Lysostaphin: A Staphylococcal Bacteriolysin with Potential Clinical Applications. Pharmaceuticals 2010, 3, 1139–1161. [Google Scholar] [CrossRef] [Green Version]
- Danis-Wlodarczyk, K.M.; Wozniak, D.J.; Abedon, S.T. Treating Bacterial Infections with Bacteriophage-Based Enzybiotics: In Vitro, in Vivo and Clinical Application. Antibiotics 2021, 10, 1497. [Google Scholar] [CrossRef]
- Ferraboschi, P.; Ciceri, S.; Grisenti, P. Applications of Lysozyme, an Innate Immune Defense Factor, as an Alternative Antibiotic. Antibiotics 2021, 10, 1534. [Google Scholar] [CrossRef] [PubMed]
- Hukić, M.; Seljmo, D.; Ramovic, A.; Ibrišimović, M.A.; Dogan, S.; Hukic, J.; Bojic, E.F. The Effect of Lysozyme on Reducing Biofilms by Staphylococcus Aureus, Pseudomonas Aeruginosa, and Gardnerella Vaginalis: An In Vitro Examination. Microb. Drug Resist. 2018, 24, 353–358. [Google Scholar] [CrossRef]
- Ellison III, R.T.; Giehl, T.J. Killing of Gram-Negative Bacteria by Lactofernn and Lysozyme. J. Clin. Investig. 1991, 88, 1080–1091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bera, A.; Biswas, R.; Herbert, S.; Götz, F. The Presence of Peptidoglycan O-Acetyltransferase in Various Staphylococcal Species Correlates with Lysozyme Resistance and Pathogenicity. Infect. Immun. 2006, 74, 4598–4604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawai, Y.; Mickiewicz, K.; Errington, J. Lysozyme Counteracts β-Lactam Antibiotics by Promoting the Emergence of L-Form Bacteria. Cell 2018, 172, 1038–1049.e10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Derde, M.; Lechevalier, V.; Guérin-Dubiard, C.; Cochet, M.F.; Jan, S.; Baron, F.; Gautier, M.; Vié, V.; Nau, F. Hen Egg White Lysozyme Permeabilizes Escherichia Coli Outer and Inner Membranes. J. Agric. Food Chem. 2013, 61, 9922–9929. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, A.; Yu, H.; Xiong, Y.; Zhou, G.; Qin, M.; Dou, J.; Wang, J. Human Lysozyme Synergistically Enhances Bactericidal Dynamics and Lowers the Resistant Mutant Prevention Concentration for Metronidazole to Helicobacter Pylori by Increasing Cell Permeability. Molecules 2016, 21, 1435. [Google Scholar] [CrossRef] [Green Version]
- Ginsburg, I. Bactericidal Cationic Peptides Can Also Function as Bacteriolysis-Inducing Agents Mimicking Beta-Lactam Antibiotics?; It Is Enigmatic Why This Concept Is Consistently Disregarded. Med. Hypotheses 2004, 62, 367–374. [Google Scholar] [CrossRef]
- Latka, A.; Maciejewska, B.; Majkowska-Skrobek, G.; Briers, Y.; Drulis-Kawa, Z. Bacteriophage-Encoded Virion-Associated Enzymes to Overcome the Carbohydrate Barriers during the Infection Process. Appl. Microbiol. Biotechnol. 2017, 101, 3103–3119. [Google Scholar] [CrossRef] [Green Version]
- Moak, M.; Molineux, I.J. Peptidoglycan Hydrolytic Activities Associated with Bacteriophage Virions. Mol. Microbiol. 2004, 51, 1169–1183. [Google Scholar] [CrossRef]
- Murray, E.; Draper, L.A.; Ross, R.P.; Hill, C. The Advantages and Challenges of Using Endolysins in a Clinical Setting. Viruses 2021, 13, 680. [Google Scholar] [CrossRef]
- Schmelcher, M.; Donovan, D.M.; Loessner, M.J. Bacteriophage Endolysins as Novel Antimicrobials. Future Microbiol. 2012, 7, 1147–1171. [Google Scholar] [CrossRef] [Green Version]
- Nelson, D.C.; Schmelcher, M.; Rodriguez-Rubio, L.; Klumpp, J.; Pritchard, D.G.; Dong, S.; Donovan, D.M. Endolysins as Antimicrobials. Adv. Virus Res. 2012, 83, 299–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abedon, S.T. Lysis from without. Bacteriophage 2011, 1, 46–49. [Google Scholar] [CrossRef] [PubMed]
- Roach, D.R.; Donovan, D.M. Antimicrobial Bacteriophage-Derived Proteins and Therapeutic Applications. Bacteriophage 2015, 5, e1062590. [Google Scholar] [CrossRef] [Green Version]
- Loessner, M.; Kramer, K.; Ebel, F.; Scherer, S. C-Terminal Domains of Listeria Monocytogenes Bacteriophage Murein Hydrolases Determine Specific Recognition and High-Affinity Binding to Bacterial Cell Wall Carbohydrates. Mol. Microbiol. 2002, 44, 335–349. [Google Scholar] [CrossRef]
- Kretzer, J.W.; Lehmann, R.; Schmelcher, M.; Banz, M.; Kim, K.P.; Korn, C.; Loessner, M.J. Use of High-Affinity Cell Wall-Binding Domains of Bacteriophage Endolysins for Immobilization and Separation of Bacterial Cells. Appl. Environ. Microbiol. 2007, 73, 1992–2000. [Google Scholar] [CrossRef] [Green Version]
- Eugster, M.R.; Haug, M.C.; Huwiler, S.G.; Loessner, M.J. The Cell Wall Binding Domain of Listeria Bacteriophage Endolysin PlyP35 Recognizes Terminal GlcNAc Residues in Cell Wall Teichoic Acid. Mol. Microbiol. 2011, 81, 1419–1432. [Google Scholar] [CrossRef]
- Loessner, M.J.; Wendlinger, G.; Scherer, S. Heterogeneous Endolysins in Listeria Monocytogenes Bacteriophages: A New Class of Enzymes and Evidence for Conserved Holin Genes within the Siphoviral Lysis Cassettes. Mol. Microbiol. 1995, 16, 1231–1241. [Google Scholar] [CrossRef]
