4.2. Synthesis of Compounds
4.2.1. 4-(Naphthalen-1-ylamino)-4-oxobutanoic Acid (9)
Naphthalen-1-amine (200 mg, 1.40 mmol) and succinic anhydride (140 mg, 1.40 mmol) were stirred in anhydrous CH2Cl2 (10 mL) for 9 h under an N2 atmosphere. The solvent was then removed under reduced pressure, and the crude product was purified by C8 reversed-phase column chromatography (100% H2O to 100% MeOH) to create 9 as a pale pink solid (330 mg, 97%). Rf = 0.43 (SiO2, CH2Cl2:10% MeOH); m.p. 165–167 °C; IR (ATR) vmax 3302, 2919, 1711, 1531, 1401, 1176, 915, 773 cm−1; 1H NMR (DMSO-d6, 400 MHz) δ 12.16 (1H, br s, OH), 9.94 (1H, br s, NH-5), 8.12–8.10 (1H, m, H-7), 7.94–7.91 (1H, m, H-10), 7.75 (1H, d, J = 7.8 Hz, H-11), 7.66 (1H, d, J = 7.8 Hz, H-13), 7.55–7.51 (2H, m, H-8, H-9), 7.47 (1H, dd, J = 7.8, 7.8 Hz, H-12), 2.75 (2H, t, J = 6.8 Hz, H2-2), 2.60 (2H, t, J = 6.8 Hz, H2-3); 13C NMR (DMSO-d6, 100 MHz) δ 173.9 (C-1), 170.8 (C-4), 133.7 (C-6, C-10a), 128.0 (C-10), 127.8 (C-6a), 125.9 (C-8/C-9), 125.7 (C-12), 125.5 (C-8/C-9), 125.1 (C-11), 122.9 (C-7), 121.6 (C-13), 30.7 (C-2), 29.1 (C-3); (+)-HRESIMS m/z 266.0783 [M+Na]+ (calcd for C14H13NNaO3, 266.0788).
4.2.2. 4-(Naphthalen-2-ylamino)-4-oxobutanoic Acid (11)
Naphthalen-2-amine (200 mg, 1.40 mmol) and succinic anhydride (140 mg, 1.40 mmol) were stirred in anhydrous CH2Cl2 (10 mL) for 9 h under an N2 atmosphere. The solvent was then removed under reduced pressure, and the crude product was purified by C8 reversed-phase column chromatography (100% H2O to 100% MeOH) to create 11 as a pink solid (260 mg, 76%). Rf = 0.19 (SiO2, CH2Cl2:10% MeOH); m.p. 170–172 °C; IR (ATR) vmax 3059, 1706, 1653, 1558, 1397, 1257, 824, 742 cm−1; 1H NMR (DMSO-d6, 400 MHz) δ 10.18 (1H, br s, NH-5), 8.30 (1H, s, H-7), 7.85–7.77 (3H, m, H-8, H-11, H-12), 7.57 (1H, dd, J = 8.9, 2.0 Hz, H-13), 7.47–7.43 (1H, m, H-9), 7.40–7.36 (1H, m, H-10), 2.62 (2H, t, J = 6.0 Hz, H2-2), 2.56 (2H, t, J = 6.0, H2-3); 13C NMR (DMSO-d6, 100 MHz) δ 173.9 (C-1), 170.4 (C-4), 136.9 (C-6), 133.5 (C-7a), 129.6 (C-11a), 128.3 (C-12), 127.4 (C-11), 127.2 (C-8), 126.3 (C-9), 124.4 (C-10), 119.8 (C-13), 114.8 (C-7), 31.2 (C-2), 29.0 (C-3); (+)-HRESIMS [M+Na]+ m/z 266.0784 (calcd for C14H13NNaO3, 266.0788).
4.2.3. 4-([1,1’-Biphenyl]-4-ylamino)-4-oxobutanoic Acid (12)
4-Aminobiphenyl (500 mg, 2.95 mmol) and succinic anhydride (295 mg, 2.95 mmol) were stirred in anhydrous CH2Cl2 (11 mL) for 24 h under an N2 atmosphere. The solvent was removed under reduced pressure, and the was product purified by recrystallisation from EtOH to create 12 as light orange crystals (356 mg, 45%). Rf = 0.17 (SiO2, CH2Cl2:10% MeOH); m.p. >230 °C; IR (ATR) vmax 3273, 3031, 2934, 2635, 1689, 1651, 1529, 1403, 1186, 832, 750, 685 cm−1; 1H NMR (DMSO-d6, 400 MHz) δ 12.13 (1H, br s, OH), 10.05 (1H, br s, NH-5), 7.70–7.65 (2H, m, H-7), 7.65–7.59 (4H, m, H-8, H-11), 7.46–7.41 (2H, m, H-12), 7.34–7.29 (1H, m, H-13), 2.61–2.57 (2H, m, H2-2/H2-3), 2.55–2.52 (2H, m, H2-2/H2-3); 13C NMR (DMSO-d6, 100 MHz) δ 173.8 (C-1), 170.1 (C-4), 139.7 (C-10), 138.8 (C-6), 134.6 (C-9), 128.9 (C-12), 127.0 (C-13), 126.9 (C-8/C-11), 126.2 (C-8/C-11), 119.3 (C-7), 31.1 (C-2/C-3), 28.8 (C-2/C-3); (+)-HRESIMS m/z 292.0950 [M+Na]+ (calcd for C16H15NNaO3, 292.0944).
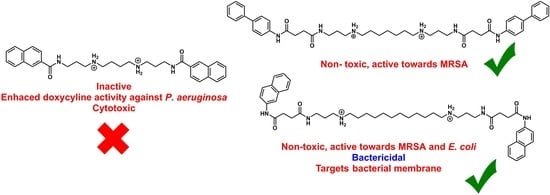
4.2.4. N1,N4-Bis(3-(1-naphthamido)propyl)butane-1,4-diaminium 2,2,2-trifluoroacetate (17a)
Following general procedure A, the reaction of 1-naphthoic acid (8) (94 mg, 0.55 mmol), di-tert-butyl butane-1,4-diylbis((3-aminopropyl)carbamate) (16a) (100 mg, 0.25 mmol), EDC·HCl (124 mg, 0.65 mmol), HOBt (87 mg, 0.64 mmol), and DIPEA (0.26 mL, 1.49 mmol) in CH2Cl2 (1.5 mL) created di-tert-butyl butane-1,4-diylbis((3-(1-naphthamido)propyl)carbamate) (42 mg, 24%) as a white solid. Following general procedure C, the reaction of a sub-sample of this material (22 mg, 0.031 mmol) in CH2Cl2 (2 mL) with TFA (0.2 mL) created the di-TFA salt 17a as a white solid after purification (12 mg, 52%). Rf = 0.48 (RP-18, MeOH:10% HCl, 7:3); m.p. 238–239 °C; IR (ATR) vmax 3307, 2839, 1646, 1451, 1203, 1116, 1015 cm−1; 1H NMR (DMSO-d6, 400 MHz) δ 8.70–8.65 (4H, m, NH2-14), 8.21–8.18 (2H, m, H-3), 8.04–7.97 (4H, m, H-6, H-7), 7.62 (2H, dd, J = 7.0, 1.0 Hz, H-9), 7.59–7.53 (6H, m, H-4, H-5, H-8), 3.43–3.39 (4H, m, H2-11), 3.03–2.97 (8H, m, H2-13, H2-15), 1.96–1.89 (4H, m, H2-12), 1.69–1.64 (4H, m, H2-16); 13C NMR (DMSO-d6, 100 MHz) δ 168.9 (C-1), 134.5 (C-6a), 133.1 (C-2), 129.9 (C-7), 129.7 (C-2a), 128.2 (C-6), 126.7 (C-4), 126.2 (C-5), 125.3 (C-3/C-8), 125.2 (C-3/C-8), 124.9 (C-9), 46.1 (C-15), 44.8 (C-13), 36.3 (C-11), 26.0 (C-12), 22.7 (C-16); (+)-HRESIMS m/z 511.3068 [M+H]+ (calcd for C32H39N4O2, 511.3068).
4.2.5. N1,N6-Bis(3-(1-naphthamido)propyl)hexane-1,6-diaminium 2,2,2-trifluoroacetate (17b)
Following general procedure A, the reaction of 1-naphthoic acid (8) (44.0 mg, 0.256 mmol), di-tert-butyl hexane-1,6-diylbis((3-aminopropyl)carbamate) (16b) (50 mg, 0.12 mmol), EDC·HCl (57.9 mg, 0.302 mmol), HOBt (40.8 mg, 0.30 mmol), and DIPEA (0.12 mL, 0.69 mmol) in CH2Cl2 (1.5 mL) created di-tert-butyl hexane-1,6-diylbis((3-(1-naphthamido)propyl)carbamate) as a colorless oil (27 mg, 30%). Following general procedure C, the reaction of a sub-sample of this material (18 mg, 0.024 mmol) in CH2Cl2 (2 mL) with TFA (0.2 mL) created the di-TFA salt 17b as a white solid (16 mg, 87%) with no further purification required. Rf = 0.47 (RP-18, MeOH:10% HCl, 7:3); m.p. 225–227 °C; IR (ATR) vmax 3293, 3057, 2944, 2856, 1675, 1542, 1313, 1201, 1132, 786, 721cm−1; 1H NMR (CD3OD, 400 MHz) δ 8.24–8.20 (2H, m, H-3), 8.03 (2H, d, J = 8.2 Hz, H-7), 7.98–7.94 (2H, m, H-6), 7.68 (2H, dd, J = 7.1, 1.4 Hz, H-9), 7.62–7.53 (6H, m, H-4, H-5, H-8), 3.59 (4H, t, J = 6.6 Hz, H2-11), 3.15 (4H, t, J = 7.4 Hz, H2-13), 3.07 (4H, t, J = 7.5 Hz, H2-15), 2.11–2.03 (4H, m, H2-12), 1.81–1.72 (4H, m, H2-16), 1.53–1.48 (4H, m, H2-17); 13C NMR (CD3OD, 100 MHz) δ 173.4 (C-1), 135.2 (C-2/C-6a), 135.0 (C-2/C-6a), 131.9 (C-7), 131.4 (C-2a), 129.6 (C-6), 128.1 (C-4), 127.6 (C-5), 126.6 (C-9), 126.1 (C-3), 126.0 (C-8), 48.9 (C-15), 46.6 (C-13), 37.5 (C-11), 27.8 (C-12), 27.1 (C-17), 27.0 (C-16); (+)-HRESIMS m/z 539.3385 [M+H]+ (calcd for C34H43N4O2, 539.3381).
