In Vivo-Acquired Resistance to Daptomycin during Methicillin-Resistant Staphylococcus aureus Bacteremia
Abstract
:1. Introduction
2. Results
2.1. Patient A
2.2. Patient B
2.3. Description of the Two DAP-R MRSA Isolates
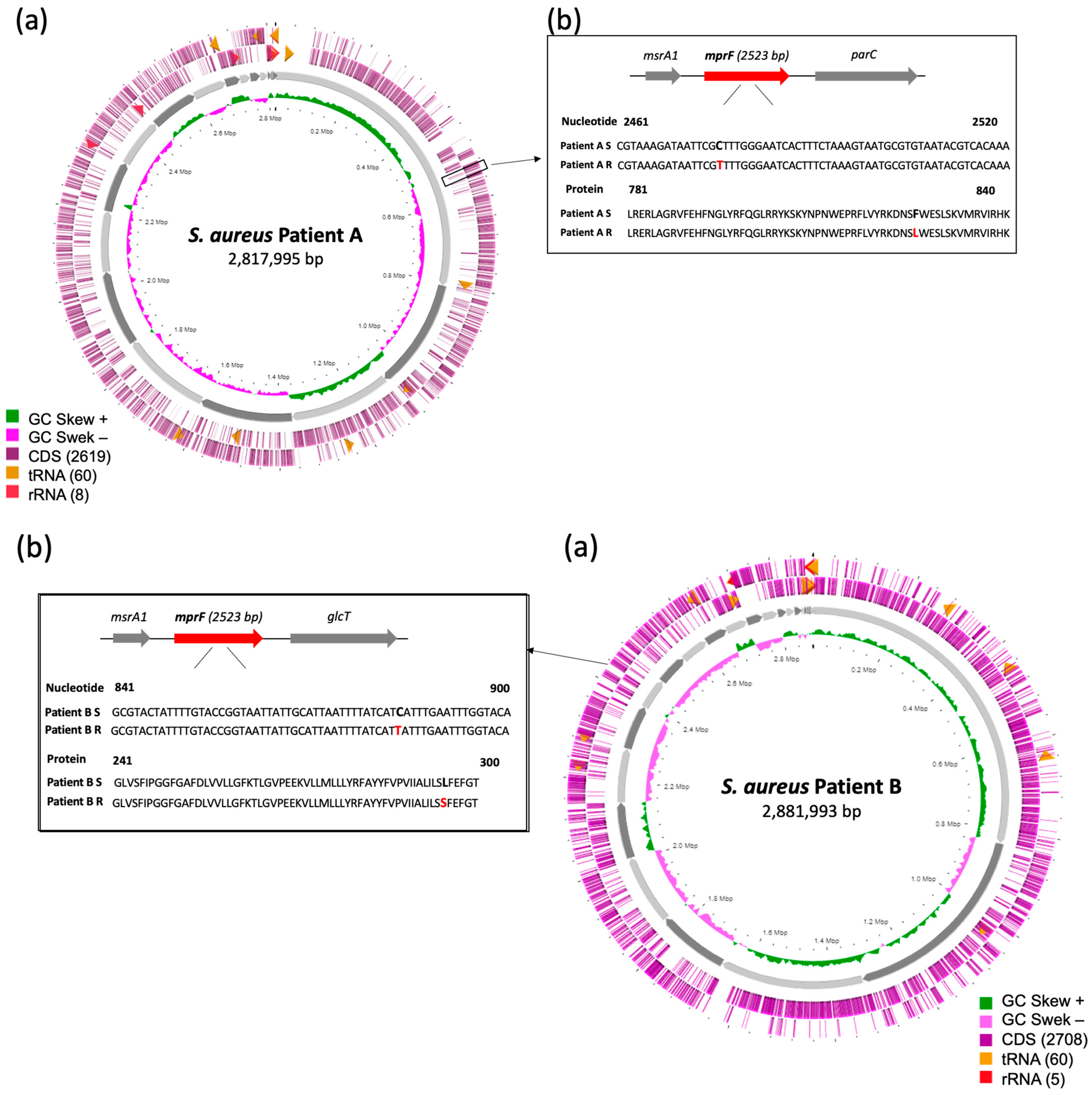
2.4. Whole Genome Analysis of the DAP-S/DAP-R Isolates
2.5. Resistome Profiling of the DAP-S/DAP-R Isolates
2.6. Virulome of the DAP-S/DAP-R Isolates
2.7. Genetic Basis of Resistance to DAP
3. Discussion
4. Materials and Methods
4.1. Bacterial Identification and Antibiotic Susceptibility Testing
4.2. Next-Generation Sequencing
4.3. In Silico Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, C.; Bayer, A.; Cosgrove, S.E.; Daum, R.S.; Fridkin, S.K.; Gorwitz, R.J.; Kaplan, S.L.; Karchmer, A.W.; Levine, D.P.; Murray, B.E.; et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: Executive summary. Clin. Infect. Dis. 2011, 52, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Kaasch, A.J.; Barlow, G.; Edgeworth, J.D.; Fowler, V.G.; Hellmich, M.; Hopkins, S.; Kern, W.V.; Llewelyn, M.J.; Rieg, S.; Rodriguez-Baño, J.; et al. Staphylococcus aureus bloodstream infection: A pooled analysis of five prospective, observational studies. J. Infect. 2014, 68, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Fowler, V.G., Jr.; Boucher, H.W.; Corey, G.R.; Abrutyn, E.; Karchmer, A.W.; Rupp, M.E.; Levine, D.P.; Chambers, H.F.; Tally, F.P.; Vigliani, G.A.; et al. S. aureus Endocarditis and Bacteremia Study Group. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N. Engl. J. Med. 2006, 355, 653–665. [Google Scholar] [CrossRef] [PubMed]
- Schweizer, M.L.; Richardson, K.; Vaughan Sarrazin, M.S.; Goto, M.; Livorsi, D.J.; Nair, R.; Alexander, B.; Beck, B.F.; Jones, M.P.; Puig-Asensio, M.; et al. Comparative Effectiveness of Switching to Daptomycin Versus Remaining on Vancomycin Among Patients With Methicillin-resistant Staphylococcus aureus (MRSA) Bloodstream Infections. Clin. Infect. Dis. 2021, 72, S68–S73. [Google Scholar] [CrossRef] [PubMed]
- Eisenstein, B.I.; Oleson, F.B., Jr.; Baltz, R.H. Daptomycin: From the mountain to the clinic, with essential help from Francis Tally, MD. Clin. Infect. Dis. 2010, 50, S10–S15. [Google Scholar] [CrossRef] [PubMed]
- Morrisette, T.; Alosaimy, S.; Abdul-Mutakabbir, J.C.; Kebriaei, R.; Rybak, M.J. The Evolving Reduction of Vancomycin and Daptomycin Susceptibility in MRSA-Salvaging the Gold Standards with Combination Therapy. Antibiotics 2020, 9, 762. [Google Scholar] [CrossRef]
- Nguyen, A.H.; Hood, K.S.; Mileykovskaya, E.; Miller, W.R.; Tran, T.T. Bacterial cell membranes and their role in daptomycin resistance: A review. Front. Mol. Biosci. 2022, 9, 1035574. [Google Scholar] [CrossRef]
- Beriashvili, D.; Spencer, N.R.; Dieckmann, T.; Overduin, M.; Palmer, M. Characterization of multimeric daptomycin bound to lipid nanodiscs formed by calcium-tolerant styrene-maleic acid copolymer. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183234. [Google Scholar] [CrossRef]