- Nelson, D.; Schuch, R.; Chahales, P.; Zhu, S.; Fischetti, V.A. PlyC: A Multimeric Bacteriophage Lysin. Proc. Natl. Acad. Sci. USA 2006, 103, 10765–10770. [Google Scholar] [CrossRef] [Green Version]
- Gilmer, D.B.; Schmitz, J.E.; Euler, C.W.; Fischetti, V.A. Novel Bacteriophage Lysin with Broad Lytic Activity Protects against Mixed Infection by Streptococcus Pyogenes and Methicillin-Resistant Staphylococcus Aureus. Agents Chemother. 2013, 57, 2743–2750. [Google Scholar] [CrossRef] [Green Version]
- Briers, Y.; Walmagh, M.; Grymonprez, B.; Biebl, M.; Pirnay, J.P.; Defraine, V.; Michiels, J.; Cenens, W.; Aertsen, A.; Miller, S.; et al. Art-175 Is a Highly Efficient Antibacterial against Multidrug-Resistant Strains and Persisters of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2014, 58, 3774–3784. [Google Scholar] [CrossRef] [Green Version]
- Borysowski, J.; Weber-Dabrowska, B.; Górski, A. Bacteriophage Endolysins as a Novel Class of Antibacterial Agents. Exp. Biol. Med. 2006, 231, 366–377. [Google Scholar] [CrossRef] [PubMed]
- Abdelkader, K.; Gerstmans, H.; Saafan, A.; Dishisha, T.; Briers, Y. The Preclinical and Clinical Progress of Bacteriophages and Their Lytic Enzymes: The Parts Are Easier than the Whole. Viruses 2019, 11, 96. [Google Scholar] [CrossRef] [Green Version]
- Schirmeier, E.; Zimmermann, P.; Hofmann, V.; Biebl, M.; Gerstmans, H.; Maervoet, V.E.T.; Briers, Y. Inhibitory and Bactericidal Effect of Artilysin® Art-175 against Colistin-Resistant Mcr-1-Positive Escherichia Coli Isolates. Int. J. Antimicrob. Agents 2018, 51, 528–529. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, Y.; Wang, J.; Zhao, Y.; Zhong, Q.; Li, G.; Fu, Z.; Lu, S. Phage Endolysin LysP108 Showed Promising Antibacterial Potential Against Methicillin-Resistant Staphylococcus Aureus. Front. Cell. Infect. Microbiol. 2021, 11, 668430. [Google Scholar] [CrossRef]
- Silva, M.D.; Oliveira, H.; Faustino, A.; Sillankorva, S. Characterization of MSlys, the Endolysin of Streptococcus Pneumoniae Phage MS1. Biotechnol. Rep. 2020, 28, e00547. [Google Scholar] [CrossRef]
- Yu, J.-H.; Lim, J.-A.; Chang, H.-J.; Park, J.-H. Characteristics and Lytic Activity of Phage-Derived Peptidoglycan Hydrolase, LysSAP8, as a Potent Alternative Biocontrol Agent for Staphylococcus Aureus. J. Microbiol. Biotechnol. 2019, 29, 1916–1924. [Google Scholar] [CrossRef]
- Linden, S.B.; Zhang, H.; Heselpoth, R.D.; Shen, Y.; Schmelcher, M.; Eichenseher, F.; Nelson, D.C. Biochemical and Biophysical Characterization of PlyGRCS, a Bacteriophage Endolysin Active against Methicillin-Resistant Staphylococcus Aureus. Appl. Microbiol. Biotechnol. 2014, 99, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, D.; Ruas-Madiedo, P.; Martínez, B.; Rodríguez, A.; García, P. Effective Removal of Staphylococcal Biofilms by the Endolysin LysH5. PLoS ONE 2014, 9, e107307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Mi, Z.; Yin, X.; Fan, H.; An, X.; Zhang, Z.; Chen, J.; Tong, Y. Characterization of Enterococcus Faecalis Phage IME-EF1 and Its Endolysin. PLoS ONE 2013, 8, e80435. [Google Scholar] [CrossRef]
- Gong, P.; Cheng, M.; Li, X.; Jiang, H.; Yu, C.; Kahaer, N.; Li, J.; Zhang, L.; Xia, F.; Hu, L.; et al. Characterization of Enterococcus Faecium Bacteriophage IME-EFm5 and Its Endolysin LysEFm5. Virology 2016, 492, 11–20. [Google Scholar] [CrossRef]
- Son, B.; Yun, J.; Lim, J.A.; Shin, H.; Heu, S.; Ryu, S. Characterization of LysB4, an Endolysin from the Bacillus Cereus-Infecting Bacteriophage B4. BMC Microbiol. 2012, 12, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Dunne, M.; Mertens, H.D.T.; Garefalaki, V.; Jeffries, C.M.; Thompson, A.; Lemke, E.A.; Svergun, D.I.; Mayer, M.J.; Narbad, A.; Meijers, R. The CD27L and CTP1L Endolysins Targeting Clostridia Contain a Built-in Trigger and Release Factor. PLoS Pathog. 2014, 10, e1004228. [Google Scholar] [CrossRef] [Green Version]
- Ghose, C.; Euler, C.W. Gram-Negative Bacterial Lysins. Antibiotics 2020, 9, 74. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez, D.; Briers, Y. Lysins Breaking down the Walls of Gram-Negative Bacteria, No Longer a No-Go. Curr. Opin. Biotechnol. 2021, 68, 15–22. [Google Scholar] [CrossRef]
- Briers, Y.; Walmagh, M.; Lavigne, R. Use of Bacteriophage Endolysin EL188 and Outer Membrane Permeabilizers against Pseudomonas aeruginosa. J. Appl. Microbiol. 2011, 110, 778–785. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Yu, J.; Wei, H. Engineered Bacteriophage Lysins as Novel Anti-Infectives. Front. Microbiol. 2014, 5, 542. [Google Scholar] [CrossRef]
- Kongari, R.; Rajaure, M.; Cahill, J.; Rasche, E.; Mijalis, E.; Berry, J.; Young, R. Phage Spanins: Diversity, Topological Dynamics and Gene Convergence. BMC Bioinform. 2018, 19, 1–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerstmans, H.; Rodríguez-Rubio, L.; Lavigne, R.; Briers, Y. From Endolysins to Artilysin®s: Novel Enzyme-Based Approaches to Kill Drug-Resistant Bacteria. Biochem. Soc. Trans. 2016, 44, 123–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Briers, Y.; Lavigne, R. Breaking Barriers: Expansion of the Use of Endolysins as Novel Antibacterials against Gram-Negative Bacteria. Future Microbiol. 2015, 10, 377–390. [Google Scholar] [CrossRef]