4.2.6. N1,N7-Bis(3-(1-naphthamido)propyl)heptane-1,7-diaminium 2,2,2-trifluoroacetate (17c)
Following general procedure A, the reaction of 1-naphthoic acid (8) (43.0 mg, 0.250 mmol), di-tert-butyl heptane-1,7-diylbis((3-aminopropyl)carbamate) (16c) (50 mg, 0.11 mmol), EDC·HCl (56.1 mg, 0.293 mmol), HOBt (39.6 mg, 0.29 mmol), and DIPEA (0.118 mL, 0.677 mmol) in CH2Cl2 (1.5 mL) created di-tert-butyl heptane-1,7-diylbis((3-aminopropyl)carbamate) as a colorless oil (45 mg, 54%). Following general procedure C, the reaction of a sub-sample of this material (6 mg, 0.008 mmol) in CH2Cl2 (2 mL) with TFA (0.2 mL) created the di-TFA salt 17c as a colorless gum after purification (5 mg, 81%). Rf = 0.63 (RP-18, MeOH:10% HCl, 7:3); IR (ATR) vmax 3752, 326, 2927, 2857, 2305, 1678, 1665, 1543, 1199, 1136, 806, 723 cm−1; 1H NMR (CD3OD, 400 MHz) δ 8.12–8.09 (2H, m, H-3), 7.90 (2H, d, J = 8.5 Hz, H-7), 7.85–7.82 (2H, m, H-6), 7.55 (2H, dd, J = 7.2, 1.4 Hz, H-9), 7.49–7.40 (6H, m, H-4, H-5, H-8), 3.47 (4H, t, J = 6.6 Hz, H2-11), 3.03 (4H, t, J = 7.4 Hz, H2-13), 2.94 (4H, t, J = 7.7 Hz, H2-15), 1.98–1.90 (4H, m, H2-12), 1.67–1.59 (4H, m, H2-16), 1.38–1.33 (6H, m, H2-17, H2-18); 13C NMR (CD3OD, 100 MHz) δ 173.5 (C-1), 135.2 (C-2/C-6a), 134.9 (C-2/C-6a), 131.9 (C-7), 131.4 (C-2a), 129.6 (C-6), 128.1 (C-4), 127.5 (C-5), 126.6 (C-9), 126.1 (C-3), 125.9 (C-8), 49.0 (C-15), 46.5 (C-13), 37.5 (C-11), 29.6 (C-18), 27.8 (C-12), 27.3 (C-16/C-17); (+)-HRESIMS m/z 553.3534 [M+H]+ (calcd for C35H45N4O2, 553.3537).
4.2.7. N1,N8-Bis(3-(1-naphthamido)propyl)octane-1,8-diaminium 2,2,2-trifluoroacetate (17d)
Following general procedure A, the reaction of 1-naphthoic acid (8) (41.3 mg, 0.240 mmol), di-tert-butyl octane-1,8-diylbis((3-aminopropyl)carbamate) (16d) (50 mg, 0.11 mmol), EDC·HCl (54.3 mg, 0.283 mmol), HOBt (38.3 mg, 0.28 mmol), and DIPEA (0.114 mL, 0.653 mmol) in CH2Cl2 (1.5 mL) created di-tert-butyl octane-1,8-diylbis((3-aminopropyl)carbamate) as a colorless oil (37 mg, 44%). Following general procedure C, the reaction of a sub-sample of this material (13 mg, 0.017 mmol) in CH2Cl2 (2 mL) with TFA (0.2 mL) created the di-TFA salt 17d as a colorless gum after purification (4 mg, 30%). Rf = 0.63 (RP-18, MeOH:10% HCl, 7:3); IR (ATR) vmax 3743, 3274, 3057, 2935, 2861, 1675, 1542, 1468, 1200, 1132, 784, 721 cm−1; 1H NMR (CD3OD, 400 MHz) δ 8.26–8.21 (2H, m, H-3), 8.03 (2H, d, J = 8.6 Hz, H-7), 7.98–7.94 (2H, m, H-6), 7.68 (2H, dd, J = 6.9, 1.2 Hz, H-9), 7.62–7.52 (6H, m, H-4, H-5, H-8), 3.60 (4H, t, J = 6.7 Hz, H2-11), 3.17 (4H, t, J = 7.5 Hz, H2-13), 3.06 (4H, t, J = 7.8 Hz, H2-15), 2.12–2.03 (4H, m, H2-12), 1.78–1.70 (4H, m, H2-16), 1.50–1.39 (8H, m, H2-17, H2-18); 13C NMR (CD3OD, 100 MHz) δ 173.5 (C-1), 135.2 (C-6a), 135.0 (C-2), 132.0 (C-7), 131.4 (C-2a), 129.6 (C-6), 128.1 (C-4), 127.6 (C-5), 126.6 (C-9), 126.1 (C-3), 126.0 (C-8), 49.0 (C-15), 46.6 (C-13), 37.5 (C-11), 30.0 (C-18), 27.8 (C-12), 27.4 (C-16/C-17), 27.3 (C-16/C-17); (+)-HRESIMS m/z 567.3680 [M+H]+ (calcd for C36H47N4O2, 567.3694).
4.2.8. N1,N10-Bis(3-(1-naphthamido)propyl)decane-1,10-diaminium 2,2,2-trifluoroacetate (17e)
Following general procedure A, the reaction of 1-napthoic acid (8) (78 mg, 0.45 mmol), di-tert-butyl decane-1,10-diylbis((3-aminopropyl)carbamate) (16e) (100 mg, 0.21 mmol), EDC·HCl (103 mg, 0.54 mmol), HOBt (72 mg, 0.53 mmol), and DIPEA (0.22 mL, 1.26 mmol) in CH2Cl2 (1.5 mL) created di-tert-butyl decane-1,10-diylbis((3-(1-naphthamido)propyl)carbamate) (120 mg, 72%) as a colorless oil. Following general procedure C, the reaction of a sub-sample of this material (99 mg, 0.13 mmol) in CH2Cl2 (2 mL) with TFA (0.2 mL) created the di-TFA salt 17e (93 mg, 87%) as a yellow oil with no further purification required. Rf = 0.27 (RP-18, MeOH:10% HCl, 7:3); IR (ATR) vmax 3306, 2944, 2832, 1685, 1448, 1113, 1022 cm−1; 1H NMR (CD3OD, 400 MHz) δ 8.23–8.20 (2H, m, H-3), 8.00 (2H, d, J = 8.3 Hz, H-7), 7.99–7.91 (2H, m, H-6), 7.65 (2H, dd, J = 7.0, 0.7 Hz, H-9), 7.58–7.50 (6H, m, H-4, H-5, H-8), 3.58 (4H, t, J = 6.6 Hz, H2-11), 3.14 (4H, t, J = 7.4 Hz, H2-13), 3.03 (4H, t, J = 7.8 Hz, H2-15), 2.09–2.02 (4H, m, H2-12), 1.75–1.67 (4H, m, H2-16), 1.42–1.33 (12H, m, H2-17, H2-18, H2-19); 13C NMR (CD3OD, 100 MHz) δ 173.5 (C-1), 135.2 (C-2/C-6a), 135.0 (C-2/C-6a), 131.9 (C-7), 131.4 (C-2a), 129.6 (C-6), 128.1 (C-4), 127.6 (C-5), 126.6 (C-9), 126.1 (C-3), 126.0 (C-8), 49.1 (C-15), 46.6 (C-13), 37.6 (C-11), 30.4 (C-18/C-19), 30.2 (C-18/C-19), 27.8 (C-12), 27.5 (C-17), 27.4 (C-16); (+)-HRESIMS m/z 595.3998 [M+H]+ (calcd for C38H51N4O2, 595.4007).
4.2.9. N1,N12-Bis(3-(1-naphthamido)propyl)dodecane-1,12-diaminium 2,2,2-trifluoroacetate (17f)
Following general procedure A, the reaction of 1-naphthoic acid (8) (52 mg, 0.30 mmol), di-tert-butyl dodecane-1,12-diylbis((3-aminopropyl)carbamate) (16f) (71 mg, 0.14 mmol), EDC·HCl (69 mg, 0.36 mmol), HOBt (47 mg, 0.35 mmol), and DIPEA (0.14 mL, 0.80 mmol) in CH2Cl2 (1.5 mL) created di-tert-butyl dodecane-1,12-diylbis((3-(1-naphthamido)propyl)carbamate) (43 mg, 37%) as a white wax. Following general procedure C, the reaction of a sub-sample of this material (19 mg, 0.023 mmol) in CH2Cl2 (2 mL) with TFA (0.2 mL) created the di-TFA salt 17f (18 mg, 92%) as a white wax with no further purification required. Rf = 0.15 (RP-18, MeOH:10% HCl, 7:3); IR (ATR) vmax 3326, 2944, 2834, 1654, 1450, 1246, 1116, 1022 cm−1; 1H NMR (CD3OD, 400 MHz) δ 8.23–8.20 (2H, m, H-3), 8.00 (2H, d, J = 8.2 Hz, H-7), 7.95–7.92 (2H, m, H-6), 7.66 (2H, dd, J = 7.0, 1.0 Hz, H-9), 7.59–7.50 (6H, m, H-4, H-5, H-8), 3.58 (4H, t, J = 6.6 Hz, H2-11), 3.15 (4H, t, J = 7.4 Hz, H2-13), 3.04 (4H, t, J = 7.8 Hz, H2-15), 2.09–2.02 (4H, m, H2-12), 1.75–1.68 (4H, m, H2-16), 1.43–1.30 (16H, m, H2-17, H2-18, H2-19, H2-20); 13C NMR (CD3OD, 100 MHz) δ 173.4 (C-1), 135.2 (C-2/C-6a), 135.0 (C-2/C-6a), 131.9 (C-7), 131.4 (C-2a), 129.6 (C-6), 128.1 (C-4), 127.5 (C-5), 126.6 (C-9), 126.1 (C-3), 125.9 (C-8), 49.1 (C-15), 46.6 (C-13), 37.6 (C-11), 30.6 (C-18/C-19/C-20), 30.5 (C-18/C-19/C-20), 30.2 (C-18/C-19/C-20), 27.8 (C-12), 27.5 (C-17), 27.4 (C-16); (+)-HRESIMS m/z 623.4321 [M+H]+ (calcd for C40H55N4O2, 623.4320).