- Zhang, T.H.; Muraih, J.K.; Tishbi, N.; Herskowitz, J.; Victor, R.L.; Silverman, J.; Uwumarenogie, S.; Taylor, S.D.; Palmer, M.; Mintzer, E. Cardiolipin prevents membrane translocation and permeabilization by daptomycin. J. Biol. Chem. 2014, 289, 11584–11591. [Google Scholar] [CrossRef]
- Silverman, J.A.; Perlmutter, N.G.; Shapiro, H.M. Correlation of daptomycin bactericidal activity and membrane depolarization in Staphylococcus aureus. Antimicrob. Agents Chemother. 2003, 47, 2538–2544. [Google Scholar] [CrossRef]
- Fair, R.J.; Tor, Y. Antibiotics and bacterial resistance in the 21st century. Perspect. Med. Chem. 2014, 6, 25–64. [Google Scholar] [CrossRef]
- Foster, T.J. Antibiotic Resistance in Staphylococcus aureus. Current Status and Future Prospects. FEMS Microbiol. Rev. 2017, 41, 430–449. [Google Scholar] [CrossRef] [PubMed]
- Sohlenkamp, C.; Geiger, O. Bacterial membrane lipids: Diversity in structures and pathways. FEMS Microbiol. Rev. 2016, 40, 133–159. [Google Scholar] [CrossRef] [PubMed]
- Mengin-Lecreulx, D.; Allen, N.E.; Hobbs, J.N.; van Heijenoort, J. Inhibition of peptidoglycan biosynthesis in Bacillus megaterium by daptomycin. FEMS Microbiol. Lett. 1990, 57, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Pogliano, J.; Pogliano, N.; Silverman, J.A. Daptomycin-mediated reorganization of membrane architecture causes mislocalization of essential cell division proteins. J. Bacteriol. 2012, 194, 4494–4504. [Google Scholar] [CrossRef]
- Müller, A.; Wenzel, M.; Strahl, H.; Grein, F.; Saaki, T.N.V.; Kohl, B.; Siersma, T.; Bandow, J.E.; Sahl, H.G.; Schneider, T.; et al. Daptomycin inhibits cell envelope synthesis by interfering with fluid membrane microdomains. Proc. Natl. Acad. Sci. USA 2016, 113, E7077–E7086. [Google Scholar] [CrossRef]
- Grein, F.; Müller, A.; Scherer, K.M.; Liu, X.; Ludwig, K.C.; Klöckner, A.; Strach, M.; Sahl, H.G.; Kubitscheck, U.; Schneider, T. Ca2+-Daptomycin targets cell wall biosynthesis by forming a tripartite complex with undecaprenyl-coupled intermediates and membrane lipids. Nat. Commun. 2020, 11, 1455. [Google Scholar] [CrossRef]
- Kullar, R.; Chin, J.N.; Edwards, D.J.; Parker, D.; Coplin, W.M.; Rybak, M.J. Pharmacokinetics of single-dose daptomycin in patients with suspected or confirmed neurological infections. Antimicrob. Agents Chemother. 2011, 55, 3505–3509. [Google Scholar] [CrossRef]
- Nikolic, P.; Mudgil, P. The Cell Wall, Cell Membrane and Virulence Factors of Staphylococcus aureus and Their Role in Antibiotic Resistance. Microorganisms 2023, 11, 259. [Google Scholar] [CrossRef]
- Bayer, A.S.; Schneider, T.; Sahl, H.G. Mechanisms of daptomycin resistance in Staphylococcus aureus: Role of the cell membrane and cell wall. Ann. N. Y. Acad. Sci. 2013, 1277, 139–158. [Google Scholar] [CrossRef]