- Poonacha, N.; Nair, S.; Desai, S.; Tuppad, D.; Hiremath, D.; Mohan, T.; Vipra, A.; Sharma, U. Efficient Killing of Planktonic and Biofilm-Embedded Coagulase-Negative Staphylococci by Bactericidal Protein P128. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef] [Green Version]
- Schuch, R.; Nelson, D.; Fischetti, V.A. A Bacteriolytic Agent That Detects and Kills Bacillus Anthracis. Nature 2002, 418, 884–889. [Google Scholar] [CrossRef]
- Pastagia, M.; Euler, C.; Chahales, P.; Fuentes-Duculan, J.; Krueger, J.G.; Fischetti, V.A. A Novel Chimeric Lysin Shows Superiority to Mupirocin for Skin Decolonization of Methicillin-Resistant and -Sensitive Staphylococcus Aureus Strains. Antimicrob. Agents Chemother. 2011, 55, 738–744. [Google Scholar] [CrossRef] [Green Version]
- Loeffler, J.M.; Nelson, D.; Fischetti, V.A. Rapid Killing of Streptococcus Pneumoniae with a Bacteriophage Cell Wall Hydrolase. Science 2001, 294, 2170–2172. [Google Scholar] [CrossRef]
- Davis, K.M.; Weiser, J.N. Modifications to the Peptidoglycan Backbone Help Bacteria to Establish Infection. Infect. Immun. 2011, 79, 562–570. [Google Scholar] [CrossRef] [Green Version]
- Kwan, B.W.; Valenta, J.A.; Benedik, M.J.; Wood, T.K. Arrested Protein Synthesis Increases Persister-like Cell Formation. Antimicrob. Agents Chemother. 2013, 57, 1468–1473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paul, V.; Rajagopalan, S.; Sundarrajan, S.; George, S.E.; Asrani, J.Y.; Pillai, R.; Chikkamadaiah, R.; Durgaiah, M.; Sriram, B.; Padmanabhan, S. A Novel Bacteriophage Tail-Associated Muralytic Enzyme (TAME) from Phage K and Its Development into a Potent Antistaphylococcal Protein. BMC Microbiol. 2011, 11, 226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Briers, Y.; Walmagh, M.; Van Puyenbroeck, V.; Cornelissen, A.; Cenens, W.; Aertsen, A.; Oliveira, H.; Azeredo, J.; Verween, G.; Pirnay, J.P.; et al. Engineered Endolysin-Based “Artilysins” to Combat Multidrug-Resistant Gram-Negative Pathogens. mBio 2014, 5, e01379-14. [Google Scholar] [CrossRef] [Green Version]
- Schuch, R.; Lee, H.M.; Schneider, B.C.; Sauve, K.L.; Law, C.; Khan, B.K.; Rotolo, J.A.; Horiuchi, Y.; Couto, D.E.; Raz, A.; et al. Combination Therapy with Lysin CF-301 and Antibiotic Is Superior to Antibiotic Alone for Treating Methicillin-Resistant Staphylococcus Aureus-Induced Murine Bacteremia. J. Infect. Dis. 2013, 209, 1469–1478. [Google Scholar] [CrossRef]
- Schuch, R.; Khan, B.K.; Raz, A.; Rotolo, J.A.; Wittekind, M. Bacteriophage Lysin CF-301, a Potent Antistaphylococcal Biofilm Agent. Antimicrob. Agents Chemother. 2017, 61, e02666-16. [Google Scholar] [CrossRef] [Green Version]
- Melo, L.D.R.; Brandão, A.; Akturk, E.; Santos, S.B.; Azeredo, J. Characterization of a New Staphylococcus Aureus Kayvirus Harboring a Lysin Active against Biofilms. Viruses 2018, 10, 182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischetti, V.A. Bacteriophage Endolysins: A Novel Anti-Infective to Control Gram-Positive Pathogens. Int. J. Med. Microbiol. 2010, 300, 357–362. [Google Scholar] [CrossRef] [Green Version]
- Kaplan, J.B. Antibiotic-Induced Biofilm Formation. Int. J. Artif. Organs 2011, 34, 737–751. [Google Scholar] [CrossRef]
- Son, J.S.; Lee, S.J.; Jun, S.Y.; Yoon, S.J.; Kang, S.H.; Paik, H.R.; Kang, J.O.; Choi, Y.J. Antibacterial and Biofilm Removal Activity of a Podoviridae Staphylococcus Aureus Bacteriophage SAP-2 and a Derived Recombinant Cell-Wall-Degrading Enzyme. Appl. Microbiol. Biotechnol. 2010, 86, 1439–1449. [Google Scholar] [CrossRef]
- Sharma, U.; Vipra, A.; Channabasappa, S. Phage-Derived Lysins as Potential Agents for Eradicating Biofilms and Persisters. Drug Discov. Today 2018, 23, 848–856. [Google Scholar] [CrossRef]
- Letrado, P.; Corsini, B.; DÍez-Martínez, R.; Bustamante, N.; Yuste, J.E.; García, P. Bactericidal Synergism between Antibiotics and Phage Endolysin Cpl-711 to Kill Multidrug-Resistant Pneumococcus. Future Microbiol. 2018, 13, 1215–1223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daniel, A.; Euler, C.; Collin, M.; Chahales, P.; Gorelick, K.J.; Fischetti, V.A. Synergism between a Novel Chimeric Lysin and Oxacillin Protects against Infection by Methicillin-Resistant Staphylococcus Aureus. Antimicrob. Agents Chemother. 2010, 54, 1603–1612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Errington, J. L-Form Bacteria, Cell Walls and the Origins of Life. Open Biol. 2013, 3, 120143. [Google Scholar] [CrossRef] [Green Version]
- Leaver, M.; Domínguez-Cuevas, P.; Coxhead, J.M.; Daniel, R.A.; Errington, J. Life without a Wall or Division Machine in Bacillus Subtilis. Nature 2009, 457, 849–853. [Google Scholar] [CrossRef] [PubMed]