4.2.10. N1,N4-Bis(3-(4-(naphthalen-1-ylamino)-4-oxobutanamido)propyl)butane-1,4-diaminium 2,2,2-trifluoroacetate (18a)
Following general procedure B, the reaction of carboxylic acid 9 (76 mg, 0.31 mmol), di-tert-butyl butane-1,4-diylbis((3-aminopropyl)carbamate) (16a) (50 mg, 0.12 mmol), EDC·HCl (67 mg, 0.35 mmol), and DMAP (76 mg, 0.62 mmol) in CH2Cl2 (1.5 mL) created di-tert-butyl butane-1,4-diylbis((3-(4-(naphthalen-1-ylamino)-4-oxobutanamido)propyl)carbamate) as a colorless oil (54 mg, 53%). Following general procedure C, the reaction of a sub-sample of this material (26 mg, 0.030 mmol) in CH2Cl2 (2 mL) with TFA (0.2 mL) created the di-TFA salt 18a as a colorless oil after purification (22 mg, 83%). Rf = 0.47 (RP-18, MeOH:10% HCl, 7:3); IR (ATR) vmax 3278, 1676, 1202, 1160, 799, 766 cm−1; 1H NMR (CD3OD, 400 MHz) δ 8.04 (2H, dd, J = 9.0, 2.0 Hz, H-10), 7.89 (2H, dd, J = 7.0, 2.0 Hz, H-7), 7.76 (2H, d, J = 8.5, Hz, H-13), 7.59–7.57 (2H, m, H-11), 7.56–7.50 (4H, m, H-8, H-9), 7.46 (2H, t, J = 7.8 Hz, H-12), 3.32–3.28 (4H, m, H2-15), 2.92–2.89 (4H, m, H2-2), 2.83 (4H, t, J = 7.0 Hz, H2-17), 2.65–2.62 (4H, m, H2-3), 2.57–2.54 (4H, m, H2-19), 1.82–1.75 (4H, m, H2-16), 1.36–1.32 (4H, m, H2-20); 13C NMR (CD3OD, 100 MHz) δ 176.3 (C-1), 174.3 (C-4), 135.7 (C-6), 134.3 (C-10a), 130.2 (C-6a), 129.4 (C-7), 127.6 (C-13), 127.4 (C-8/C-9), 127.3 (C-8/C-9), 126.6 (C-12), 124.0 (C-11), 123.6 (C-10), 47.8 (C-19), 46.0 (C-17), 36.6 (C-15), 32.0 (C-2), 31.6 (C-3), 27.7 (C-16), 23.9 (C-20); (+)-HRESIMS m/z 653.3807 [M+H]+ (calcd for C38H49N6O4, 653.3810).
4.2.11. N1,N6-Bis(3-(4-(naphthalen-1-ylamino)-4-oxobutanamido)propyl)hexane-1,6-diaminium 2,2,2-trifluoroacetate (18b)
Following general procedure B, the reaction of carboxylic acid 9 (71 mg, 0.29 mmol), di-tert-butyl hexane-1,6-diylbis((3-aminopropyl)carbamate) (16b) (50 mg, 0.12 mmol), EDC·HCl (62 mg, 0.32 mmol), and DMAP (71 mg, 0.58 mmol) in CH2Cl2 (1.5 mL) created di-tert-butyl hexane-1,6-diylbis((3-(4-(naphthalen-1-ylamino)-4-oxobutanamido)propyl)carbamate) as a colorless oil (97 mg, 92%). Following general procedure C, the reaction of a sub-sample of this material (44 mg, 0.050 mmol) in CH2Cl2 (2 mL) with TFA (0.2 mL) created the di-TFA salt 18b as an orange oil after purification (39 mg, 86%). Rf = 0.47 (RP-18, MeOH:10% HCl, 7:3); IR (ATR) vmax 3279, 3050, 1671, 1542, 1201, 1132, 800, 777 cm−1; 1H NMR (CD3OD, 400 MHz) δ 8.01 (2H, dd, J = 9.0, 2.5 Hz, H-10), 7.85 (2H, dd, J = 7.5, 3.5 Hz, H-7), 7.71 (2H, d, J = 8.5, Hz, H-13), 7.57 (2H, dd, J = 7.5, 1.0 Hz, H-11), 7.51–7.47 (4H, m, H-8, H-9), 7.42 (2H, t, J = 7.8 Hz, H-12), 3.31–3.26 (4H, m, H2-15), 2.88 (8H, t, J = 6.5 Hz, H2-2, H2-17), 2.61–2.53 (8H, m, H2-3, H2-19), 1.82–1.75 (4H, m, H2-16), 1.33–1.26 (4H, m, H2-20), 0.91–0.87 (4H, m, H2-21); 13C NMR (CD3OD, 100 MHz) δ 176.3 (C-1), 174.2 (C-4), 135.7 (C-6), 134.4 (C-10a), 130.0 (C-6a), 129.4 (C-7), 127.5 (C-13), 127.33 (C-8/C-9), 127.27 (C-8/C-9), 126.5 (C-12), 123.7 (C-11), 123.6 (C-10), 48.7 (C-19), 46.0 (C-17), 36.5 (C-15), 32.0 (C-2), 31.5 (C-3), 27.8 (C-16), 26.8 (C-20/C-21), 26.7 (C-20/C-21); (+)-HRESIMS [M+H]+ m/z 681.4104 (calcd for C40H53N6O4, 681.4123).
4.2.12. N1,N7-Bis(3-(4-(naphthalen-1-ylamino)-4-oxobutanamido)propyl)heptane-1,7-diaminium 2,2,2-trifluoroacetate (18c)
Following general procedure B, the reaction of carboxylic acid 9 (68 mg, 0.29 mmol), di-tert-butyl heptane-1,7-diylbis((3-aminopropyl)carbamate) (16c) (50 mg, 0.11 mmol), EDC·HCl (60 mg, 0.31 mmol), and DMAP (69 mg, 0.57 mmol) in CH2Cl2 (1.5 mL) created di-tert-butyl heptane-1,7-diylbis((3-(4-(naphthalen-1-ylamino)-4-oxobutanamido)propyl)carbamate) as a colorless oil (94 mg, 95%). Following general procedure C, the reaction of a sub-sample of this material (82 mg, 0.092 mmol) in CH2Cl2 (2 mL) with TFA (0.2 mL) created the di-TFA salt 18c as a colorless oil after purification (59 mg, 69%). Rf = 0.42 (RP-18, MeOH:10% HCl, 7:3); IR (ATR) vmax 3279, 3053, 1671, 1537, 1201, 1131, 800, 777 cm−1; 1H NMR (CD3OD, 400 MHz) δ 8.05 (2H, dd, J = 9.5, 1.5 Hz, H-10), 7.87 (2H, dd, J = 7.5, 2.5 Hz, H-7), 7.74 (2H, d, 8.0 Hz, H-13), 7.61 (2H, dd, J = 7.5, 1.0 Hz, H-11), 7.55–7.48 (4H, m, H-8, H-9), 7.45 (2H, t, J = 8.0 Hz, H-12), 3.33–3.30 (4H, m, H2-15), 2.91 (8H, t, J = 6.5 Hz, H2-2, H2-17), 2.65–2.61 (8H, m, H2-3, H2-19), 1.85–1.79 (4H, m, H2-16), 1.42–1.35 (4H, m, H2-20), 1.01–0.98 (6H, m, H2-21, H2-22); 13C NMR (CD3OD, 100 MHz) δ 176.2 (C-1), 174.1 (C-4), 135.7 (C-6), 134.3 (C-10a), 130.0 (C-6a), 129.4 (C-7), 127.5 (C-13), 127.3 (C-8/C-9), 127.2 (C-8/C-9), 126.5 (C-12), 123.7 (C-11), 123.6 (C-10), 48.8 (C-19), 46.0 (C-17), 36.6 (C-15), 32.0 (C-2), 31.6 (C-3), 29.4 (C-22), 27.7 (C-16), 27.0 (C-20/C-21), 26.9 (C-20/C-21); (+)-HRESIMS [M+H]+ m/z 695.4265 (calcd for C41H55N6O4, 695.4279).
4.2.13. N1,N8-Bis(3-(4-(naphthalen-1-ylamino)-4-oxobutanamido)propyl)octane-1,8-diaminium 2,2,2-trifluoroacetate (18d)
Following general procedure B, the reaction of carboxylic acid 9 (67 mg, 0.28 mmol), di-tert-butyl octane-1,8-diylbis((3-aminopropyl)carbamate) (16d) (50 mg, 0.11 mmol), EDC·HCl (59 mg, 0.31 mmol), and DMAP (67 mg, 0.55 mmol) in CH2Cl2 (1.5 mL) created di-tert-butyl octane-1,8-diylbis((3-(4-(naphthalen-1-ylamino)-4-oxobutanamido)propyl)carbamate) as a colorless oil (69 mg, 69%). Following general procedure C, the reaction of this material (69 mg, 0.076 mmol) in CH2Cl2 (2 mL) with TFA (0.2 mL) created the di-TFA salt 18d as a colorless oil after purification (37 mg, 54%). Rf = 0.40 (RP-18, MeOH:10% HCl, 7:3); IR (ATR) vmax 3052, 1655, 1542, 1201, 1132, 799, 777 cm−1; 1H NMR (CD3OD, 400 MHz) δ 8.05 (2H, dd, J = 8.0, 1.5 Hz, H-10), 7.88 (2H, dd, J = 7.5, 2.0 Hz, H-7), 7.75 (2H, d, J = 8.0 Hz, H-13), 7.62 (2H, dd, J = 7.5, 1.0 Hz, H-11), 7.56–7.49 (4H, m, H-8, H-9), 7.46 (2H, t, J = 8.0 Hz, H-12), 3.34–3.31 (4H, m, H2-15), 2.95–2.91 (8H, m, H2-2, H2-17), 2.67–2.62 (8H, m, H2-3, H2-19), 1.86–1.80 (4H, m, H2-16), 1.45–1.39 (4H, m, H2-20), 1.07–1.01 (8H, m, H2-21, H2-22); 13C NMR (CD3OD, 100 MHz) δ 176.2 (C-1), 174.1 (C-4), 135.7 (C-6), 134.3 (C-10a), 130.0 (C-6a), 129.4 (C-7), 127.4 (C-13), 127.3 (C-8/C-9), 127.2 (C-8/C-9), 126.5 (C-12), 123.6 (C-11), 123.5 (C-10), 48.9 (C-19), 46.0 (C-17), 36.6 (C-15), 32.0 (C-2), 31.6 (C-3), 29.8 (C-22), 27.8 (C-16), 27.2 (C-20/C-21), 27.1 (C-20/C-21); (+)-HRESIMS [M+2H]2+ m/z 355.2250 (calcd for C42H58N6O4, 355.2254).
