Antifungal Biofilm Inhibitory Effects of Combinations of Diclofenac and Essential Oils
Abstract
:1. Introduction
2. Results
2.1. EOs’ Chemical Compositions
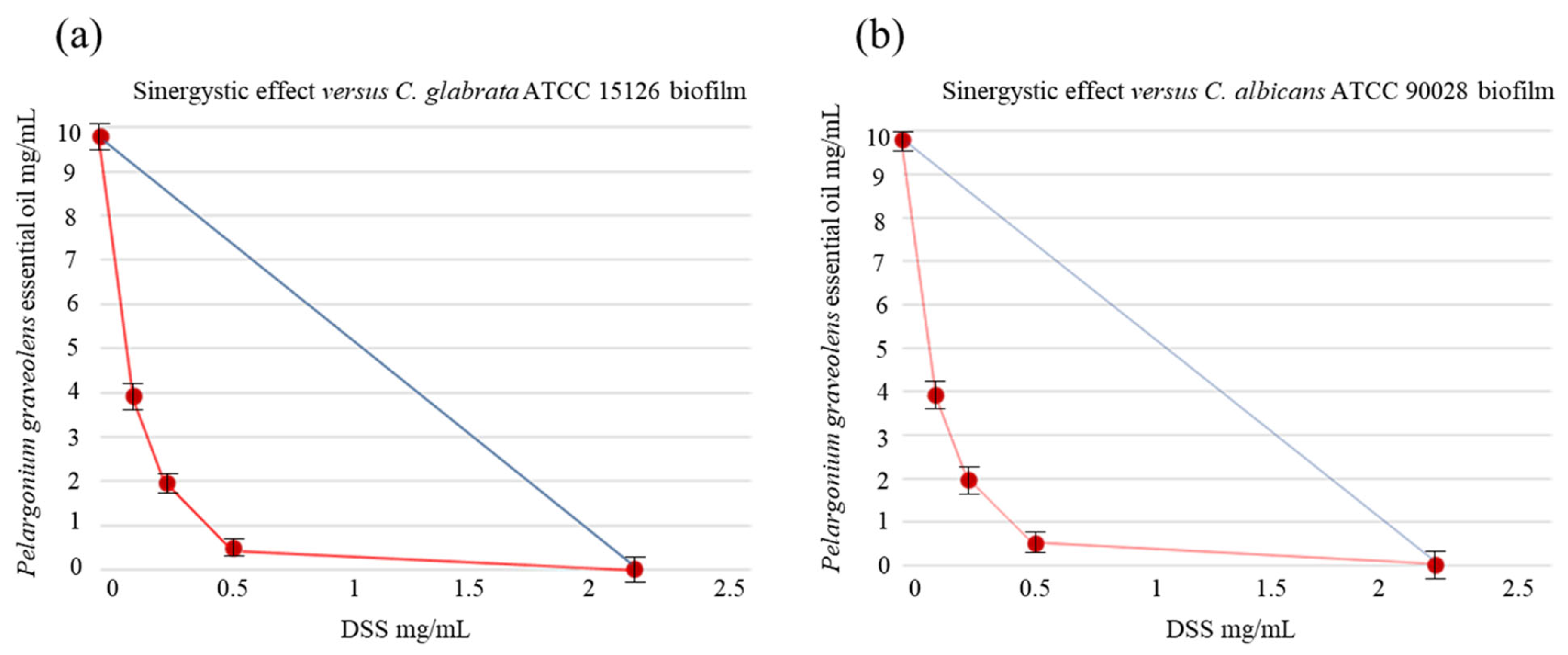
2.2. Antifungal Activity
3. Discussion
4. Material and Methods
4.1. Materials
4.1.1. Essential Oils
4.1.2. Chemicals
4.1.3. Fungal Strains
4.2. Methods
4.2.1. Fungal Strains and Antifungal Testing
4.2.2. Medium and Culture Conditions
4.2.3. Preparation of the Test Solution
4.2.4. Biofilm Biomass Measurement and Reduction
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Dadar, M.; Tiwari, R.; Karthik, K.; Chakraborty, S.; Shahali, Y.; Dhama, K. Candida albicans-Biology, molecular characterization, pathogenicity, and advances in diagnosis and control—An update. Microb. Pathog. 2018, 117, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Nami, S.; Mohammadi, R.; Vakili, M.; Khezripour, K.; Mirzaei, H.; Morovati, H. Fungal vaccines, mechanism of actions and immunology: A comprehensive review. Biomed. Pharmacother. 2019, 109, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Rodrigues, C.F.; Araújo, D.; Rodrigues, M.E.; Henriques, M. Candida Species Biofilms’ Antifungal Resistance. J. Fungi 2017, 3, 8. [Google Scholar] [CrossRef] [PubMed]
- Spampinato, C.; Leonardi, D. Candida infections, causes, targets, and resistance mechanisms: Traditional and alternative antifungal agents. BioMed Res. Int. 2013, 2013, 204237. [Google Scholar] [CrossRef]
- Rodrigues, M.E.; Gomes, F.; Rodrigues, C.F. Candida spp./bacteria mixed biofilms. J. Fungi 2019, 6, 5. [Google Scholar] [CrossRef] [PubMed]
- Ellis, D. Amphotericin B: Spectrum and resistance. J. Antimicrob. Chemother. 2002, 49 (Suppl. 1), 7–10. [Google Scholar] [CrossRef]
- Roemer, T.; Krysan, D.J. Antifungal drug development: Challenges, unmet clinical needs, and new approaches. Cold Spring Harb. Perspect. 2014, 4, a019703. [Google Scholar] [CrossRef]
- Kaur, J.; Nobile, C.J. Antifungal drug-resistance mechanisms in Candida biofilms. Curr. Opin. Microbiol. 2023, 71, 102237. [Google Scholar] [CrossRef]
- Berman, J.; Krysan, D.J. Drug resistance and tolerance in fungi. Nat. Rev. Microbiol. 2020, 18, 319–331. [Google Scholar] [CrossRef]
- Harriott, M.M.; Noverr, M.C. Candida albicans and Staphylococcus aureus form polymicrobial biofilms: Effects on antimicrobial resistance. Antimicrob. Agents Chemother. 2009, 53, 3914–3922. [Google Scholar] [CrossRef]