- Dell’Era, S.; Buchrieser, C.; Couvé, E.; Schnell, B.; Briers, Y.; Schuppler, M.; Loessner, M.J. Listeria Monocytogenes L-Forms Respond to Cell Wall Deficiency by Modifying Gene Expression and the Mode of Division. Mol. Microbiol. 2009, 73, 306–322. [Google Scholar] [CrossRef]
- Wohlfarth, J.C.; Feldmüller, M.; Schneller, A.; Kilcher, S.; Burkolter, M.; Meile, S.; Pilhofer, M.; Schuppler, M.; Loessner, M.J. L-Form Conversion in Gram-Positive Bacteria Enables Escape from Phage Infection. Nat. Microbiol. 2023, 8, 387–399. [Google Scholar] [CrossRef]
- Errington, J.; Mickiewicz, K.; Kawai, Y.; Wu, L.J. L-Form Bacteria, Chronic Diseases and the Origins of Life. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 1707. [Google Scholar] [CrossRef] [Green Version]
- Ongenae, V.; Mabrouk, A.S.; Crooijmans, M.; Rozen, D.; Briegel, A.; Claessen, D. Reversible Bacteriophage Resistance by Shedding the Bacterial Cell Wall. Open Biol. 2022, 12, 210379. [Google Scholar] [CrossRef]
- Petrovic Fabijan, A.; Martinez-Martin, D.; Venturini, C.; Mickiewicz, K.; Flores-Rodriguez, N.; Errington, J.; Iredell, J. L-Form Switching in Escherichia Coli as a Common β-Lactam Resistance Mechanism. Microbiol. Spectr. 2022, 10, e0241922. [Google Scholar] [CrossRef]
- Zou, J.; Kou, S.H.; Xie, R.; VanNieuwenhze, M.S.; Qu, J.; Peng, B.; Zheng, J. Non-Walled Spherical Acinetobacter Baumannii Is an Important Type of Persister upon β-Lactam Antibiotic Treatment. Emerg. Microbes Infect. 2020, 9, 1149–1159. [Google Scholar] [CrossRef]
- Schito, G.C. The Importance of the Development of Antibiotic Resistance in Staphylococcus Aureus. Clin. Microbiol. Infect. 2006, 12, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mickiewicz, K.M.; Kawai, Y.; Drage, L.; Gomes, M.C.; Davison, F.; Pickard, R.; Hall, J.; Mostowy, S.; Aldridge, P.D.; Errington, J. Possible Role of L-Form Switching in Recurrent Urinary Tract Infection. Nat. Commun. 2019, 10, 4379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dörr, T.; Davis, B.M.; Waldor, M.K. Endopeptidase-Mediated Beta Lactam Tolerance. PLoS Pathog. 2015, 11, e1004850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cross, T.; Ransegnola, B.; Shin, J.H.; Weaver, A.; Fauntleroy, K.; VanNieuwenhze, M.S.; Westblade, L.F.; Dörra, T. Spheroplast-Mediated Carbapenem Tolerance in Gram-Negative Pathogens. Antimicrob. Agents Chemother. 2019, 63, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monahan, L.G.; Turnbull, L.; Osvath, S.R.; Birch, D.; Charles, I.G.; Whitchurch, C.B. Rapid Conversion of Pseudomonas Aeruginosa to a Spherical Cell Morphotype Facilitates Tolerance to Carbapenems and Penicillins but Increases Susceptibility to Antimicrobial Peptides. Antimicrob. Agents Chemother. 2014, 58, 1956–1962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domingue, G.J.; Woody, H.B. Bacterial Persistence and Expression of Disease. Clin. Microbiol. Rev. 1997, 10, 320–344. [Google Scholar] [CrossRef] [PubMed]
- Grosboillot, V.; Keller, I.; Ernst, C.; Loessner, M.J.; Schuppler, M. Ampicillin Treatment of Intracellular Listeria Monocytogenes Triggers Formation of Persistent, Drug-Resistant L-Form Cells. Front. Cell. Infect. Microbiol. 2022, 12, 869339. [Google Scholar] [CrossRef]
- Sun, Y.; Sun, T.L.; Huang, H.W. Mode of Action of Antimicrobial Peptides on E. Coli Spheroplasts. Biophys. J. 2016, 111, 132–139. [Google Scholar] [CrossRef] [Green Version]
- Rakhuba, D.V.; Kolomiets, E.I.; Dey, E.; Novik, G. Bacteriophage Receptors, Mechanisms of Phage Adsorption and Penetration into Host Cell. Pol. J. Microbiol. 2010, 59, 145–155. [Google Scholar] [CrossRef]
- Fernandes, S.; São-José, C. Enzymes and Mechanisms Employed by Tailed Bacteriophages to Breach the Bacterial Cell Barriers. Viruses 2018, 10, 396. [Google Scholar] [CrossRef] [Green Version]
- Azeredo, J.; García, P.; Drulis-Kawa, Z. Targeting Biofilms Using Phages and Their Enzymes. Curr. Opin. Biotechnol. 2021, 68, 251–261. [Google Scholar] [CrossRef]
- Pires, D.P.; Oliveira, H.; Melo, L.D.R.; Sillankorva, S.; Azeredo, J. Bacteriophage-Encoded Depolymerases: Their Diversity and Biotechnological Applications. Appl. Microbiol. Biotechnol. 2016, 100, 2141–2151. [Google Scholar] [CrossRef] [Green Version]
- Chan, B.K.; Abedon, S.T. Bacteriophages and Their Enzymes in Biofilm Control. Curr. Pharm. Des. 2015, 21, 85–99. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Paff, M.L.; Molineux, I.J.; Bull, J.J. Therapeutic Application of Phage Capsule Depolymerases against K1, K5, and K30 Capsulated E. Coli in Mice. Front. Microbiol. 2017, 8, 2257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yian Wong, T.; Preston, L.A.; Schiller, N.L. ALGINATE LYASE: Review of Major Sources and Enzyme Characteristics, Structure-Function Analysis, Biological Roles, and Applications. Annu. Rev. Microbiol. 2000, 54, 289–340. [Google Scholar] [CrossRef] [PubMed]
