Sanguiin H-6 Fractionated from Cloudberry (Rubus chamaemorus) Seeds Can Prevent the Methicillin-Resistant Staphylococcus aureus Biofilm Development during Wound Infection
Abstract
:1. Introduction
2. Results
2.1. Minimum Inhibitory Concentration and Minimal Bactericidal Concentration
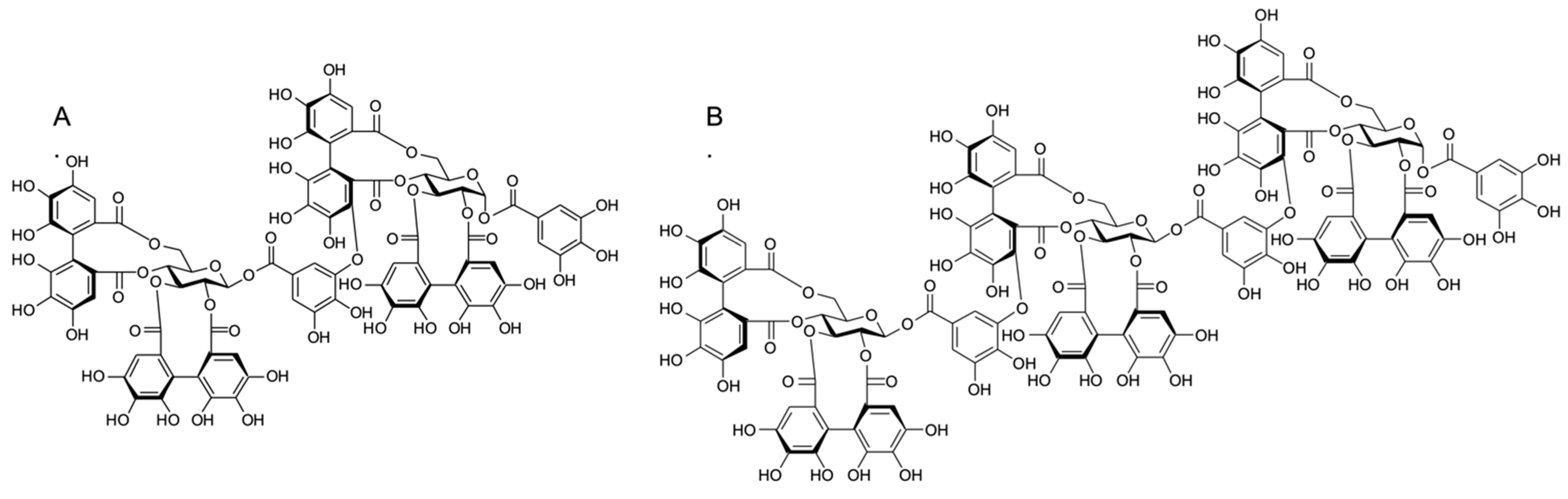