4.2.14. N1,N10-Bis(3-(4-(naphthalen-1-ylamino)-4-oxobutanamido)propyl)decane-1,10-diaminium 2,2,2-trifluoroacetate (18e)
Following general procedure B, the reaction of carboxylic acid 9 (63 mg, 0.26 mmol), di-tert-butyl decane-1,10-diylbis((3-aminopropyl)carbamate) (16e) (50 mg, 0.10 mmol), EDC·HCl (55 mg, 0.29 mmol), and DMAP (63 mg, 0.52 mmol) in CH2Cl2 (1.5 mL) created di-tert-butyl decane-1,10-diylbis((3-(4-(naphthalen-1-ylamino)-4-oxobutanamido)propyl)carbamate) as a colorless oil (67 mg, 72%). Following general procedure C, the reaction of a sub-sample of this material (27 mg, 0.029 mmol) in CH2Cl2 (2 mL) with TFA (0.2 mL) created the di-TFA salt 18e as a colorless oil after purification (26 mg, 93%). Rf = 0.23 (RP-18, MeOH: 10% HCl, 7:3); IR (ATR) vmax 2937, 1673, 1538, 1202, 1133, 800, 776, 721 cm−1; 1H NMR (CD3OD, 400 MHz) δ 8.05 (2H, dd, J = 8.0, 1.5 Hz, H-10), 7.88 (2H, dd, J = 7.4, 2.4 Hz, H-7), 7.76 (2H, d, J = 8.5, Hz, H-13), 7.62 (2H, d, J = 7.5 Hz, H-11), 7.56–7.50 (4H, m, H-8, H-9), 7.46 (2H, t, J = 8.0 Hz, H-12), 3.34–3.30 (4H, m, H2-15), 2.95–2.91 (8H, m, H2-2, H2-17), 2.68–2.61 (8H, m, H2-3, H2-19), 1.86–1.79 (4H, m, H2-16), 1.47–1.40 (4H, m, H2-20), 1.14–1.08 (12H, m, H2-21, H2-22, H2-23); 13C NMR (CD3OD, 100 MHz) δ 176.3 (C-1), 174.2 (C-4), 135.8 (C-6), 134.4 (C-10a), 130.1 (C-6a), 129.5 (C-7), 127.5 (C-13), 127.34 (C-8/C-9), 127.26 (C-8/C-9), 126.5 (C-12), 123.7 (C-11), 123.6 (C-10), 49.0 (C-19), 46.1(C-17), 36.6 (C-15), 32.0 (C-2), 31.6 (C-3), 30.3 (C-23), 30.1 (C-22), 27.8 (C-16), 27.4 (C-20/C-21), 27.2 (C-20/C-21); (+)-HRESIMS m/z 737.4699 [M+H]+ (calcd for C44H61N6O4, 737.4749).
4.2.15. N1,N12-Bis(3-(4-(Naphthalen-1-ylamino)-4-oxobutanamido)propyl)dodecane-1,12-diaminium 2,2,2-trifluoroacetate (18f)
Following general procedure B, the reaction of carboxylic acid 9 (58 mg, 0.24 mmol), di-tert-butyl dodecane-1,12-diylbis((3-aminopropyl)carbamate) (16f) (57 mg, 0.11 mmol), EDC·HCl (52 mg, 0.27 mmol), and DMAP (60 mg, 0.49 mmol) in CH2Cl2 (1.5 mL) created di-tert-butyl dodecane-1,12-diylbis((3-(4-(naphthalen-1-ylamino)-4-oxobutanamido)propyl)carbamate) as a colorless oil (62 mg, 58%). Following general procedure C, the reaction of a sub-sample of this material (26 mg, 0.027 mmol) in CH2Cl2 (2 mL) with TFA (0.2 mL) created the di-TFA salt 18f as a pale-yellow oil after purification (20 mg, 75%). Rf = 0.20 (RP-18, MeOH:10% HCl, 7:3); IR (ATR) vmax 3083, 2927, 1667, 1537, 1201, 1132, 800, 775 cm−1; 1H NMR (CD3OD, 400 MHz) δ 8.05 (2H, dd, J = 8.0, 1.5 Hz, H-10), 7.88 (2H, dd, J = 7.5, 2.0 Hz, H-7), 7.75 (2H, d, J = 8.0 Hz, H-13), 7.61 (2H, dd, J = 7.5, 1.0 Hz, H-11), 7.56–7.50 (4H, m, H-8, H-9), 7.46 (2H, t, J = 7.8 Hz, H-12), 3.34–3.30 (4H, m, H2-15), 2.95–2.91 (8H, m, H2-2, H2-17), 2.68–2.62 (8H, m, H2-3, H2-19), 1.86–1.80 (4H, m, H2-16), 1.48–1.41 (4H, m, H2-20), 1.21–1.08 (16H, m, H2-21, H2-22, H2-23, H2-24); 13C NMR (CD3OD, 100 MHz) δ 176.2 (C-1), 174.2 (C-4), 135.7 (C-6), 134.3 (C-10a), 130.0 (C-6a) 129.4 (C-7), 127.5 (C-13), 127.3 (C-8/C-9), 127.2 (C-8/C-9), 126.5 (C-12), 123.7 (C-11), 123.5 (C-10), 49.0 (C-19), 46.0 (C-17), 36.6 (C-15), 32.0 (C-2), 31.6 (C-3), 30.5 (C-22/C-23/C-24), 30.4 (C-22/C-23/C-24), 30.1 (C-22/C-23/C-24), 27.7 (C-16), 27.3 (C-20/C-21), 27.2 (C-20/C-21); (+)-HRESIMS [M+2H]2+ m/z 383.2575 (calcd for C46H66N6O4, 383.2567).
4.2.16. N1,N4-Bis(3-(2-naphthamido)propyl)butane-1,4-diaminium 2,2,2-trifluoroacetate (19a)
Following general procedure A, the reaction of 2-napthoic acid (10) (94 mg, 0.55 mmol), di-tert-butyl butane-1,4-diylbis((3-aminopropyl)carbamate) (16a) (100 mg, 0.25 mmol), EDC·HCl (124 mg, 0.65 mmol), HOBt (87 mg, 0.64 mmol), and DIPEA (0.26 mL, 1.49 mmol) in CH2Cl2 (1.5 mL) created di-tert-butyl butane-1,4-diylbis((3-(2-naphthamido)propyl)carbamate) (91 mg, 51%) as a white solid. Following general procedure C, the reaction of a sub-sample of this material (72 mg, 0.10 mmol) in CH2Cl2 (2 mL) with TFA (0.2 mL) created the di-TFA salt 19a as a beige solid after purification (71 mg, 96%). Rf = 0.33 (RP-18, MeOH:10% HCl, 7:3); m.p. 132–135 °C; IR (ATR) vmax 3332, 2948, 2835, 1674, 1639, 1544, 1435, 1313, 1201, 1133, 1018, 721 cm−1; 1H NMR (CD3OD, 400 MHz) δ 8.38 (2H, br s, H-3), 7.96–7.87 (8H, m, H-4, H-7, H-8, H-9), 7.61–7.53 (4H, m, H-5, H-6), 3.57 (4H, t, J = 6.4 Hz, H2-11), 3.14–3.10 (8H, m, H2-13, H2-15), 2.08–2.01 (4H, m, H2-12), 1.90–1.87 (4H, m, H2-16); 13C NMR (CD3OD, 100 MHz) δ 171.2 (C-1), 136.4 (C-7a), 134.0 (C-3a), 132.2 (C-2), 130.0 (C-4), 129.5 (C-8), 129.1 (C-3/C-6), 129.0 (C-3/C-6), 128.8 (C-7), 128.0 (C-5), 124.8 (C-9), 48.2 (C-15), 46.5 (C-13), 37.5 (C-11), 27.9 (C-12), 24.4 (C-16); (+)-HRESIMS m/z 511.3071 [M+H]+ (calcd for C32H39N4O2, 511.3068).
4.2.17. N1,N6-Bis(3-(2-naphthamido)propyl)hexane-1,6-diaminium 2,2,2-trifluoroacetate (19b)
Following general procedure A, the reaction of 2-naphthoic acid (10) (44.0 mg, 0.256 mmol), di-tert-butyl hexane-1,6-diylbis((3-aminopropyl)carbamate) (16b) (50.0 mg, 0.12 mmol), EDC·HCl (57.9 mg, 0.302 mmol), HOBt (40.8 mg, 0.30 mmol), and DIPEA (0.12 mL, 0.69 mmol) in CH2Cl2 (1.5 mL) created di-tert-butyl hexane-1,6-diylbis((3-(2-naphthamido)propyl)carbamate) as a colorless oil (48.0 mg, 54%). Following general procedure C, the reaction of a sub-sample of this material (6 mg, 0.0081 mmol) in CH2Cl2 (2 mL) with TFA (0.2 mL) created the di-TFA salt 19b as a colorless gum (6 mg, 97%) with no further purification required. Rf = 0.43 (RP-18, MeOH:10% HCl, 7:3); IR (ATR) vmax 3747, 3325, 2922, 2853, 2354, 1635, 1541, 1465, 1199 cm-1; 1H NMR (CD3OD, 400 MHz) δ 8.39 (2H, br s, H-3), 7.98–7.88 (8H, m, H-4, H-7, H-8, H-9), 7.62–7.55 (4H, m, H-5, H-6), 3.57 (4H, t, J = 6.5 Hz, H2-11), 3.09 (4H, t, J = 7.2 Hz, H2-13), 3.05 (4H, t, J = 8.0 Hz, H2-15), 2.06–1.99 (4H, m, H2-12), 1.80–1.74 (4H, m, H2-16), 1.54–1.50 (4H, m, H2-17); 13C NMR (CD3OD, 100 MHz) δ 171.3 (C-1), 136.5 (C-7a), 134.1 (C-3a), 131.5 (C-2), 130.0 (C-4), 129.5 (C-8), 129.1 (C-3/C-6), 129.0 (C-3/C-6), 128.9 (C-7), 128.0 (C-5), 124.7 (C-9), 49.0 (C-15), 46.4 (C-13), 37.5 (C-11), 28.0 (C-12), 27.2 (C-16/C-17), 27.1 (C-16/C-17); (+)-HRESIMS m/z 539.3387[M+H]+ (calcd for C34H43N4O2, 539.3381).