- McCallum, K.L.; Laakso, D.H.; Field^, C.W.; Mccallum, K.L. Use of a Bacteriophage-Encoded Glycanase Enzyme in the Generation of Lipopolysaccharide 0 Side Chain Deficient Mutants of Escherichia Coli 09:K30 and Klebsiella 01:K20: Role of 0 and K Antigens in Resistance to Complement-Mediated Serum Killing. Can. J. Microbiol. 1989, 35, 994–999. [Google Scholar] [CrossRef] [PubMed]
- Volozhantsev, N.V.; Shpirt, A.M.; Borzilov, A.I.; Komisarova, E.V.; Krasilnikova, V.M.; Shashkov, A.S.; Verevkin, V.V.; Knirel, Y.A. Characterization and Therapeutic Potential of Bacteriophage-Encoded Polysaccharide Depolymerases with β Galactosidase Activity against Klebsiella Pneumoniae K57 Capsular Type. Antibiotics 2020, 9, 732. [Google Scholar] [CrossRef]
- Bendary, M.M.; Ibrahim, D.; Mosbah, R.A.; Mosallam, F.; Hegazy, W.A.H.; Awad, N.F.S.; Alshareef, W.A.; Alomar, S.Y.; Zaitone, S.A.; Abd El-Hamid, M.I. Thymol Nanoemulsion: A New Therapeutic Option for Extensively Drug Resistant Foodborne Pathogens. Antibiotics 2021, 10, 25. [Google Scholar] [CrossRef]
- Gutiérrez, D.; Briers, Y.; Rodríguez-Rubio, L.; Martínez, B.; Rodríguez, A.; Lavigne, R.; García, P. Role of the Pre-Neck Appendage Protein (Dpo7) from Phage VB_SepiS-PhiIPLA7 as an Anti-Biofilm Agent in Staphylococcal Species. Front. Microbiol. 2015, 6, 1315. [Google Scholar] [CrossRef] [Green Version]
- Guo, Z.; Huang, J.; Yan, G.; Lei, L.; Wang, S.; Yu, L.; Zhou, L.; Gao, A.; Feng, X.; Han, W.; et al. Identification and Characterization of Dpo42, a Novel Depolymerase Derived from the Escherichia Coli Phage VB_EcoM_ECOO78. Front. Microbiol. 2017, 8, 1460. [Google Scholar] [CrossRef] [Green Version]
- Hernandez-Morales, A.C.; Lessor, L.L.; Wood, T.L.; Migl, D.; Mijalis, E.M.; Cahill, J.; Russell, W.K.; Young, R.F.; Gill, J.J. Genomic and Biochemical Characterization of Acinetobacter Podophage Petty Reveals a Novel Lysis Mechanism and Tail-Associated Depolymerase Activity. J. Virol. 2018, 92, e01064–e01117. [Google Scholar] [CrossRef] [Green Version]
- Mi, L.; Liu, Y.; Wang, C.; He, T.; Gao, S.; Xing, S.; Huang, Y.; Fan, H.; Zhang, X.; Yu, W.; et al. Identification of a Lytic Pseudomonas Aeruginosa Phage Depolymerase and Its Anti-Biofilm Effect and Bactericidal Contribution to Serum. Virus Genes 2019, 55, 394–405. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, R.; Xu, M.; Liu, Y.; Zhu, X.; Qiu, J.; Liu, Q.; He, P.; Li, Q. A Novel Polysaccharide Depolymerase Encoded by the Phage SH-KP152226 Confers Specific Activity Against Multidrug-Resistant Klebsiella Pneumoniae via Biofilm Degradation. Front. Microbiol. 2019, 10, 2768. [Google Scholar] [CrossRef] [Green Version]
- Chhibber, S.; Bansal, S.; Kaur, S. Disrupting the Mixed-Species Biofilm of Klebsiella Pneumoniae B5055 and Pseudomonas Aeruginosa PAO Using Bacteriophages Alone or in Combination with Xylitol. Microbiology 2015, 161, 1369–1377. [Google Scholar] [CrossRef]
- Oliveira, A.; Ribeiro, H.G.; Silva, A.C.; Silva, M.D.; Sousa, J.C.; Rodrigues, C.F.; Melo, L.D.R.; Henriques, A.F.; Sillankorva, S. Synergistic Antimicrobial Interaction between Honey and Phage against Escherichia Coli Biofilms. Front. Microbiol. 2017, 8, 2407. [Google Scholar] [CrossRef] [Green Version]
- Chai, Z.; Wang, J.; Tao, S.; Mou, H. Application of Bacteriophage-Borne Enzyme Combined with Chlorine Dioxide on Controlling Bacterial Biofilm. LWT 2014, 59, 1159–1165. [Google Scholar] [CrossRef]
- Tait, K.; Skillman, L.C.; Sutherland, I.W. The Efficacy of Bacteriophage as a Method of Biofilm Eradication. Biofouling 2002, 18, 305–311. [Google Scholar] [CrossRef] [Green Version]
- Latka, A.; Drulis-Kawa, Z. Advantages and Limitations of Microtiter Biofilm Assays in the Model of Antibiofilm Activity of Klebsiella Phage KP34 and Its Depolymerase. Sci. Rep. 2020, 10, 20338. [Google Scholar] [CrossRef] [PubMed]
- Pertics, B.Z.; Cox, A.; Nyúl, A.; Szamek, N.; Kovács, T.; Schneider, G. Isolation and Characterization of a Novel Lytic Bacteriophage against the K2 Capsule-Expressing Hypervirulent Klebsiella Pneumoniae Strain 52145, and Identification of Its Functional Depolymerase. Microorganisms 2021, 9, 650. [Google Scholar] [CrossRef] [PubMed]
- Pertics, B.Z.; Kovács, T.; Schneider, G. Characterization of a Lytic Bacteriophage and Demonstration of Its Combined Lytic Effect with a K2 Depolymerase on the Hypervirulent Klebsiella Pneumoniae Strain 52145. Microorganisms 2023, 11, 669. [Google Scholar] [CrossRef] [PubMed]
- Olsen, N.M.C.; Thiran, E.; Hasler, T.; Vanzieleghem, T.; Belibasakis, G.N.; Mahillon, J.; Loessner, M.J.; Schmelcher, M. Synergistic Removal of Static and Dynamic Staphylococcus Aureus Biofilms by Combined Treatment with a Bacteriophage Endolysin and a Polysaccharide Depolymerase. Viruses 2018, 10, 438. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Li, X.; Wang, S.; Guan, L.; Li, X.; Hu, D.; Gao, D.; Song, J.; Chen, H.; Qiana, P. A Novel Tail-Associated O91-Specific Polysaccharide Depolymerase from a Podophage Reveals Lytic Efficacy of Shiga Toxin-Producing Escherichia Coli. Appl. Environ. Microbiol. 2020, 86, 9. [Google Scholar] [CrossRef]