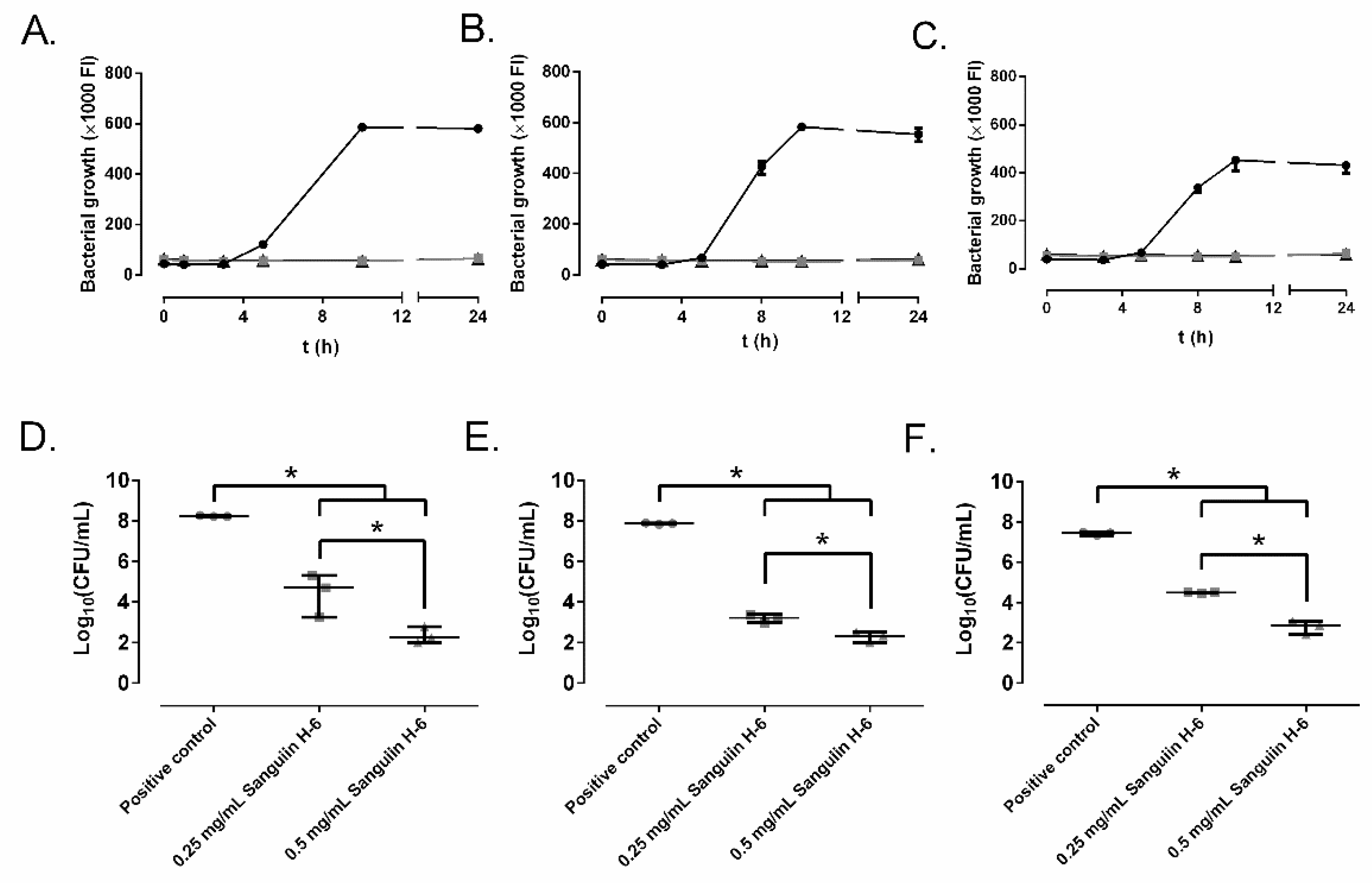
2.2. Effect of Ellagitannins on the MRSA Growth
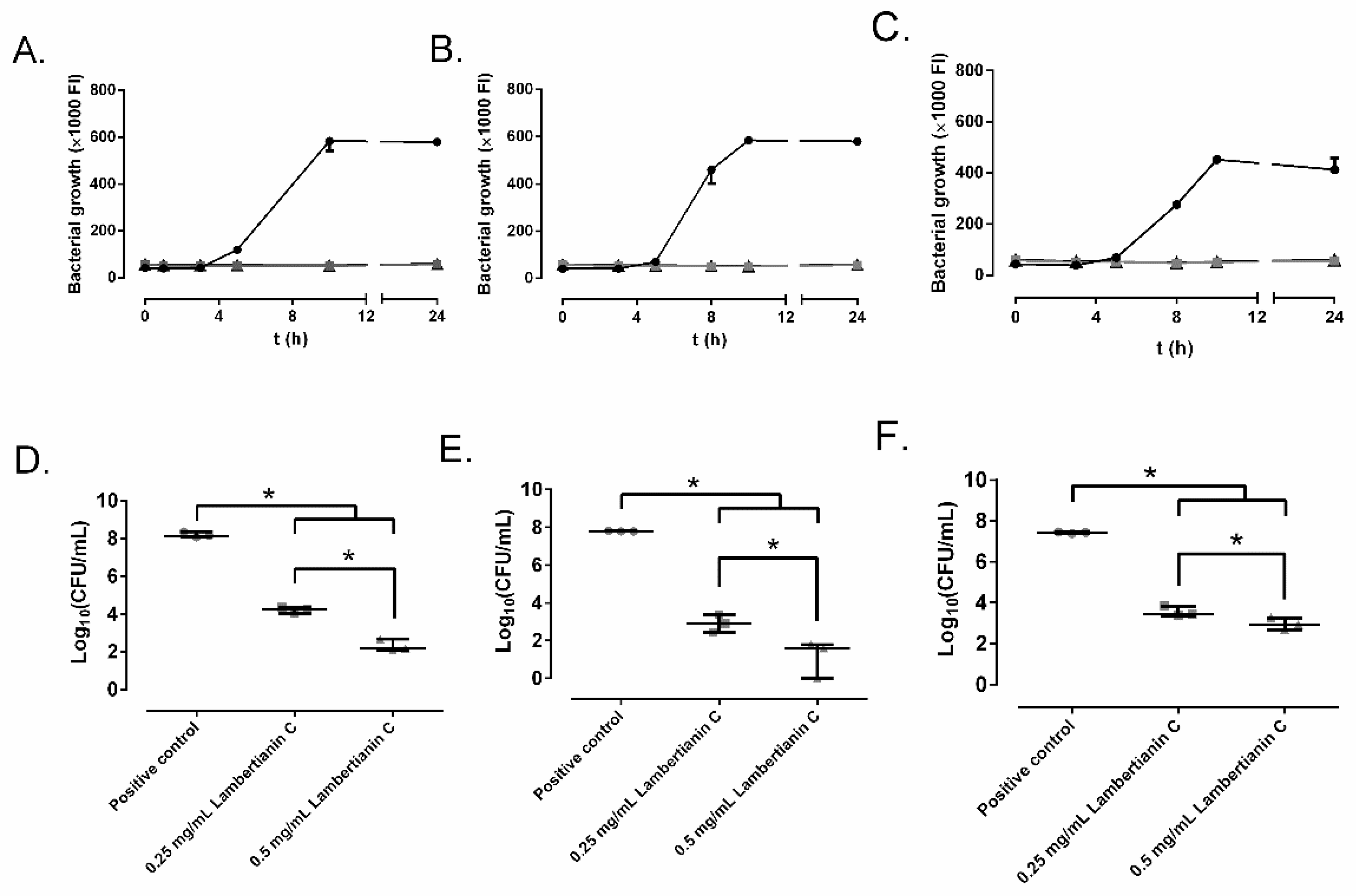
2.3. Effect on Biofilm Development