- de Barros, P.P.; Rossoni, R.D.; de Souza, C.M.; Scorzoni, L.; Fenley, J.D.C.; Junqueira, J.C. Candida biofilms: An update on developmental mechanisms and therapeutic challenges. Mycopathologia 2020, 185, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Moraes, D.C.; Ferreira-Pereira, A. Insights on the anticandidal activity of non-antifungal drugs. J. Mycol. Med. 2019, 29, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Foletto, V.S.; da Rosa, T.F.; Serafin, M.B.; Bottega, A.; Hörner, R. Repositioning of non-antibiotic drugs as an alternative to microbial resistance: A systematic review. Int. Antimicrob. Agents 2021, 58, 106380. [Google Scholar] [CrossRef] [PubMed]
- Barbarossa, A.; Rosato, A.; Corbo, F.; Clodoveo, M.L.; Fracchiolla, G.; Carrieri, A.; Carocci, A. Non-Antibiotic Drug Repositioning as an Alternative Antimicrobial Approach. Antibiotics 2022, 11, 816. [Google Scholar] [CrossRef] [PubMed]
- Paes Leme, R.C.; da Silva, R.B. Antimicrobial activity of non-steroidal anti-inflammatory drugs on biofilm: Current evidence and potential for drug repurposing. Front. Microbiol. 2021, 12, 707629. [Google Scholar] [CrossRef]
- Babaei, F.; Mirzababaei, M.; Tavakkoli, A.; Nassiri-Asl, M.; Hosseinzadeh, H. Can nonsteroidal anti-inflammatory drugs (NSAIDs) be repurposed for fungal infection? Naunyn-Schmiedebergs Arch. Pharmacol. 2023, 1–17. [Google Scholar] [CrossRef]
- Arif, T.; Bhosale, J.D.; Kumar, N.; Mandal, T.K.; Bendre, R.S.; Lavekar, G.S.; Dabur, R. Natural products—Antifungal agents derived from plants. J. Asian Nat. Prod. Res. 2009, 11, 621–638. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; Coppola, R.; De Feo, V. Essential oils and antifungal activity. Pharmaceuticals 2017, 10, 86. [Google Scholar] [CrossRef]
- Angane, M.; Swift, S.; Huang, K.; Butts, C.A.; Quek, S.Y. Essential oils and their major components: An updated review on antimicrobial activities, mechanism of action and their potential application in the food industry. Foods 2022, 11, 464. [Google Scholar] [CrossRef]
- Hyldgaard, M.; Mygind, T.; Rikke, L.M. Essential oils in food preservation: Mode of action, synergies, and interactions with food matrix components. Front. Microbiol. 2012, 3, 12. [Google Scholar] [CrossRef]