- Jado, I.; López, R.; García, E.; Fenoll, A.; Casal, J.; García, P.; Pallares, R.; de la Campa, A.G.; Bouza, E.; Baquero, F.; et al. Phage Lytic Enzymes as Therapy for Antibiotic-Resistant Streptococcus Pneumoniae Infection in a Murine Sepsis Model. J. Antimicrob. Chemother. 2003, 52, 967–973. [Google Scholar] [CrossRef] [Green Version]
- Huan, Y.; Kong, Q.; Mou, H.; Yi, H. Antimicrobial Peptides: Classification, Design, Application and Research Progress in Multiple Fields. Front. Microbiol. 2020, 11, 582779. [Google Scholar] [CrossRef]
- Cattoir, V.; Felden, B. Future Antibacterial Strategies: From Basic Concepts to Clinical Challenges. J. Infect. Dis. 2019, 220, 350–360. [Google Scholar] [CrossRef]
- Kumar, P.; Kizhakkedathu, J.N.; Straus, S.K. Antimicrobial Peptides: Diversity, Mechanism of Action and Strategies to Improve the Activity and Biocompatibility in Vivo. Biomolecules 2018, 8, 4. [Google Scholar] [CrossRef] [Green Version]
- Di Somma, A.; Moretta, A.; Canè, C.; Cirillo, A.; Duilio, A. Antimicrobial and Antibiofilm Peptides. Biomolecules 2020, 10, 652. [Google Scholar] [CrossRef] [Green Version]
- Zhou, M.; Zheng, M.; Cai, J. Small Molecules with Membrane-Active Antibacterial Activity. ACS Appl. Mater. Interfaces 2020, 12, 21292–21299. [Google Scholar] [CrossRef]
- Boparai, J.K.; Sharma, P.K. Mini Review on Antimicrobial Peptides, Sources, Mechanism and Recent Applications. Protein Pept. Lett. 2020, 27, 4–16. [Google Scholar] [CrossRef]
- Bechinger, B.; Gorr, S.U. Antimicrobial Peptides: Mechanisms of Action and Resistance. J. Dent. Res. 2017, 96, 254–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenberger, C.M.; Gallo, R.L.; Finlay, B.B. Interplay between Antibacterial Effectors: A Macrophage Antimicrobial Peptide Impairs Intracellular Salmonella Replication. Proc. Natl. Acad. Sci. USA 2004, 101, 2422–2427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gottschalk, S.; Ifrah, D.; Lerche, S.; Gottlieb, C.T.; Cohn, M.T.; Hiasa, H.; Hansen, P.R.; Gram, L.; Ingmer, H.; Thomsen, L.E. The Antimicrobial Lysine-Peptoid Hybrid LP5 Inhibits DNA Replication and Induces the SOS Response in Staphylococcus Aureus. BMC Microbiol. 2013, 13, 192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gottschalk, S.; Gottlieb, C.T.; Vestergaard, M.; Hansen, P.R.; Gram, L.; Ingmer, H.; Thomsen, L.E. Amphibian Antimicrobial Peptide Fallaxin Analogue FL9 Affects Virulence Gene Expression and DNA Replication in Staphylococcus Aureus. J. Med. Microbiol. 2015, 64, 1504–1513. [Google Scholar] [CrossRef] [Green Version]
- Mardirossian, M.; Pérébaskine, N.; Benincasa, M.; Gambato, S.; Hofmann, S.; Huter, P.; Müller, C.; Hilpert, K.; Innis, C.A.; Tossi, A.; et al. The Dolphin Proline-Rich Antimicrobial Peptide Tur1A Inhibits Protein Synthesis by Targeting the Bacterial Ribosome. Cell Chem. Biol. 2018, 25, 530–539.e7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graf, M.; Wilson, D.N. Intracellular Antimicrobial Peptides Targeting the Protein Synthesis Machinery. Adv. Exp. Med. Biol. 2019, 1117, 73–89. [Google Scholar] [CrossRef] [PubMed]
- Park, C.B.; Kim, H.S.; Kim, S.C. Mechanism of Action of the Antimicrobial Peptide Buforin II: Buforin II Kills Microorganisms by Penetrating the Cell Membrane and Inhibiting Cellular Functions. Biochem. Biophys. Res. Commun. 1998, 244, 253–257. [Google Scholar] [CrossRef] [Green Version]
- Hancock, R.E.W.; Haney, E.F.; Gill, E.E. The Immunology of Host Defence Peptides: Beyond Antimicrobial Activity. Nat. Rev. Immunol. 2016, 16, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Maloy, W.L.; Kari, U.P. Structure-Activity Studies on Defense Peptides Magainins and Other Host Defense Peptides. Biopolymers 1995, 37, 105–122. [Google Scholar] [CrossRef]
- Pereira, A.M.; da Costa, A.; Dias, S.C.; Casal, M.; Machado, R. Production and Purification of Two Bioactive Antimicrobial Peptides Using a Two-Step Approach Involving an Elastin-like Fusion Tag. Pharmaceuticals 2021, 14, 956. [Google Scholar] [CrossRef]
- Petri, G.L.; Di Martino, S.; De Rosa, M. Peptidomimetics: An Overview of Recent Medicinal Chemistry Efforts toward the Discovery of Novel Small Molecule Inhibitors. J. Med. Chem. 2022, 65, 7438–7475. [Google Scholar] [CrossRef]
- Sørensen, O.E.; Follin, P.; Johnsen, A.H.; Calafat, J.; Sandra Tjabringa, G.; Hiemstra, P.S.; Borregaard, N. Human Cathelicidin, HCAP-18, Is Processed to the Antimicrobial Peptide LL-37 by Extracellular Cleavage with Proteinase 3. Blood 2001, 97, 3951–3959. [Google Scholar] [CrossRef] [Green Version]
- Semple, F.; Dorin, J.R. β-Defensins: Multifunctional Modulators of Infection, Inflammation and More? J. Innate Immun. 2012, 4, 337–348. [Google Scholar] [CrossRef]
- Matos De Opitz, C.L.; Sass, P. Tackling Antimicrobial Resistance by Exploring New Mechanisms of Antibiotic Action. Future Microbiol. 2020, 15, 703–708. [Google Scholar] [CrossRef]