4.2.18. N1,N7-Bis(3-(2-naphthamido)propyl)heptane-1,7-diaminium 2,2,2-trifluoroacetate (19c)
Following general procedure A, the reaction of 2-naphthoic acid (10) (42.6 mg, 0.247 mmol), di-tert-butyl heptane-1,7-diylbis((3-aminopropyl)carbamate) (16c) (50 mg, 0.11 mmol), EDC·HCl (56.1 mg, 0.293 mmol), HOBt (39.6 mg, 0.29 mmol), and DIPEA (0.118 mL, 0.677 mmol) in CH2Cl2 (1.5 mL) created di-tert-butyl heptane-1,7-diylbis((3-(2-naphthamido)propyl)carbamate) as a colorless oil (48 mg, 58%). Following general procedure C, the reaction of a sub-sample of this material (12 mg, 0.016 mmol) in CH2Cl2 (2 mL) with TFA (0.2 mL) created the di-TFA salt 19c as a colorless gum after purification (6 mg, 48%). Rf = 0.33 (RP-18, MeOH:10% HCl, 7:3); IR (ATR) vmax 3311, 2927, 2857, 1674, 1639, 1544, 1505, 1434, 1312, 1200, 1179, 1131, 876, 832, 799, 780, 762, 721 cm−1; 1H NMR (CD3OD, 400 MHz) δ 8.43 (2H, s, H-3), 8.03–7.90 (8H, m, H-4, H-7, H-8, H-9), 7.66–7.56 (4H, m, H-5, H-6), 3.59 (4H, t, J = 6.5 Hz, H2-11), 3.11 (4H, t, J = 7.0 Hz, H2-13), 3.05 (4H, t, J = 7.5 Hz, H2-15), 2.09–2.02 (4H, m, H2-12), 1.81–1.73 (4H, m, H2-16), 1.51–1.47 (6H, m, H2-17, H2-18); 13C NMR (CD3OD, 100 MHz) δ 171.2 (C-1), 136.4 (C-7a), 134.1 (C-3a), 132.2 (C-2), 130.0 (C-4), 129.5 (C-8), 129.1 (C-3/C-6), 129.0 (C-3/C-6), 128.8 (C-7), 128.0 (C-5), 124.8 (C-9), 49.0 (C-15), 46.4 (C-13), 37.5 (C-11), 29.7 (C-18), 27.9 (C-12), 27.3 (C-16/C-17), 27.2 (C-16/C-17); (+)-HRESIMS m/z 553.3520 [M+H]+ (calcd for C35H45N4O2, 553.3537).
4.2.19. N1,N8-Bis(3-(2-naphthamido)propyl)octane-1,8-diaminium 2,2,2-trifluoroacetate (19d)
Following general procedure A, the reaction of 2-naphthoic acid (10) (41.3 mg, 0.240 mmol), di-tert-butyl octane-1,8-diylbis((3-aminopropyl)carbamate) (16d) (50 mg, 0.11 mmol), EDC·HCl (54.3 mg, 0.283 mmol), HOBt (38.3 mg, 0.28 mmol), and DIPEA (0.114 mL, 0.653 mmol) in CH2Cl2 (1.5 mL) created di-tert-butyl octane-1,8-diylbis((3-(2-naphthamido)propyl)carbamate) as a colorless oil (44 mg, 52%). Following general procedure C, the reaction of a sub-sample of this material (18 mg, 0.024 mmol) in CH2Cl2 (2 mL) with TFA (0.2 mL) created the di-TFA salt 19d as a colorless gum after purification (16 mg, 84%). Rf = 0.33 (RP-18, MeOH:10% HCl, 7:3); IR (ATR) vmax 3716, 2927, 2343, 1739, 1463, 1263, 801 cm-1; 1H NMR (CD3OD, 400 MHz) δ 8.40 (2H, s, H-3), 7.99–7.88 (8H, m, H-4, H-7, H-8, H-9), 7.62–7.56 (4H, m, H-5, H-6), 3.57 (4H, t, J = 6.5 Hz, H2-11), 3.09 (4H, t, J = 7.4 Hz, H2-13), 3.02 (4H, t, J = 7.8 Hz, H2-15), 2.10–1.99 (4H, m, H2-12), 1.80–1.69 (4H, m, H2-16), 1.50–1.43 (8H, m, H2-17, H2-18); 13C NMR (CD3OD, 100 MHz) δ 171.2 (C-1), 136.4 (C-7a), 134.1 (C-3a), 132.2 (C-2), 130.0 (C-4), 129.5 (C-8), 129.1 (C-3/C-6), 129.0 (C-3/C-6), 128.9 (C-7), 128.0 (C-5), 124.7 (C-9), 49.0 (C-15), 46.4 (C-13), 37.5 (C-11), 30.0 (C-18), 27.9 (C-12), 27.5 (C-16/C-17), 27.4 (C-16/C-17); (+)-HRESIMS m/z 567.3691 [M+H]+ (calcd for C36H47N4O2, 567.3694).
4.2.20. N1,N10-Bis(3-(2-naphthamido)propyl)decane-1,10-diaminium 2,2,2-trifluoroacetate (19e)
Following general procedure A, the reaction of 2-napthoic acid (10) (78 mg, 0.45 mmol), di-tert-butyl decane-1,10-diylbis((3-aminopropyl)carbamate) (16e) (100 mg, 0.21 mmol), EDC·HCl (103 mg, 0.54 mmol), HOBt (72 mg, 0.53 mmol), and DIPEA (0.22 mL, 1.26 mmol) in CH2Cl2 (1.5 mL) created di-tert-butyl decane-1,10-diylbis((3-(2-naphthamido)propyl)carbamate) (99 mg, 59%) as a colorless oil. Following general procedure C, the reaction of a sub-sample of this material (78 mg, 0.098 mmol) in CH2Cl2 (2 mL) with TFA (0.2 mL) created the di-TFA salt 19e (76 mg, 94%) as a yellow oil with no further purification required. Rf = 0.14 (RP-18, MeOH:10% HCl, 7:3); IR (ATR) vmax 3307,2944, 2832, 1685, 1448, 1115, 1022 cm−1; 1H NMR (CD3OD, 400 MHz) δ 8.40 (2H, br s, H-3), 7.98–7.89 (8H, m, H-4, H-7, H-8, H-9), 7.62–7.54 (4H, m, H-5, H-6), 3.57 (4H, t, J = 6.5 Hz, H2-11), 3.08 (4H, t, J = 7.2 Hz, H2-13), 3.01 (4H, t, J = 7.7 Hz, H2-15), 2.07–2.00 (4H, m, H2-12), 1.75–1.67 (4H, m, H2-16), 1.43–1.34 (12H, m, H2-17, H2-18, H2-19); 13C NMR (CD3OD, 100 MHz) δ 171.1 (C-1), 136.4 (C-7a), 134.1 (C-3a), 132.2 (C-2), 130.1 (C-4), 129.5 (C-8), 129.1 (C-3/C-6), 129.0 (C-3/C-6), 128.8 (C-7), 128.0 (C-5), 124.8 (C-9), 49.0 (C-15), 46.4 (C-13), 37.6 (C-11), 30.4 (C-18/C-19), 30.2 (C-18/C-19), 27.8 (C-12), 27.5 (C-17), 27.4 (C-16); (+)-HRESIMS m/z 595.3993 [M+H]+ (calcd for C38H51N4O2, 595.4007).
4.2.21. N1,N12-Bis(3-(2-naphthamido)propyl)dodecane-1,12-diaminium 2,2,2-trifluoroacetate (19f)
Following general procedure A, the reaction of 2-naphthoic acid (10) (55 mg, 0.32 mmol), di-tert-butyl dodecane-1,12-diylbis((3-aminopropyl)carbamate) (16f) (75 mg, 0.15 mmol), EDC·HCl (73 mg, 0.38 mmol), HOBt (51 mg, 0.38 mmol), and DIPEA (0.15 mL, 0.86 mmol) in CH2Cl2 (1.5 mL) created di-tert-butyl dodecane-1,12-diylbis((3-(2-naphthamido)propyl)carbamate) (35 mg, 28%) as a colorless oil. Following general procedure C, the reaction of a sub-sample of this material (20 mg, 0.024 mmol) in CH2Cl2 (2 mL) with TFA (0.2 mL) created the di-TFA salt 19f (16 mg, 78%) as an orange oil with no further purification required. Rf = 0.12 (RP-18, MeOH:10% HCl, 7:3); IR (ATR) vmax 3308, 2945, 2833, 1655, 1450, 1246, 1115, 1022 cm−1; 1H NMR (CD3OD, 400 MHz) δ 8.43 (2H, s, H-3), 8.01–7.91 (8H, m, H-4, H-7, H-8, H-9), 7.65–7.57 (4H, m, H-5, H-6), 3.59 (4H, t, J = 6.5 Hz, H2-11), 3.11 (4H, t, J = 7.3 Hz, H2-13), 3.04 (4H, t, J = 7.8 Hz, H2-15), 2.08–2.02 (4H, m, H2-12), 1.77–1.70 (4H, m, H2-16) 1.46–1.32 (16H, m, H2-17, H2-18, H2-19, H2-20); 13C NMR (CD3OD, 100 MHz) δ 171.2 (C-1), 136.4 (C-7a), 134.1 (C-3a), 132.2 (C-2), 130.0 (C-4), 129.5 (C-8), 129.1 (C-3/C-6), 129.0 (C-3/C-6), 128.8 (C-7), 128.0 (C-5), 124.7 (C-9), 49.1 (C-15), 46.4 (C-13), 37.5 (C-11), 30.7 (C-18/C-19/C-20), 30.5 (C-18/C-19/C-20), 30.3 (C-18/C-19/C-20), 27.9 (C-12), 27.5 (C-17), 27.4 (C-16); (+)-HRESIMS m/z 623.4306 [M+H]+ (calcd for C40H55N4O2, 623.4320).