- El-Tarabily, K.A.; El-Saadony, M.T.; Alagawany, M.; Arif, M.; Batiha, G.E.; Khafaga, A.F.; Elwan, H.A.M.; Elnesr, S.S.; El-Hack, M.E.A. Using essential oils to overcome bacterial biofilm formation and their antimicrobial resistance. Saudi J. Biol. Sci. 2021, 28, 5145–5156. [Google Scholar] [CrossRef] [PubMed]
- Ayaz, M.; Ullah, F.; Sadiq, A.; Ullah, F.; Ovais, M.; Ahmed, J.; Devkota, H.P. Synergistic interactions of phytochemicals with antimicrobial agents: Potential strategy to counteract drug resistance. Chem. Biol. Interac. 2019, 308, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Rosato, A.; Sblano, S.; Salvagno, L.; Carocci, A.; Clodoveo, M.L.; Corbo, F.; Fracchiolla, G. Anti-biofilm inhibitory synergistic effects of combinations of essential oils and antibiotics. Antibiotics 2020, 9, 637. [Google Scholar] [CrossRef]
- Barbarossa, A.; Sblano, S.; Rosato, A.; Carrieri, A.; Corbo, F.; Clodoveo, M.L.; Fracchiolla, G.; Carocci, A. Synergistic action of Cinnamomum verum essential oil with sertraline. Antibiotics 2022, 11, 1617. [Google Scholar] [CrossRef]
- Rosato, A.; Altini, E.; Sblano, S.; Salvagno, L.; Maggi, F.; de Michele, G.; Carocci, A.; Clodoveo, M.L.; Corbo, F.; Fracchiolla, G. Synergistic Activity of New Diclofenac and Essential Oils Combinations against Different Candida spp. Antibiotics 2021, 10, 688. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Qu, X.; Tang, H.; Wang, Y.; Yang, H.; Yuan, W.; Yue, B. Diclofenac Resensitizes Methicillin-Resistant Staphylococcus aureus to β-Lactams and Prevents Implant Infections. Adv. Sci. 2021, 8, 2100681. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, H.A.; da Silva, C.R.; de Andrade Neto, J.B.; do Av Sá, L.G.; do Nascimento, F.B.; Moreno, L.S.; Barroso, F.D.; da Silva, L.J.; Cândido, T.M.; de Oliveira, L.C.; et al. Synergistic activity of diclofenac sodium with oxacillin against planktonic cells and biofilm of methicillin-resistant Staphylococcus aureus strains. Future Microbiol. 2021, 16, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Brilhante, R.S.N.; Brasil, J.A.; Oliveira, J.S.D.; Pereira, V.S.; Pereira-Neto, W.D.A.; Sidrim, J.J.C.; Rocha, M.F.G. Diclofenac exhibits synergism with azoles against planktonic cells and biofilms of Candida tropicalis. Biofouling 2020, 36, 528–536. [Google Scholar] [CrossRef]
- Santos, A.L.S.; de Mello, T.P.; de Souza, R.L.; Branquinha, M.H. Biofilm: A robust and efficient barrier to antifungal chemotherapy. J. Antimicrob. Agents 2015, 1, e101. [Google Scholar] [CrossRef]