- Miller, W.R.; Bayer, A.S.; Arias, C.A. Mechanism of action and resistance to daptomycin in Staphylococcus aureus and enterococci. Cold Spring Harb. Perspect. Med. 2016, 6, a026997. [Google Scholar] [CrossRef] [PubMed]
- Casanova, G.N.; Ruiz, S.M.; Bellido, M.J.L. Mechanisms of Resistance to Daptomycin in Staphylococcus aureus. Rev. Española Quimioter. 2017, 30, 391–396. [Google Scholar]
- Tran, T.T.; Munita, J.M.; Arias, C.A. Mechanisms of drug resistance: Daptomycin resistance. Ann. N. Y. Acad. Sci. 2015, 1354, 32–53. [Google Scholar] [CrossRef] [PubMed]
- Capone, A.; Cafiso, V.; Campanile, F.; Parisi, G.; Mariani, B.; Petrosillo, N.; Stefani, S. In vivo development of daptomycin resistance in vancomycin-susceptible methicillin-resistant Staphylococcus aureus severe infections previously treated with glycopeptides. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 625–631. [Google Scholar] [CrossRef]
- Bertsche, U.; Yang, S.J.; Kuehner, D.; Wanner, S.; Mishra, N.N.; Roth, T.; Nega, M.; Schneider, A.; Mayer, C.; Grau, T.; et al. Increased cell wall teichoic acid production and D-alanylation are common phenotypes among daptomycin-resistant methicillin-resistant Staphylococcus aureus (MRSA) clinical isolates. PLoS ONE 2013, 8, e67398. [Google Scholar] [CrossRef]
- Boyle-Vavra, S.; Jones, M.; Gourley, B.L.; Holmes, M.; Ruf, R.; Balsam, A.R.; Boulware, D.R.; Kline, S.; Jawahir, S.; Devries, A.; et al. Comparative genome sequencing of an isogenic pair of USA800 clinical methicillin-resistant Staphylococcus aureus isolates obtained before and after daptomycin treatment failure. Antimicrob. Agents Chemother. 2011, 55, 2018–2025. [Google Scholar] [CrossRef] [PubMed]
- Stefani, S.; Campanile, F.; Santagati, M.; Mezzatesta, M.L.; Cafiso, V.; Pacini, G. Insights and clinical perspectives of daptomycin resistance in Staphylococcus aureus: A review of the available evidence. Int. J. Antimicrob. Agents 2015, 46, 278–289. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, M.; Villa, G.; Ansaldi, F.; De Florentiis, D.; Tascini, C.; Cojutti, P.; Righi, E.; Sartor, A.; Crapis, M.; De Rosa, F.G.; et al. Risk factors associated with the onset of daptomycin non-susceptibility in Staphylococcus aureus infections in critically ill patients. Intensive Care Med. 2015, 41, 366–368. [Google Scholar] [CrossRef]
- Moise, P.A.; North, D.; Steenbergen, J.N.; Sakoulas, G. Susceptibility relationship between vancomycin and daptomycin in Staphylococcus aureus: Facts and assumptions. Lancet Infect. Dis. 2009, 9, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Smirnova, M.V.; Vostrov, S.N.; Strukova, E.V.; Dovzhenko, S.A.; Kobrin, M.B.; Portnoy, Y.A.; Zinner, S.H.; Firsov, A.A. The impact of duration of antibiotic exposure on bacterial resistance predictions using in vitro dynamic models. J. Antimicrob. Chemother. 2009, 64, 815–820. [Google Scholar] [CrossRef]