4.2.22. N1,N4-Bis(3-(4-(naphthalen-2-ylamino)-4-oxobutanamido)propyl)butane-1,4-diaminium 2,2,2-trifluoroacetate (20a)
Following general procedure B, the reaction of carboxylic acid 11 (62 mg, 0.25 mmol), di-tert-butyl butane-1,4-diylbis((3-aminopropyl)carbamate) (16a) (48 mg, 0.12 mmol), EDC·HCl (55 mg, 0.29 mmol), and DMAP (62 mg, 0.51 mmol) in CH2Cl2 (1.5 mL) created di-tert-butyl butane-1,4-diylbis((3-(4-(naphthalen-2-ylamino)-4-oxobutanamido)propyl)carbamate) as a pale-pink oil (84 mg, 82%). Following general procedure C, the reaction of a sub-sample of this material (16 mg, 0.019 mmol) in CH2Cl2 (2.0 mL) with TFA (0.2 mL) created the di-TFA salt 20a as a colorless oil after purification (15 mg, 90%). Rf = 0.28 (RP-18, MeOH:10% HCl, 7:3); IR (ATR) vmax 3278, 1676, 1202, 1160, 799, 766 cm−1; 1H NMR (CD3OD, 400 MHz) δ 8.18 (2H, d, J = 2.0 Hz, H-7), 7.80–7.73 (6H, m, H-8, H-11, H-12), 7.54 (2H, dd, J = 9.0, 2.0 Hz, H-13), 7.46–7.42 (2H, m, H-9), 7.40–7.36 (2H, m, H-10), 3.32–3.28 (4H, m, H2-15), 2.87 (4H, t, J = 7.0 Hz, H2-17), 2.80–2.77 (4H, m, H2-2), 2.70–2.66 (4H, m, H2-19), 2.61–2.58 (4H, m, H2-3), 1.84–1.77 (4H, m, H2-16), 1.46–1.42 (4H, m, H2-20); 13C NMR (CD3OD, 100 MHz) δ 176.5 (C-1), 173.2 (C-4), 137.5 (C-6), 135.2 (C-7a), 131.9 (C-11a), 129.7 (C-12), 128.7 (C-11), 128.4 (C-8), 127.6 (C-9), 126.0, (C-10), 121.2 (C-13), 117.4 (C-7), 47.9 (C-19), 45.8 (C-17), 36.4 (C-15), 32.4 (C-2), 31.3 (C-3), 27.8 (C-16), 24.0 (C-20); (+)-HRESIMS m/z 653.3823 [M+H]+ (calcd for C38H49N6O4, 653.3810).
4.2.23. N1,N6-Bis(3-(4-(naphthalen-2-ylamino)-4-oxobutanamido)propyl)hexane-1,6-diaminium 2,2,2-trifluoroacetate (20b)
Following general procedure B, the reaction of carboxylic acid 11 (71 mg, 0.29 mmol), di-tert-butyl hexane-1,6-diylbis((3-aminopropyl)carbamate) (16b) (50 mg, 0.12 mmol), EDC·HCl (62 mg, 0.32 mmol), and DMAP (71 mg, 0.58 mmol) in CH2Cl2 (1.5 mL) created di-tert-butylhexane-1,6-diylbis((3-(4-(naphthalen-2-ylamino)-4-oxobutanamido)propyl)carbamate) as a pale brown oil (53 mg, 50%). Following general procedure C, the reaction of a sub-sample of this material (26 mg, 0.030 mmol) in CH2Cl2 (2 mL) with TFA (0.2 mL) created the di-TFA salt 20b as a yellow oil after purification (24 mg, 88%). Rf = 0.32 (RP-18, MeOH:10% HCl, 7:3); IR (ATR) vmax 3279, 1671, 1542, 1201, 1132, 800, 777 cm−1; 1H NMR (CD3OD, 400 MHz) δ 8.20 (2H, d, J = 2.0 Hz, H-7), 7.78–7.71 (6H, m, H-8, H-11, H-12), 7.54 (2H, dd, J = 8.5, 2.0 Hz, H-13), 7.43 (2H, td, J = 7.5, 1.5 Hz, H-9), 7.37 (2H, td, J = 7.5, 1.5 Hz, H-10), 3.35–3.30 (4H, m, H2-15), 2.96 (4H, t, J = 6.8 Hz, H2-17), 2.80 (4H, t, J = 6.5 Hz, H2-2), 2.63–2.59 (8H, m, H2-3, H2-19), 1.88–1.82 (4H, m, H2-16), 1.31–1.27 (4H, m, H2-20), 0.83–0.81 (4H, m, H2-21); 13C NMR (CD3OD, 100 MHz) δ 176.5 (C-1), 173.2 (C-4), 137.6 (C-6), 135.3 (C-7a), 131.9 (C-11a), 129.6 (C-12), 128.7 (C-11), 128.5 (C-8), 127.6 (C-9), 126.0, (C-10), 121.1 (C-13), 117.3 (C-7), 48.7 (C-19), 45.8 (C-17), 36.4 (C-15), 32.4 (C-2), 31.2 (C-3), 27.9 (C-16), 26.8 (C-20/C-21), 26.6 (C-20/C-21); (+)-HRESIMS [M+H]+ m/z 681.4106 (calcd for C40H53N6O4, 681.4123).
4.2.24. N1,N7-Bis(3-(4-(naphthalen-2-ylamino)-4-oxobutanamido)propyl)heptane-1,7-diaminium 2,2,2-trifluoroacetate (20c)
Following general procedure B, the reaction of carboxylic acid 11 (68 mg, 0.29 mmol), di-tert-butyl heptane-1,7-diylbis((3-aminopropyl)carbamate) (16c) (50 mg, 0.11 mmol), EDC·HCl (60 mg, 0.31 mmol), and DMAP (69 mg, 0.57 mmol) in CH2Cl2 (1.5 mL) created di-tert-butyl heptane-1,7-diylbis((3-(4-(naphthalen-2-ylamino)-4-oxobutanamido)propyl)carbamate) as a colorless oil (64 mg, 65%). Following general procedure C, the reaction of a sub-sample of this material (44 mg, 0.049 mmol) in CH2Cl2 (2 mL) with TFA (0.2 mL) created the di-TFA salt 20c as a colorless oil after purification (42 mg, 93%). Rf = 0.29 (RP-18, MeOH:10% HCl, 7:3); IR (ATR) vmax 3289, 3027 1666, 1537, 1201, 1131, 800, 723 cm−1; 1H NMR (CD3OD, 400 MHz) δ 8.20 (2H, d, J = 2.0 Hz, H-7), 7.77–7.71 (6H, m, H-8, H-11, H-12), 7.54 (2H, dd, J = 9.0, 2.0 Hz, H-13), 7.42 (2H, td, J = 7.5, 1.3 Hz, H-9), 7.36 (2H, td, J = 8.3, 1.5 Hz, H-10), 3.35–3.30 (4H, m, H2-15), 3.00 (4H, t, J = 7.0 Hz, H2-17) 2.81–2.78 (4H, m, H2-2), 2.67 (4H, t, J = 8.0 Hz, H2-19), 2.62–2.59 (4H, m, H2-3), 1.89–1.83 (4H, m, H2-16), 1.37–1.29 (4H, m, H2-20), 0.81–0.76 (6H, m, H2-21, H2-22); 13C NMR (CD3OD, 100 MHz) δ 176.6 (C-1), 173.2 (C-4), 137.6 (C-6), 135.3 (C-7a), 131.9 (C-11a), 129.6 (C-12), 128.6 (C-8/C-11), 128.5 (C-8/C-11), 127.6 (C-9), 126.0, (C-10), 121.0 (C-13), 117.2 (C-7), 48.9 (C-19), 45.8 (C-17), 36.4 (C-15), 32.4 (C-2), 31.2 (C-3), 29.4 (C-22), 27.9 (C-16), 27.01 (C-20/C-21), 26.96 (C-20/C-21); (+)-HRESIMS [M+H]+ m/z 695.4283 (calcd for C41H55N6O4, 695.4279).
4.2.25. N1,N8-Bis(3-(4-(naphthalen-2-ylamino)-4-oxobutanamido)propyl)octane-1,8-diaminium 2,2,2-trifluoroacetate (20d)
Following general procedure B, the reaction of carboxylic acid 11 (67 mg, 0.27 mmol), di-tert-butyl octane-1,8-diylbis((3-aminopropyl)carbamate) (16d) (50 mg, 0.11 mmol), EDC·HCl (59 mg, 0.31 mmol) and DMAP (67 mg, 0.55 mmol) in CH2Cl2 (1.5 mL) created di-tert-butyl octane-1,8-diylbis((3-(4-(naphthalen-2-ylamino)-4-oxobutanamido)propyl)carbamate) as a colorless oil (40 mg, 40%). Following general procedure C, the reaction of this material (40 mg, 0.044 mmol) in CH2Cl2 (2 mL) with TFA (0.2 mL) created the di-TFA salt 20d as a pale purple oil after purification (37 mg, 90%). Rf = 0.28 (RP-18, MeOH:10% HCl, 7:3); IR (ATR) vmax 3288, 3061 1669, 1553, 1200, 1132, 800, 721 cm−1; 1H NMR (CD3OD, 400 MHz) δ 8.20 (2H, d, J = 2.0 Hz, H-7), 7.77–7.71 (6H, m, H-8, H-11, H-12), 7.55 (2H, dd, J = 9.0, 2.0 Hz, H-13), 7.44–7.40 (2H, m, H-9), 7.39–7.34 (2H, m, H-10), 3.36–3.30 (4H, m, H2-15), 3.01 (4H, t, J = 7.0 Hz, H2-17), 2.82–2.78 (4H, m, H2-2), 2.71 (4H, t, J = 8.0 Hz, H2-19), 2.62–2.59 (4H, m, H2-3), 1.90–1.84 (4H, m, H2-16), 1.42–1.36 (4H, m, H2-20), 0.85–0.80 (8H, m, H2-21, H2-22); 13C NMR (CD3OD, 100 MHz) δ 176.5 (C-1), 173.2 (C-4), 137.6 (C-6), 135.3 (C-7a), 131.9 (C-11a), 129.6 (C-12), 128.6 (C-8/C-11), 128.4 (C-8/C-11), 127.5 (C-9), 125.9, (C-10), 121.0 (C-13), 117.2 (C-7), 48.9 (C-19), 45.8 (C-17), 36.3 (C-15), 32.3 (C-2), 31.2 (C-3), 29.7 (C-22), 27.9 (C-16), 27.14 (C-20/C-21), 27.07 (C-20/C-21); (+)-HRESIMS [M+2H]2+ m/z 355.2252 (calcd for C42H58N6O4, 355.2254).