- Shariati, A.; Didehdar, M.; Razavi, S.; Heidary, M.; Soroush, F.; Chegini, Z. Natural Compounds: A Hopeful Promise as an Antibiofilm Agent Against Candida Species. Front. Pharmacol. 2022, 13, 917787. [Google Scholar] [CrossRef]
- Tardugno, R.; Spagnoletti, A.; Grandini, A.; Maresca, I.; Sacchetti, G.; Pellati, F.; Benvenuti, S. Chemical profile and biological activities of Cedrelopsis grevei H. Baillon bark essential oil. Plant Biosyst. 2018, 152, 120–129. [Google Scholar] [CrossRef]
- Giske, C.G.; Turnidge, J.; Cantón, R.; Kahlmeter, G. Update from the European committee on antimicrobial susceptibility testing (EUCAST). J. Clin. Microbiol. 2022, 60, e00276-21. [Google Scholar] [CrossRef]
- Eloff, J.N. Quantifying the bioactivity of plant extracts during screening and bioassay-guided fractionation. Phytomedicine 2004, 11, 370–371. [Google Scholar] [CrossRef]
- Williamson, E.M. Synergy and other interactions in phytomedicines. Phytomedicine 2001, 8, 401–409. [Google Scholar] [CrossRef]
- White, D.C.; Arrage, A.A.; Nivens, D.E.; Palmer, R.J.; Rice, J.F.; Sayler, G.S. Biofilm ecology: On-line methods bring new insights into mic and microbial biofouling. Biofouling 1996, 10, 3–16. [Google Scholar] [CrossRef]
- Stepanović, S.; Vuković, D.; Dakić, I.; Savić, B.; Švabić-Vlahović, M. A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J. Microbiol. Methods 2000, 40, 175–179. [Google Scholar] [CrossRef]

| EO mg/mL | DSS mg/mL | Synergism | Anidulafungin µg/mL | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Strains | Essential Oil | sMIC50 a | %Red ± SD b | sMIC50 c | %Red ± SD d | EO mg/mL e | DSS mg/mL f | DSS + EO %Red ± SD g | FICI h | sMIC5 i,c | %Red ± SD l |
| C. albicans ATCC 10231 | M. alternifolia | 9.4 | 51.00 ± 0.82 | 1.25 | 40.00 ± 0.82 | 1.2 | 0.5 | 43.00 ± 0.82 | 0.53 | 2.00 | 33.98 ± 0.41 |
| M. piperita | 4.9 | 68.00 ± 2.04 | 1.23 | 0.25 | 60.77 ± 0.21 | 0.45 | |||||
| P. graveolens | 9.6 | 45.50 ± 0.85 | 2.40 | 0.13 | 58.87 ± 0.64 | 0.35 | |||||
| C. albicans ATCC 90028 | M. alternifolia | 9.4 | 53.00 ± 0.47 | 1.25 | 63.00 ± 1.25 | 2.4 | 0.5 | 48.47 ± 0.41 | 0.65 | 2.00 | 16.09 ± 0.21 |
| M. piperita | 9.8 | 45.50 ± 0.85 | 2.45 | 0.25 | 66.27 ± 0.52 | 0.45 | |||||
| P. graveolens | 9.6 | 64.80 ± 0.08 | 1.20 | 0.3 | 74.27 ± 0.21 | 0.33 | |||||