- Cavera, V.L.; Arthur, T.D.; Kashtanov, D.; Chikindas, M.L. Bacteriocins and Their Position in the next Wave of Conventional Antibiotics. Int. J. Antimicrob. Agents 2015, 46, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Brul, S.; Zaat, S.A.J. Bacterial Persister-Cells and Spores in the Food Chain: Their Potential Inactivation by Antimicrobial Peptides (AMPs). Int. J. Mol. Sci. 2020, 21, 8967. [Google Scholar] [CrossRef] [PubMed]
- Gruenheid, S.; Le Moual, H. Resistance to Antimicrobial Peptides in Gram-Negative Bacteria. FEMS Microbiol. Lett. 2012, 330, 81–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidtchen, A.; Frick, I.M.; Andersson, E.; Tapper, H.; Björck, L. Proteinases of Common Pathogenic Bacteria Degrade and Inactivate the Antibacterial Peptide LL-37. Mol. Microbiol. 2002, 46, 157–168. [Google Scholar] [CrossRef] [Green Version]
- Assoni, L.; Milani, B.; Carvalho, M.R.; Nepomuceno, L.N.; Waz, N.T.; Guerra, M.E.S.; Converso, T.R.; Darrieux, M. Resistance Mechanisms to Antimicrobial Peptides in Gram-Positive Bacteria. Front. Microbiol. 2020, 11, 593215. [Google Scholar] [CrossRef]
- Shprung, T.; Wani, N.A.; Wilmes, M.; Mangoni, M.L.; Bitler, A.; Shimoni, E.; Sahl, H.G.; Shai, Y. Opposing Effects of PhoPQ and PmrAB on the Properties of Salmonella Enterica Serovar Typhimurium: Implications on Resistance to Antimicrobial Peptides. Biochemistry 2021, 60, 2943–2955. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, M.; Zhou, C.; Kallenbach, N.R.; Ren, D. Control of Bacterial Persister Cells by Trp/Arg-Containing Antimicrobial Peptides. Appl. Environ. Microbiol. 2011, 77, 4878–4885. [Google Scholar] [CrossRef] [Green Version]
- De Breij, A.; Riool, M.; Cordfunke, R.A.; Malanovic, N.; De Boer, L.; Koning, R.I.; Ravensbergen, E.; Franken, M.; Van Der Heijde, T.; Boekema, B.K.; et al. The Antimicrobial Peptide SAAP-148 Combats Drug-Resistant Bacteria and Biofilms. Sci. Transl. Med. 2018, 10, 423. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Brul, S.; Zaat, S.A.J. Isolation of Persister Cells of Bacillus Subtilis and Determination of Their Susceptibility to Antimicrobial Peptides. Int. J. Mol. Sci. 2021, 22, 10059. [Google Scholar] [CrossRef]
- Omardien, S.; Drijfhout, J.W.; Zaat, S.A.; Brul, S. Cationic Amphipathic Antimicrobial Peptides Perturb the Inner Membrane of Germinated Spores Thus Inhibiting Their Outgrowth. Front. Microbiol. 2018, 9, 2277. [Google Scholar] [CrossRef] [Green Version]
- Hou, S.; Liu, Z.; Young, A.W.; Mark, S.L.; Kallenbach, N.R.; Ren, D. Effects of Trp- and Arg-Containing Antimicrobial-Peptide Structure on Inhibition of Escherichia Coli Planktonic Growth and Biofilm Formation. Appl. Environ. Microbiol. 2010, 76, 1967–1974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duong, L.; Gross, S.P.; Siryaporn, A. Developing Antimicrobial Synergy with AMPs. Front. Med Technol. 2021, 3, 640981. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, R.; Esmaeili Gouvarchin Ghaleh, H.; Ranjbar, R. Antibiofilm Effect of Melittin Alone and in Combination with Conventional Antibiotics toward Strong Biofilm of MDR-MRSA and -Pseudomonas Aeruginosa. Front. Microbiol. 2023, 14, 1030401. [Google Scholar] [CrossRef] [PubMed]
- Rishi, P.; Bhagat, N.R.; Thakur, R.; Pathania, P. Tackling Salmonella Persister Cells by Antibiotic–Nisin Combination via Mannitol. Indian J. Microbiol. 2018, 58, 239–243. [Google Scholar] [CrossRef] [PubMed]
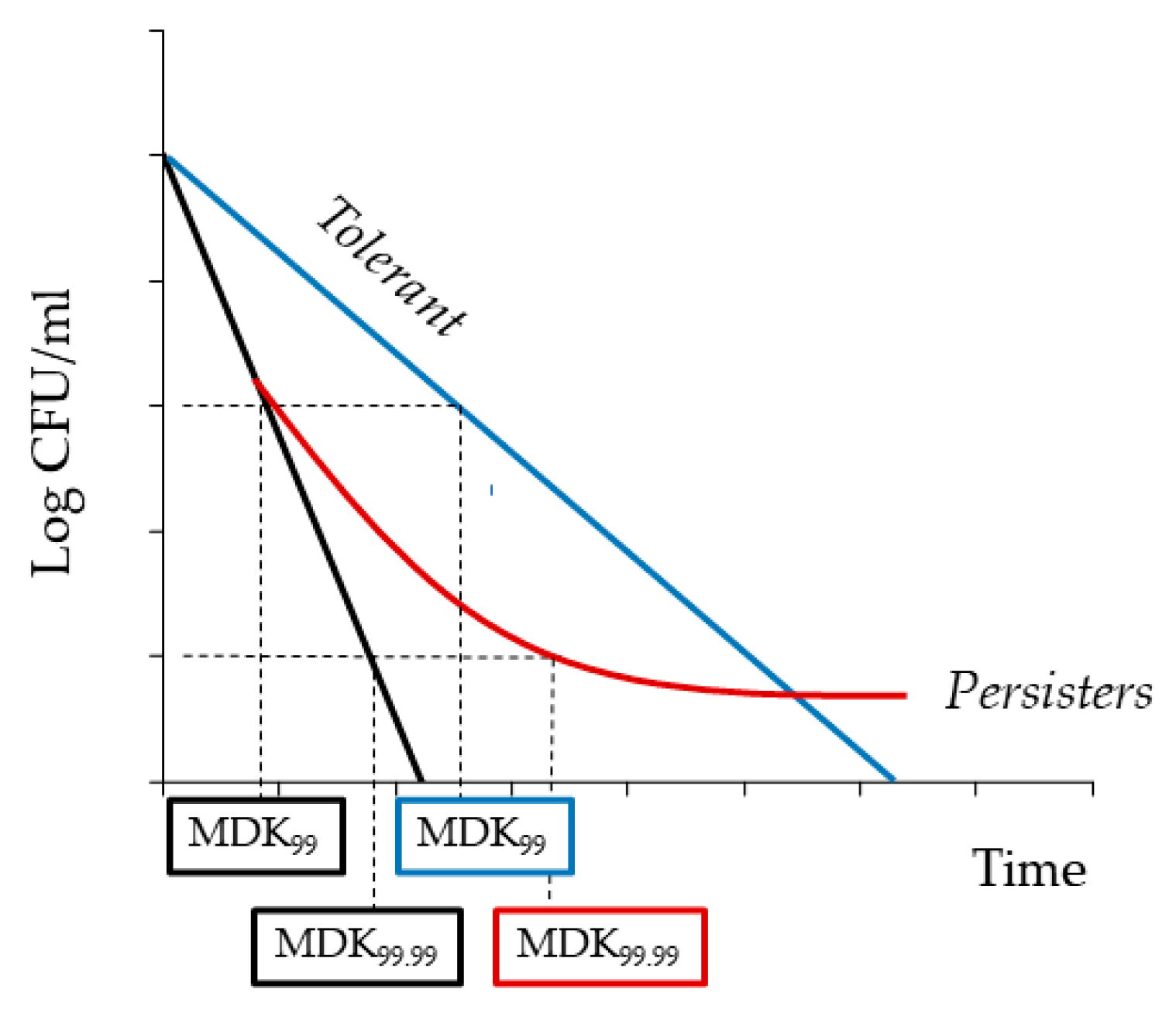
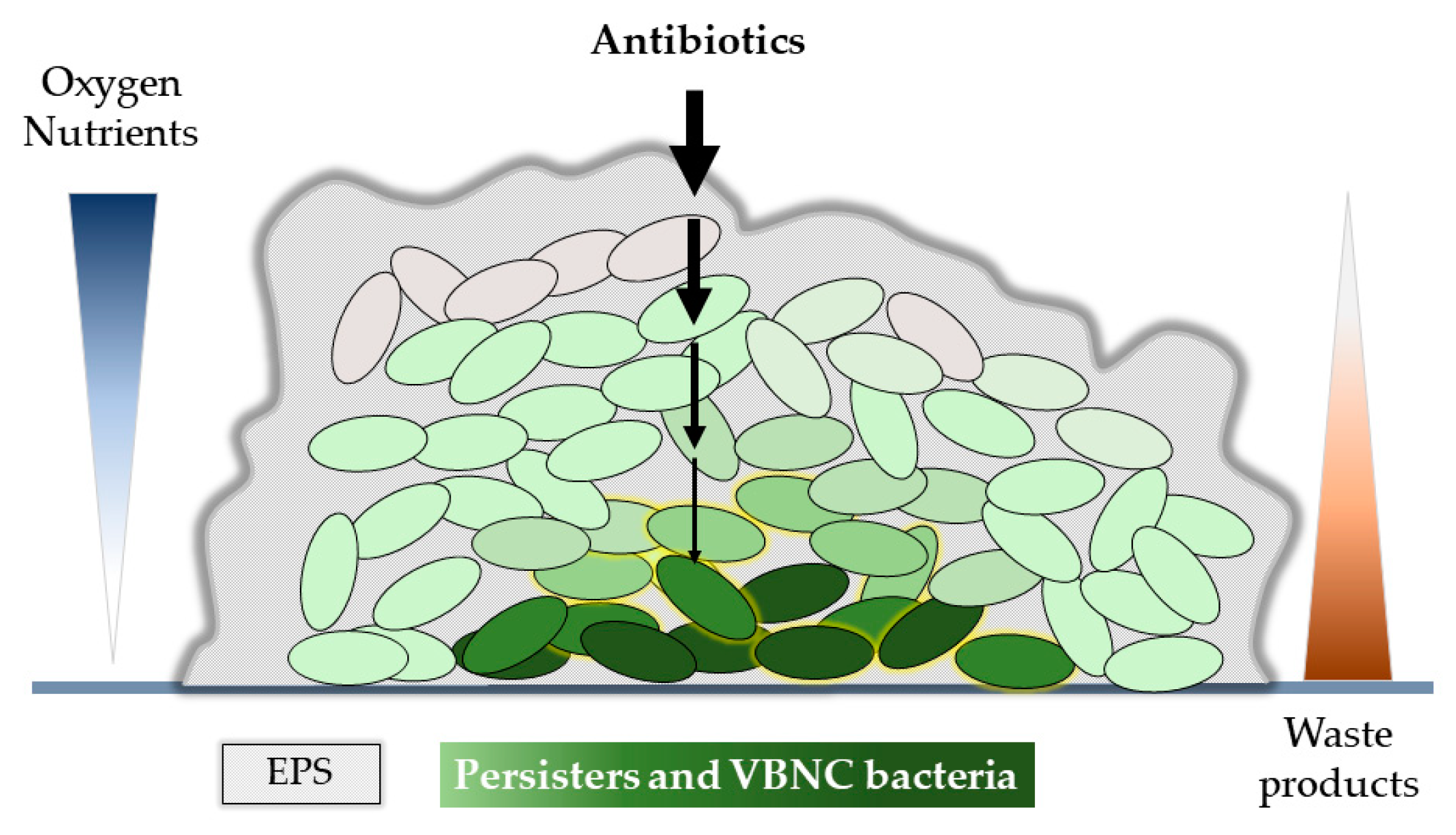
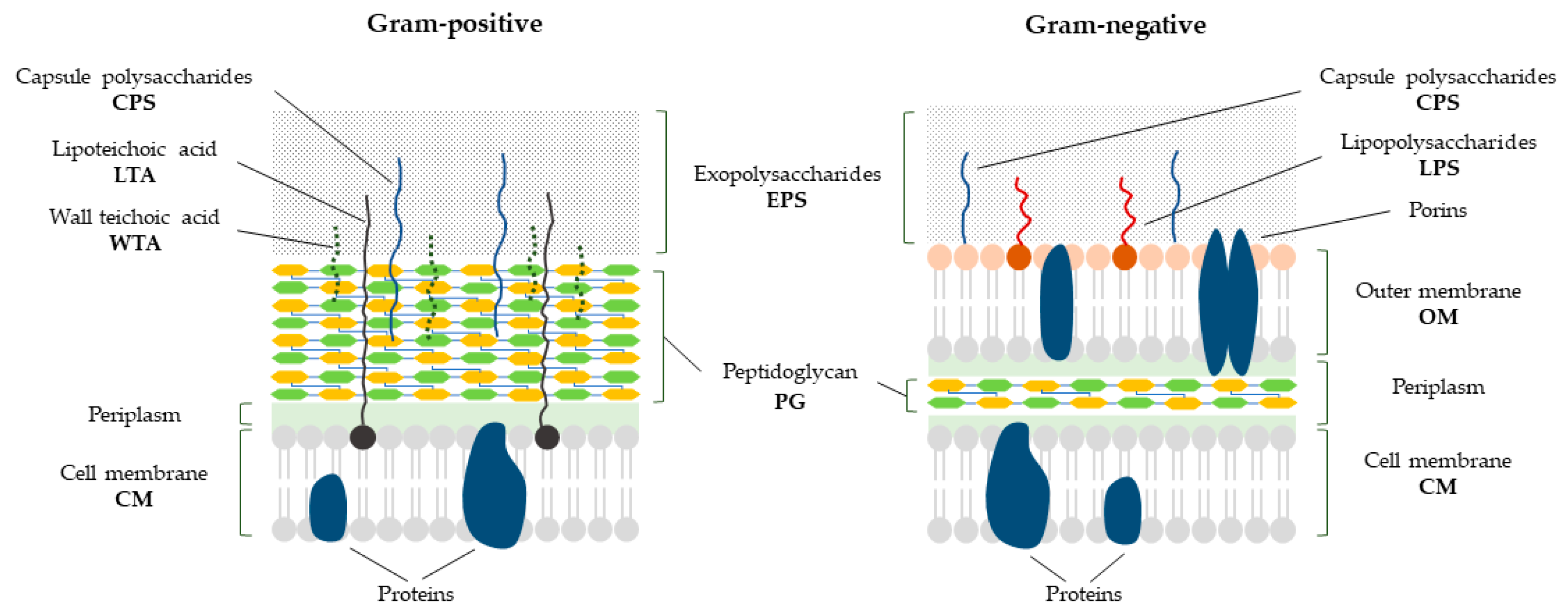

| Name and Origin | Target | Planktonic Cells | Biofilm | Persister Cells | Ref. |
|---|---|---|---|---|---|
| LysH5 native endolysin from S. aureus phage vB_SauS-phiIPLA88. | S. aureus S. epidermidis | MIC (strain dependent) 0.05–0.1 µM. Killing assay: 10× MIC (0.5 µM) of LysH5/for 3 h/37 °C. No viable cells detected. Re-growth: Minimal re-growth was detected under conditions: 10× MIC (0.5 µM) of LysH5/for 24 h/37 °C. Cells remained sensitive to LysH5 and were effectively killed with second dose of LysH5 10× MIC (0.5 µM) of LysH5/for 3 h/37 °C. Sub-MIC concentration: 0.25× MIC or 0.5× MIC. Bacterial growth was not inhibited. No induction of persister cells. | Disrupting assay: 24 h-old biofilm, 3× MIC (0.15 µM) of LysH5/for 6 h/37 °C. Destruction of a matrix structure. No viable cells detected. Inhibition in biofilm formation: Formation of biofilm is fully inhibited Even in the presence of sub-MIC concentrations of LysH5 (0.1 µM) Sub-MIC concentration: 0.25× MIC or 0.5× MIC Bacterial growth was not inhibited No induction of biofilm formation | Starting population: 108 CFU. Persister cells isolation: treatment with 100× MIC of rifampicin (2 µg/mL) or 10× MIC of ciprofloxacin (3 µg/mL) for 4 h/37 °C. Persister population: 103CFU. Killing assay: 10× MIC (0.5 µM) of LysH5/for 3 h/37 °C. No viable cells detected. | [105] |