4.2.26. N1,N10-Bis(3-(4-(naphthalen-2-ylamino)-4-oxobutanamido)propyl)decane-1,10-diaminium 2,2,2-trifluoroacetate (20e)
Following general procedure B, the reaction of carboxylic acid 11 (63 mg, 0.26 mmol), di-tert-butyl decane-1,10-diylbis((3-aminopropyl)carbamate) (16e) (50 mg, 0.10 mmol), EDC·HCl (55 mg, 0.29 mmol), and DMAP (63 mg, 0.52 mmol) in CH2Cl2 (1.5 mL) created di-tert-butyl decane-1,10-diylbis((3-(4-(naphthalen-2-ylamino)-4-oxobutanamido)propyl)carbamate) as a colorless oil (78 mg, 83%). Following general procedure C, the reaction of a sub-sample of this material (41 mg, 0.044 mmol) in CH2Cl2 (2.0 mL) with TFA (0.2 mL) created the di-TFA salt 20e as a colorless oil after purification (38 mg, 89%). Rf = 0.17 (RP-18, MeOH: 10% HCl, 7:3); IR (ATR) vmax 3293, 1679, 1538, 1202, 1132, 721 cm−1; 1H NMR (CD3OD, 400 MHz) δ 8.21 (2H, d, J = 2.0 Hz, H-7), 7.78–7.72 (6H, m, H-8, H-11, H-12), 7.55 (2H, dd, J = 8.5, 2.0 Hz, H-13), 7.44–7.39 (2H, m, H-9), 7.37–7.33 (2H, m, H-10), 3.36–3.30 (4H, m, H2-15), 3.02 (4H, t, J = 7.0 Hz, H2-17), 2.81–2.74 (8H, m, H2-2, H2-19), 2.62–2.59 (4H, m, H2-3), 1.90–1.83 (4H, m, H2-16), 1.49–1.41 (4H, m, H2-20), 0.96–0.88 (12H, m, H2-21, H2-22, H2-23); 13C NMR (CD3OD, 100 MHz) δ 176.5 (C-1), 173.2 (C-4), 137.7 (C-6), 135.3 (C-7a), 132.0 (C-11a), 129.7 (C-12), 128.7 (C-11), 128.5 (C-8), 127.6 (C-9), 126.0 (C-10), 121.1 (C-13), 117.3 (C-7), 49.1 (C-19), 45.9 (C-17), 36.4 (C-15), 32.4 (C-2), 31.3 (C-3), 30.2 (C-23), 30.0 (C-22), 27.9 (C-16), 27.32 (C-20/C-21), 27.28 (C-20/C-21); (+)-HRESIMS m/z 737.4736 [M+H]+ (calcd for C44H61N6O4, 737.4749).
4.2.27. N1,N12-Bis(3-(4-(naphthalen-2-ylamino)-4-oxobutanamido)propyl)dodecane-1,12-diaminium 2,2,2-trifluoroacetate (20f)
Following general procedure B, the reaction of carboxylic acid 11 (58 mg, 0.24 mmol), di-tert-butyl dodecane-1,12-diylbis((3-aminopropyl)carbamate) (16f) (57 mg, 0.11 mmol), EDC·HCl (52 mg, 0.27 mmol), and DMAP (60 mg, 0.49 mmol) in CH2Cl2 (1.5 mL) created di-tert-butyl dodecane-1,12-diylbis((3-(4-(naphthalen-2-ylamino)-4-oxobutanamido)propyl)carbamate) as a colorless oil (81 mg, 76%). Following general procedure C, the reaction of a sub-sample of this material (28 mg, 0.029 mmol) in CH2Cl2 (2 mL) with TFA (0.2 mL) created the di-TFA salt 20f as a colorless oil after purification (26 mg, 90%). Rf = 0.13 (RP-18, MeOH:10% HCl, 7:3); IR (ATR) vmax 2916, 1769, 1671, 1503, 1202, 1037, 802 cm−1; 1H NMR (CD3OD, 400 MHz) δ 8.21 (2H, d, J = 1.5 Hz, H-7), 7.79–7.72 (6H, m, H-8, H-11, H-12), 7.56 (2H, dd, J = 9.0, 2.0 Hz, H-13), 7.44–7.40 (2H, m, H-9), 7.38–7.34 (2H, m, H-10), 3.36–3.30 (4H, m, H2-15), 3.02 (4H, t, J = 7.0 Hz, H2-17), 2.81–2.75 (4H, m, H2-2, H2-19), 2.62–2.59 (4H, m, H2-3), 1.90–1.84 (4H, m, H2-16), 1.51–1.44 (4H, m, H2-20), 1.04–0.99 (16H, m, H2-21, H2-22, H2-23, H2-24); 13C NMR (CD3OD, 100 MHz) δ 176.5 (C-1), 173.2 (C-4), 137.6 (C-6), 135.3 (C-7a), 131.9 (C-11a), 129.6 (C-12), 128.6 (C-8/C-11), 128.4 (C-8/C-11), 127.5 (C-9), 125.9 (C-10), 121.0 (C-13), 117.2 (C-7), 49.1 (C-19), 45.8 (C-17), 36.4 (C-15), 32.4 (C-2), 31.2 (C-3), 30.4 (C-22/C-23/C-24), 30.3 (C-22/C-23/C-24), 30.1 (C-22/C-23/C-24), 27.9 (C-16), 27.3 (C-20/C-21), 27.2 (C-20/C-21); (+)-HRESIMS [M+2H]2+ m/z 383.2578 (calcd for C46H66N6O4, 383.2567).
4.2.28. N1,N4-Bis(3-(4-([1,1’-biphenyl]-4-ylamino)-4-oxobutanamido)propyl)butane-1,4-diaminium 2,2,2-trifluoroacetate (21a)
Following general procedure A, the reaction of carboxylic acid 12 (74 mg, 0.27 mmol) with di-tert-butyl butane-1,4-diylbis((3-aminopropyl)carbamate) (16a) (50 mg, 0.12 mmol), EDC·HCl (62 mg, 0.32 mmol), HOBt (44 mg, 0.32 mmol), and DIPEA (0.13 mL, 0.75 mmol) in CH2Cl2 (1.5 mL) created di-tert-butyl butane-1,4-diylbis((3-(4-([1,1’-biphenyl]-4-ylamino)-4-oxobutanamido)propyl)carbamate) as a pink solid (65 mg, 60%). Following general procedure C, the reaction of a sub-sample of this material (20 mg, 0.022 mmol) in CH2Cl2 (2 mL) with TFA (0.2 mL) created the di-TFA salt 21a as a yellow solid after purification (15 mg, 73%). Rf = 0.37 (RP-18, MeOH:10% HCl, 5:1); m.p. 203–205 °C; IR (ATR) vmax 3290, 3099, 3033, 2831, 1669, 1599, 1531, 1200, 1178, 1130, 835, 764, 720 cm−1; 1H NMR (DMSO-d6, 400 MHz) δ 10.08 (2H, br s, NH-5), 8.61–8.47 (4H, m, NH2-18), 8.11 (2H, t, J = 6.0 Hz, NH-14), 7.71–7.68 (4H, m, H-7), 7.65–7.58 (8H, m, H-8, H-11), 7.46–7.40 (4H, m, H-12), 7.35–7.29 (2H, m, H-13), 3.13 (4H, dt, J = 6.4, 6.2 Hz, H2-15), 2.93–2.82 (8H, m, H2-17, H2-19), 2.61 (4H, t, J = 7.0 Hz, H2-2/H2-3), 2.43 (4H, t, J = 7.0 Hz, H2-2/H2-3), 1.72 (4H, tt, J = 7.4, 6.4 Hz, H2-16), 1.63–1.54 (4H, m, H2-20); 13C NMR (DMSO-d6, 100 MHz) δ 172.0 (C-1), 170.5 (C-4), 139.7 (C-10), 138.8 (C-6), 134.6 (C-9), 128.9 (C-12), 127.0 (C-13), 126.8 (C-8), 126.2 (C-11), 119.3 (C-7), 46.1 (C-19), 44.5 (C-17), 35.5 (C-15), 31.5 (C-2/C-3), 30.1 (C-2/C-3), 26.1 (C-16), 22.6 (C-20); (+)-HRESIMS m/z 705.4118 [M+H]+ (calcd for C42H53N6O4, 705.4123).
4.2.29. N1,N6-Bis(3-(4-([1,1’-biphenyl]-4-ylamino)-4-oxobutanamido)propyl)hexane-1,6-diaminium 2,2,2-trifluoroacetate (21b)
Following general procedure A, the reaction of carboxylic acid 12 (69 mg, 0.26 mmol) with di-tert-butyl hexane-1,6-diylbis((3-aminopropyl)carbamate) (16b) (50 mg, 0.12 mmol), EDC·HCl (58 mg, 0.30 mmol), HOBt (41 mg, 0.30 mmol), and DIPEA (0.12 mL, 0.69 mmol) in CH2Cl2 (1.5 mL) created di-tert-butyl hexane-1,6-diylbis((3-(4-([1,1’-biphenyl]-4-ylamino)-4-oxobutanamido)propyl)carbamate) as a pink solid (54 mg, 48%). Following general procedure C, the reaction of a sub-sample of this material (20 mg, 0.021 mmol) in CH2Cl2 (2 mL) with TFA (0.2 mL) created the di-TFA salt 21b as a yellow solid after purification (15 mg, 74%). Rf = 0.43 (RP-18, MeOH:10% HCl, 5:1); m.p. 200–202 °C; IR (ATR) vmax 3296, 3031, 2947, 2832, 2528, 1681, 1657, 1637, 1529, 1198, 1173, 1123, 1000, 759, 718 cm−1; 1H NMR (DMSO-d6, 400 MHz) δ 10.08 (2H, br s, NH-5), 8.54–8.29 (4H, m, NH2-18), 8.12 (2H, t, J = 5.8 Hz, NH-14), 7.71–7.65 (4H, m, H-7), 7.65–7.58 (8H, m, H-8, H-11), 7.46–7.40 (4H, m, H-12), 7.35–7.29 (2H, m, H-13), 3.14 (4H, dt, J = 6.4, 6.2 Hz, H2-15), 2.86 (4H, t, J = 7.2 Hz, H2-17), 2.77 (4H, t, J = 7.8 Hz, H2-19), 2.61 (4H, t, J = 6.8 Hz, H2-2/H2-3), 2.44 (4H, t, J = 6.8 Hz, H2-2/H2-3), 1.71 (4H, tt, J = 7.2, 6.7 Hz, H2-16), 1.53–1.43 (4H, m, H2-20), 1.23–1.15 (4H, m, H2-21); 13C NMR (DMSO-d6, 100 MHz) δ 172.1 (C-1), 170.6 (C-4), 139.7 (C-10), 138.8 (C-6), 134.5 (C-9), 128.9 (C-12), 127.0 (C-13), 126.8 (C-8), 126.1 (C-11), 119.2 (C-7), 46.7 (C-19), 44.5 (C-17), 35.4 (C-15), 31.4 (C-2/C-3), 30.1 (C-2/C-3), 26.2 (C-16), 25.4 (C-21), 25.3 (C-20); (+)-HRESIMS m/z 733.4415 [M+H]+ (calcd for C44H57N6O4, 733.4436).