| C. glabrata ATCC 15126 | M. alternifolia | 9.4 | 44.30 ± 0.92 | 2.05 | 52.05 ± 0.76 | 2.35 | 0.82 | 48.80 ± 0.22 | 0.65 | 2.00 | 27.41 ± 0.64 |
| M. piperita | 9.8 | 49.30 ± 0.17 | 2.45 | 0.41 | 74.10 ± 0.12 | 0.45 | |||||
| P. graveolens | 9.6 | 62.44 ± 1.29 | 1.20 | 0.21 | 56.02 ± 0.73 | 0.23 | |||||
| C. kefyr ATCC 204093 | M. alternifolia | 4.7 | 45.50 ± 0.39 | 2.05 | 53.24 ± 1.13 | 1.18 | 0.41 | 45.60 ± 0.43 | 0.45 | 2.00 | 51.24 ± 0.41 |
| M. piperita | 9.8 | 68.00 ± 0.71 | 0.31 | 0.82 | 57.14 ± 0.69 | 0.43 | |||||
| P. graveolens | 9.6 | 46.00 ± 0.85 | 1.20 | 0.41 | 58.50 ± 0.41 | 0.33 | |||||
| C. krusei ATCC 14243 | M. alternifolia | 9.4 | 44.00 ± 0.47 | 2.05 | 42.00 ± 0.82 | 2.35 | 0.41 | 50.00 ± 0.82 | 0.45 | 2.00 | 47.51 ± 0.88 |
| M. piperita | 4.9 | 55.60 ± 2.05 | 0.61 | 0.41 | 65.67 ± 0.34 | 0.33 | |||||
| P. graveolens | 4.8 | 68.00 ± 0.95 | 1.20 | 0.10 | 54.76 ± 0.88 | 0.30 | |||||
| C. krusei ATCC 6528 | M. alternifolia | 9.4 | 46.00 ± 4.75 | 2.05 | 41.33 ± 0.47 | 2.35 | 0.82 | 55.23 ± 1.60 | 0.65 | 2.00 | 28.64 ± 0.30 |
| M. piperita | 4.9 | 44.00 ± 2.50 | 1.23 | 0.10 | 75.16 ± 0.24 | 0.30 | |||||
| P. graveolens | 9.6 | 53.00 ± 0.94 | 2.40 | 0.41 | 55.65 ± 1.71 | 0.45 | |||||
| C. tropicalis ATCC 750 | M. alternifolia | 4.7 | 4.00 ± 0.54 | 2.05 | 46.00 ± 0.82 | 0.59 | 0.82 | 55.20 ± 0.51 | 0.53 | 2.00 | 32.44 ± 0.67 |
| M. piperita | 9.8 | 63.76 ± 0.87 | 2.45 | 0.10 | 57.70 ± 0.36 | 0.30 | |||||
| P. graveolens | 4.8 | 76.10 ± 0.29 | 0.60 | 0.21 | 75.41 ± 0.31 | 0.23 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbarossa, A.; Rosato, A.; Carrieri, A.; Tardugno, R.; Corbo, F.; Clodoveo, M.L.; Fracchiolla, G.; Carocci, A. Antifungal Biofilm Inhibitory Effects of Combinations of Diclofenac and Essential Oils. Antibiotics 2023, 12, 1673. https://doi.org/10.3390/antibiotics12121673
Barbarossa A, Rosato A, Carrieri A, Tardugno R, Corbo F, Clodoveo ML, Fracchiolla G, Carocci A. Antifungal Biofilm Inhibitory Effects of Combinations of Diclofenac and Essential Oils. Antibiotics. 2023; 12(12):1673. https://doi.org/10.3390/antibiotics12121673
Chicago/Turabian StyleBarbarossa, Alexia, Antonio Rosato, Antonio Carrieri, Roberta Tardugno, Filomena Corbo, Maria Lisa Clodoveo, Giuseppe Fracchiolla, and Alessia Carocci. 2023. "Antifungal Biofilm Inhibitory Effects of Combinations of Diclofenac and Essential Oils" Antibiotics 12, no. 12: 1673. https://doi.org/10.3390/antibiotics12121673