| P128 chimeric ectolysin created by the fusion of Lys16 ectolysin from Staphylococcus phage K, and SH3b lysostaphin CBD from S. simultans [123]. | MRSA, MSSA and CoNS strains of S. epidermidis S. haemolyticus S. lugdunensis | MIC (strain dependent) 0.017–4.64 µM. Killing assay: 1× MIC of P128/for 1 h/37 °C, 2- to 3-log CFU reduction. 1× MIC of P128/for 2–4 h/37 °C, >4-log CFU reduction. Re-growth: 10× MIC (0.5 µM) of LysH5/for 24 h/37 °C. Minimal re-growth was detected. Cells remained sensitive to P128 and were effectively killed with second dose of P128. | Disrupting assay: 24 h-old biofilm, 1× MIC of P128/for 2 h/37 °C. Destruction of a matrix structure. No viable cells detected. P128 was able to eradicate the biofilm mass from the surface of microtiter plates and catheters with equal efficiency. | Starting population: 108 CFU. Persister cells isolation: treatment with 50× MIC of vancomycin or 100× MIC of daptomycin for 6 h/37 °C. Persister population: 103 to 105 CFU. Killing assay: 4× MIC of P128/for 1 h/37 °C. No viable cells detected. | [117] |
| Art-175 chimeric endolysin created by fusion of the sheep myeloid antimicrobial peptide of 29 amino acids (SMAP-29) and N-terminus of endolysin KZ144 [124]. | MDR and laboratory strains of P. aeruginosa. | MIC 10 µg/mL. 2 µg/mL (with the addition of 500 µM EDTA). Killing assay: 25× MIC of Art-175 (+0.5 mM EDTA)/for 30 h/37 °C, >4-log CFU reduction. Time-laps microscopy: Cells from mid-exponential phase (OD600 0.6), concentrated five times, 25× MIC of Art-175 (0.1 mg/mL). Complete lysis and dispersion of cellular debris after 6 min. | No data. | Starting population: overnight culture. Persister cells isolation: treatment with 5× MIC of ofloxacin for 5 h/37 °C. Persister population: 105 CFU. Killing assay: 10× MIC Art-175 (40 µg/mL) (+0.5 mM EDTA)/for 1 h/37 °C, >5-log CFU reduction. No viable cells detected. | [97] |
| MDR and laboratory strains of A. baumannii. | MIC (strain dependent) from 4 to 20 µg/mL or from 4 to 10 µg/mL (with the addition of 500 µM EDTA). Killing assay: 1× MIC of Art-175/for 1 h/37 °C. No viable cells detected. Time-laps microscopy: Cells from mid-exponential phase (OD600 0.6), concentrated five times, Art-175 (0.4 mg/mL) (+0.5 mM EDTA). Complete lysis and dispersion of cellular debris after 3 s. | No data. | Starting population: overnight culture. Persister cells isolation: treatment with 60× MIC of tobramycin for 5 h/37 °C. Persister population: 103 CFU. Killing assay: 30× MIC Art-175 (120 µg/mL) (+0.5 mM EDTA)/for 1 h/37 °C, >5-log CFU reduction No viable cells detected. | [69] | |
| CF-301 (PlySs2) native endolysin from prophage of Streptococcus suis genome [125]. | MSSA and MRSA strains of S. aureus S. epidermidis. | Killing assay: 0.32 µg/mL of CF-301/for 24 h/37 °C. No viable cells detected. Re-growth: Minimal re-growth was detected under conditions: 0.032 µg/mL of CF-301/for 24 h/37 °C. Cells remained sensitive to CF-301 and were effectively killed with second dose of CF-301, 0.32 µg/mL of CF-301/for 24 h/37 °C. Sub-MIC concentration: 0.032 µg/mL. Bacterial growth slightly inhibited. No induction of persister cells. | MBEC (strain dependent) 0.125–0.25 µg/mL. Disrupting assay: 24 h-old biofilm: 32 µg/mL of CF-301/for 2 h/37 °C. 2-week-old biofilm: 32 µg/mL of CF-301/for 4 h/37 °C. Destruction of a matrix structure >5-log drop of viable cells after treatment. Inhibition in biofilm formation: formation of biofilm is fully inhibited at concentration down to 0.032 µg/mL. Mix-species biofilm: complete disruption of biofilm at 0.032 µg/mL. MIC of CF-301/for 24 h/37 °C. CF-301 was able to eradicate the biofilm mass from the surface of microtiter plates and catheters with equal efficiency. | Starting population: 108–109 CFU. Persister cells isolation: treatment with 100× MIC of daptomycin (100 µg/mL) or 3× MIC of ciprofloxacin (3 µg/mL) for 4 h/37 °C. Persister population: ~107CFU for daptomycin, ~106CFU for ciprofloxacin. Killing assay: 5× MIC (160 µg/mL) of CF-301/for 1–2 h/37 °C. No viable cells detected. | [126] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stojowska-Swędrzyńska, K.; Kuczyńska-Wiśnik, D.; Laskowska, E. New Strategies to Kill Metabolically-Dormant Cells Directly Bypassing the Need for Active Cellular Processes. Antibiotics 2023, 12, 1044. https://doi.org/10.3390/antibiotics12061044
Stojowska-Swędrzyńska K, Kuczyńska-Wiśnik D, Laskowska E. New Strategies to Kill Metabolically-Dormant Cells Directly Bypassing the Need for Active Cellular Processes. Antibiotics. 2023; 12(6):1044. https://doi.org/10.3390/antibiotics12061044
Chicago/Turabian StyleStojowska-Swędrzyńska, Karolina, Dorota Kuczyńska-Wiśnik, and Ewa Laskowska. 2023. "New Strategies to Kill Metabolically-Dormant Cells Directly Bypassing the Need for Active Cellular Processes" Antibiotics 12, no. 6: 1044. https://doi.org/10.3390/antibiotics12061044