4.2.30. N1,N7-Bis(3-(4-([1,1’-biphenyl]-4-ylamino)-4-oxobutanamido)propyl)heptane-1,7-diaminium 2,2,2-trifluoroacetate (21c)
Following general procedure A, the reaction of carboxylic acid 12 (66 mg, 0.25 mmol) with di-tert-butyl heptane-1,7-diylbis((3-aminopropyl)carbamate) (16c) (50 mg, 0.11 mmol), EDC·HCl (56 mg, 0.29 mmol), HOBt (39 mg, 0.29 mmol), and DIPEA (0.12 mL, 0.69 mmol) in CH2Cl2 (1.5 mL) created di-tert-butyl heptane-1,7-diylbis((3-(4-([1,1’-biphenyl]-4-ylamino)-4-oxobutanamido)propyl)carbamate) as a pink solid (52 mg, 50%). Following general procedure C, the reaction of a sub-sample of this material (20 mg, 0.021 mmol) in CH2Cl2 (2 mL) with TFA (0.2 mL) created the di-TFA salt 21c as a yellow solid after purification (14 mg, 68%). Rf = 0.51 (RP-18, MeOH:10% HCl, 5:1); m.p. 178–180 °C; IR (ATR) vmax 3288, 3034, 2939, 2859, 2527, 1658, 1637, 1533, 1198, 1173, 1134, 763, 719 cm−1; 1H NMR (DMSO-d6, 400 MHz) δ 10.08 (2H, br s, NH-5), 8.48–8.33 (4H, m, NH2-18), 8.12 (2H, t, J = 5.8 Hz, NH-14), 7.71–7.65 (4H, m, H-7), 7.65–7.57 (8H, m, H-8, H-11), 7.46–7.40 (4H, m, H-12), 7.35–7.29 (2H, m, H-13), 3.14 (4H, dt, J = 6.4, 6.2 Hz, H2-15), 2.86 (4H, t, J = 7.0 Hz, H2-17), 2.75 (4H, t, J = 7.0 Hz, H2-19), 2.61 (4H, t, J = 6.6 Hz, H2-2/H2-3), 2.44 (4H, t, J = 6.6 Hz, H2-2/H2-3), 1.71 (4H, tt, J = 7.0, 6.4 Hz, H2-16), 1.53–1.42 (4H, m, H2-20), 1.20–1.10 (6H, m, H2-21, H2-22); 13C NMR (DMSO-d6, 100 MHz) δ 172.3 (C-1), 170.6 (C-4), 139.7 (C-10), 138.8 (C-6), 134.5 (C-9), 128.9 (C-12), 127.0 (C-13), 126.8 (C-8), 126.1 (C-11), 119.2 (C-7), 46.7 (C-19), 44.4 (C-17), 35.4 (C-15), 31.4 (C-2/C-3), 30.1 (C-2/C-3), 28.0 (C-21/C-22), 26.2 (C-16), 25.7 (C-21/C-22), 25.4 (C-20); (+)-HRESIMS m/z 747.4566 [M+H]+ (calcd for C45H59N6O4, 747.4592).
4.2.31. N1,N8-Bis(3-(4-([1,1’-biphenyl]-4-ylamino)-4-oxobutanamido)propyl)octane-1,8-diaminium 2,2,2-trifluoroacetate (21d)
Following general procedure A, the reaction of carboxylic acid 12 (65 mg, 0.24 mmol) with di-tert-butyl octane-1,8-diylbis((3-aminopropyl)carbamate) (16d) (50 mg, 0.11 mmol), EDC·HCl (54 mg, 0.28 mmol), HOBt (38 mg, 0.28 mmol), and DIPEA (0.11 mL, 0.63 mmol) in CH2Cl2 (1.5 mL) created di-tert-butyl octane-1,8-diylbis((3-(4-([1,1’-biphenyl]-4-ylamino)-4-oxobutanamido)propyl)carbamate) as a pink solid (56 mg, 53%). Following general procedure C, the reaction of a sub-sample of this material (20 mg, 0.021 mmol) in CH2Cl2 (2 mL) with TFA (0.2 mL) created the di-TFA salt 21d as a yellow solid after purification (14 mg, 67%). Rf = 0.51 (RP-18, MeOH:10% HCl, 5:1); m.p. 182–184 °C; IR (ATR) vmax 3411, 3292, 3049, 2937, 2860, 2255, 1674, 1534, 1200, 1173, 1129, 1024, 1003, 764, 719 cm−1; 1H NMR (DMSO-d6, 400 MHz) δ 10.08 (2H, br s, NH-5), 8.48–8.34 (4H, m, NH2-18), 8.13 (2H, t, J = 6.0 Hz, NH-14), 7.71–7.65 (4H, m, H-7), 7.65–7.57 (8H, m, H-8, H-11), 7.46–7.40 (4H, m, H-12), 7.35–7.29 (2H, m, H-13), 3.15 (4H, dt, J = 6.4, 6.0 Hz, H2-15), 2.87 (4H, t, J = 7.0 Hz, H2-17), 2.76 (4H, t, J = 7.5 Hz, H2-19), 2.61 (4H, t, J = 6.8 Hz, H2-2/H2-3), 2.43 (4H, t, J = 6.8 Hz, H2-2/H2-3), 1.71 (4H, tt, J = 7.0, 6.4 Hz, H2-16), 1.53–1.41 (4H, m, H2-20), 1.19–1.08 (8H, m, H2-21, H2-22); 13C NMR (DMSO-d6, 100 MHz) δ 172.2 (C-1), 170.6 (C-4), 139.7 (C-10), 138.8 (C-6), 134.5 (C-9), 128.9 (C-12), 127.0 (C-13), 126.8 (C-8), 126.1 (C-11), 119.2 (C-7), 46.8 (C-19), 44.4 (C-17), 35.4 (C-15), 31.4 (C-2/C-3), 30.0 (C-2/C-3), 28.3 (C-21/C-22), 26.2 (C-16), 25.8 (C-21/C-22), 25.4 (C-20); (+)-HRESIMS m/z 761.4739 [M+H]+ (calcd for C46H61N6O4, 761.4749).
4.2.32. N1,N10-Bis(3-(4-([1,1’-biphenyl]-4-ylamino)-4-oxobutanamido)propyl)decane-1,10-diaminium 2,2,2-trifluoroacetate (21e)
Following general procedure A, the reaction of carboxylic acid 12 (61 mg, 0.23 mmol) with di-tert-butyl decane-1,10-diylbis((3-aminopropyl)carbamate) (16e) (50 mg, 0.10 mmol), EDC·HCl (51 mg, 0.27 mmol), HOBt (36 mg, 0.27 mmol), and DIPEA (0.11 mL, 0.63 mmol) in CH2Cl2 (1.5 mL) created di-tert-butyl decane-1,10-diylbis((3-(4-([1,1’-biphenyl]-4-ylamino)-4-oxobutanamido)propyl)carbamate) as a pale yellow solid (56 mg, 57%). Following general procedure C, the reaction of a sub-sample of this material (20 mg, 0.020 mmol) in CH2Cl2 (2 mL) with TFA (0.2 mL) created the di-TFA salt 21e as a yellow solid after purification (15 mg, 74%). Rf = 0.34 (RP-18, MeOH:10% HCl, 5:1); m.p. 184–186 °C; IR (ATR) vmax 3287, 3099, 3033, 2930, 2854, 1668, 1599, 1533, 1200, 1175, 1130, 764, 720 cm−1; 1H NMR (DMSO-d6, 400 MHz) δ 10.09 (2H, br s, NH-5), 8.49–8.37 (4H, m, NH2-18), 8.14 (2H, t, J = 6.0 Hz, NH-14), 7.71–7.66 (4H, m, H-7), 7.64–7.57 (8H, m, H-8, H-11), 7.46–7.39 (4H, m, H-12), 7.34–7.28 (2H, m, H-13), 3.15 (4H, dt, J = 6.4, 6.2 Hz, H2-15), 2.93–2.83 (4H, m, H2-17), 2.82–2.72 (4H, m, H2-19), 2.62 (4H, t, J = 6.8 Hz, H2-2/H2-3), 2.44 (4H, t, J = 6.8 Hz, H2-2/H2-3), 1.72 (4H, tt, J = 7.0, 6.4 Hz, H2-16), 1.53–1.42 (4H, m, H2-20), 1.20–1.07 (12H, m, H2-21, H2-22, H2-23); 13C NMR (DMSO-d6, 100 MHz) δ 172.3 (C-1), 170.6 (C-4), 139.7 (C-10), 138.8 (C-6), 134.5 (C-9), 128.9 (C-12), 127.0 (C-13), 126.8 (C-8), 126.1 (C-11), 119.2 (C-7), 46.9 (C-19), 44.4 (C-17), 35.4 (C-15), 31.4 (C-2/C-3), 30.1 (C-2/C-3), 28.8 (C-21/C-22/C-23), 28.5 (C-21/C-22/C-23), 26.2 (C-16), 25.9 (C-21/C-22/C-23), 25.5 (C-20); (+)-HRESIMS m/z 789.5055 [M+H]+ (calcd for C48H65N6O4, 789.5062).