- Ernst, C.M.; Peschel, A. MprF-mediated daptomycin resistance. Int. J. Med. Microbiol. 2019, 309, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yin, Y.; Chen, H.; Wang, Q.; Wang, X.; Wang, H. Fitness Cost of Daptomycin-Resistant Staphylococcus aureus Obtained from in Vitro Daptomycin Selection Pressure. Front. Microbiol. 2017, 8, 2199. [Google Scholar] [CrossRef] [PubMed]
- Sabat, A.J.; Tinelli, M.; Grundmann, H.; Akkerboom, V.; Monaco, M.; Del Grosso, M.; Errico, G.; Pantosti, A.; Friedrich, A.W. Daptomycin Resistant Staphylococcus aureus Clinical Strain With Novel Non-synonymous Mutations in the mprF and vraS Genes: A New Insight Into Daptomycin Resistance. Front. Microbiol. 2018, 9, 2705. [Google Scholar] [CrossRef] [PubMed]
- Peleg, A.Y.; Miyakis, S.; Ward, D.V.; Earl, A.M.; Rubio, A.; Cameron, D.R.; Pillai, S.; Moellering, R.C., Jr.; Eliopoulos, G.M. Whole genome characterization of the mechanisms of daptomycin resistance in clinical and laboratory derived isolates of Staphylococcus aureus. PLoS ONE 2012, 7, e28316. [Google Scholar] [CrossRef] [PubMed]
- Thitiananpakorn, K.; Aiba, Y.; Tan, X.E.; Watanabe, S.; Kiga, K.; Sato’o, Y.; Boonsiri, T.; Li, F.Y.; Sasahara, T.; Taki, Y.; et al. Association of mprF mutations with cross-resistance to daptomycin and vancomycin in methicillin-resistant Staphylococcus aureus (MRSA). Sci. Rep. 2020, 10, 16107. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Yu, F.; Lin, H.; Murugesan, K.; Huang, W.; Hoss, A.G.; Dhand, A.; Lee, L.Y.; Zhuge, J.; Yin, C.; et al. Evolution and mutations predisposing to daptomycin resistance in vancomycin-resistant Enterococcus faecium ST736 strains. PLoS ONE 2018, 13, e0209785. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Hu, J.; Li, L.; Zhang, M.; Cui, Q.; Ma, Y.; Su, H.; Zhang, X.; Xu, H.; Wang, M. New Mutations in cls Lead to Daptomycin Resistance in a Clinical Vancomycin- and Daptomycin-Resistant Enterococcus faecium Strain. Front. Microbiol. 2022, 13, 896916. [Google Scholar] [CrossRef]
- Pouget, C.; Chatre, C.; Lavigne, J.P.; Pantel, A.; Reynes, J.; Dunyach-Remy, C. Effect of Antibiotic Exposure on Staphylococcus epidermidis Responsible for Catheter-Related Bacteremia. Int. J. Mol. Sci. 2023, 24, 1547. [Google Scholar] [CrossRef]
- Carattoli, A.; Zankari, E.; García-Fernández, A.; Voldby Larsen, M.; Lund, O.; Villa, L.; Møller Aarestrup, F.; Hasman, H. In Silico detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing. Antimicrob. Agents Chemother. 2014, 58, 3895–3903. [Google Scholar] [CrossRef]
- Zankari, E.; Allesøe, R.; Joensen, K.G.; Cavaco, L.M.; Lund, O.; Aarestrup, F.M. PointFinder: A novel web tool for WGS-based detection of antimicrobial resistance associated with chromosomal point mutations in bacterial pathogens. J. Antimicrob. Chemother. 2017, 72, 2764–2768. [Google Scholar] [CrossRef]
- Bortolaia, V.; Kaas, R.S.; Ruppe, E.; Roberts, M.C.; Schwarz, S.; Cattoir, V.; Philippon, A.; Allesoe, R.L.; Rebelo, A.R.; Florensa, A.F.; et al. ResFinder 4.0 for Predictions of Phenotypes from Genotypes. J. Antimicrob. Chemother. 2020, 75, 3491–3500. [Google Scholar] [CrossRef]
- Bartels, M.D.; Petersen, A.; Worning, P.; Nielsen, J.B.; Larner-Svensson, H.; Johansen, H.K.; Andersen, L.P.; Jarløv, J.O.; Boye, K.; Larsen, A.R.; et al. Comparing Whole-Genome Sequencing with Sanger Sequencing for Spa Typing of Methicillin-Resistant Staphylococcus aureus. J. Clin. Microbiol. 2014, 52, 4305–4308. [Google Scholar] [CrossRef]
- Alikhan, N.F.; Petty, N.K.; Ben Zakour, N.L.; Beatson, S.A. BLAST Ring Image Generator (BRIG): Simple prokaryote genome comparisons. BMC Genom. 2011, 12, 402. [Google Scholar] [CrossRef]
- Snippy: Fast Bacterial Variant Calling from NGS Reads. Available online: https://github.com/tseemann/snippy (accessed on 24 June 2023).
- Ankrum, A.; Hall, B.G. Population dynamics of Staphylococcus aureus in cystic fibrosis patients to determine transmission events by use of whole-genome sequencing. J. Clin. Microbiol. 2017, 55, 2143–2152. [Google Scholar] [CrossRef]
| Strain | Location | Collection Date | Specimen Type | ST | spa Type | MICs Values (mg/L): | Resistance Profile | % Sequence Similarities | Mutation in MprF and Cls Proteins * | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DAP | VAN | TEI | DAL | CPT | CBP | |||||||||
| A_DAP-S | Nîmes | 26 April 2021 | Blood | 5 | t777 | 0.5 | 1 | 0.5 | 0.06 | 0.25 | 1 | PEN, OXA, OFX | 99.96 | - |
| A_DAP-R | Nîmes | 16 May 2021 | Blood | 5 | t777 | 2 | 1 | 1 | 0.06 | 0.38 | 1 | PEN, OXA, OFX | L826F | |
| B_DAP-S | Sète | 3 September 2021 | Blood | 8 | t622 | 0.5 | 1 | 0.5 | 0.06 | 0.38 | 1.5 | PEN, OXA, OFX, RIF | 99.93 | - |
| B_DAP-R | Sète | 26 September 2021 | Blood | 8 | t622 | 2 | 1 | 0.5 | 0.06 | 0.38 | 1.5 | PEN, OXA, OFX, RIF | S295L/R228H | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boutet-Dubois, A.; Magnan, C.; Lienard, A.; Pouget, C.; Bouchet, F.; Marchandin, H.; Larcher, R.; Lavigne, J.-P.; Pantel, A. In Vivo-Acquired Resistance to Daptomycin during Methicillin-Resistant Staphylococcus aureus Bacteremia. Antibiotics 2023, 12, 1647. https://doi.org/10.3390/antibiotics12121647
Boutet-Dubois A, Magnan C, Lienard A, Pouget C, Bouchet F, Marchandin H, Larcher R, Lavigne J-P, Pantel A. In Vivo-Acquired Resistance to Daptomycin during Methicillin-Resistant Staphylococcus aureus Bacteremia. Antibiotics. 2023; 12(12):1647. https://doi.org/10.3390/antibiotics12121647
Chicago/Turabian StyleBoutet-Dubois, Adeline, Chloé Magnan, Alexi Lienard, Cassandra Pouget, Flavien Bouchet, Hélène Marchandin, Romaric Larcher, Jean-Philippe Lavigne, and Alix Pantel. 2023. "In Vivo-Acquired Resistance to Daptomycin during Methicillin-Resistant Staphylococcus aureus Bacteremia" Antibiotics 12, no. 12: 1647. https://doi.org/10.3390/antibiotics12121647