2.4. Biofilm Treatment
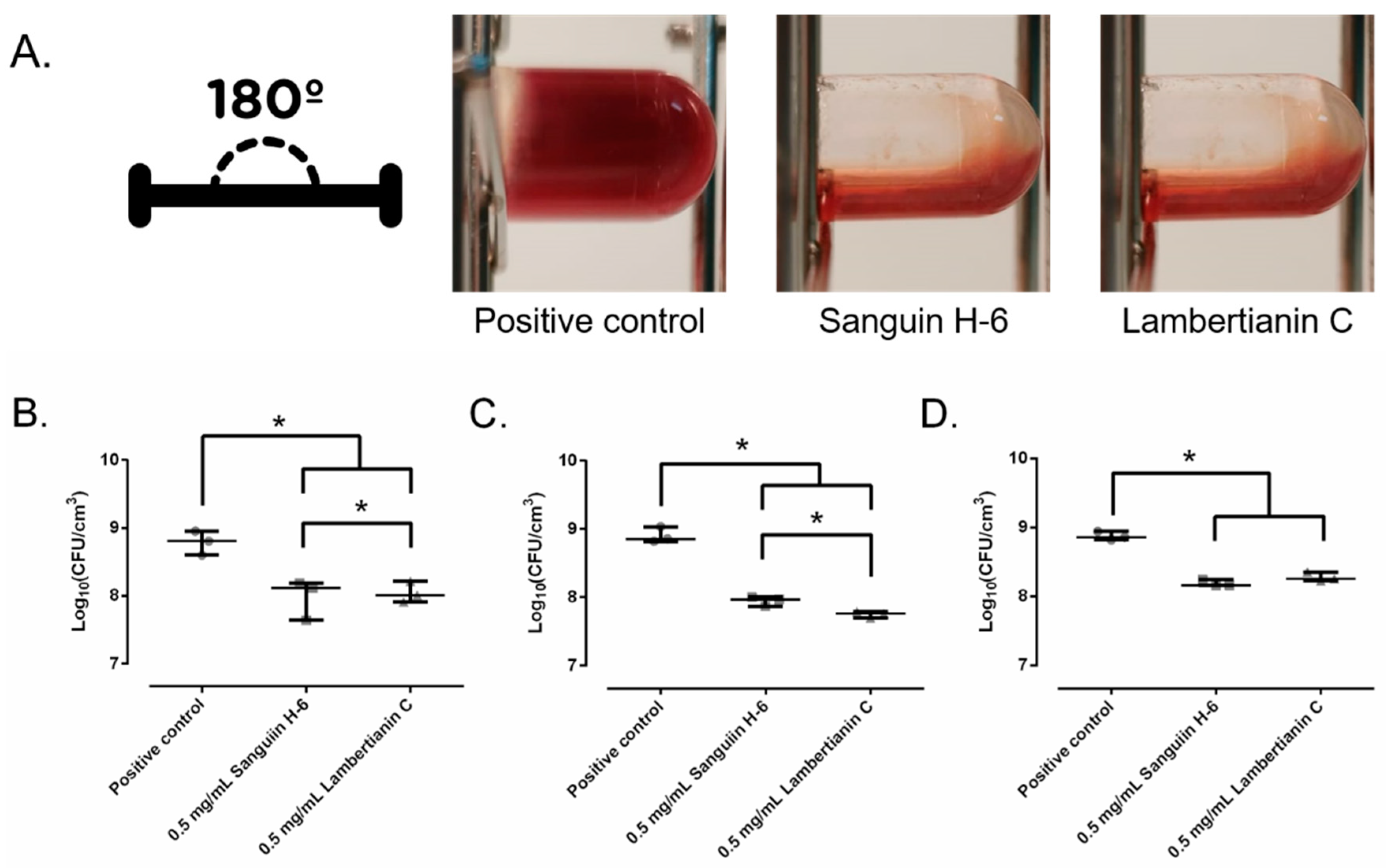
2.5. Biofilm Development in Wound-Like Medium
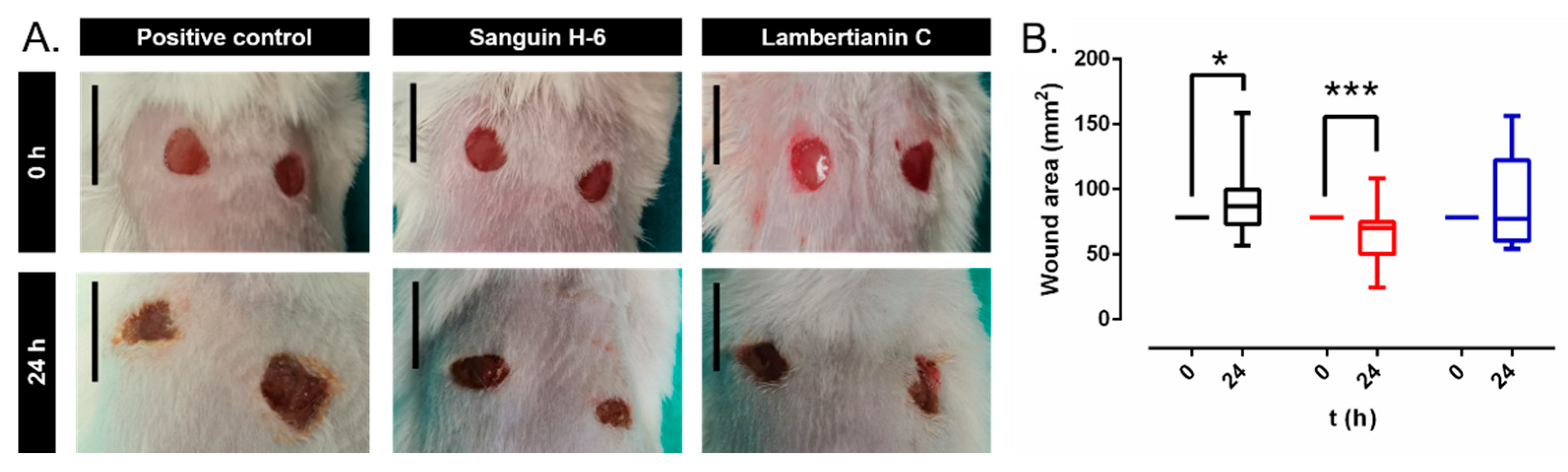
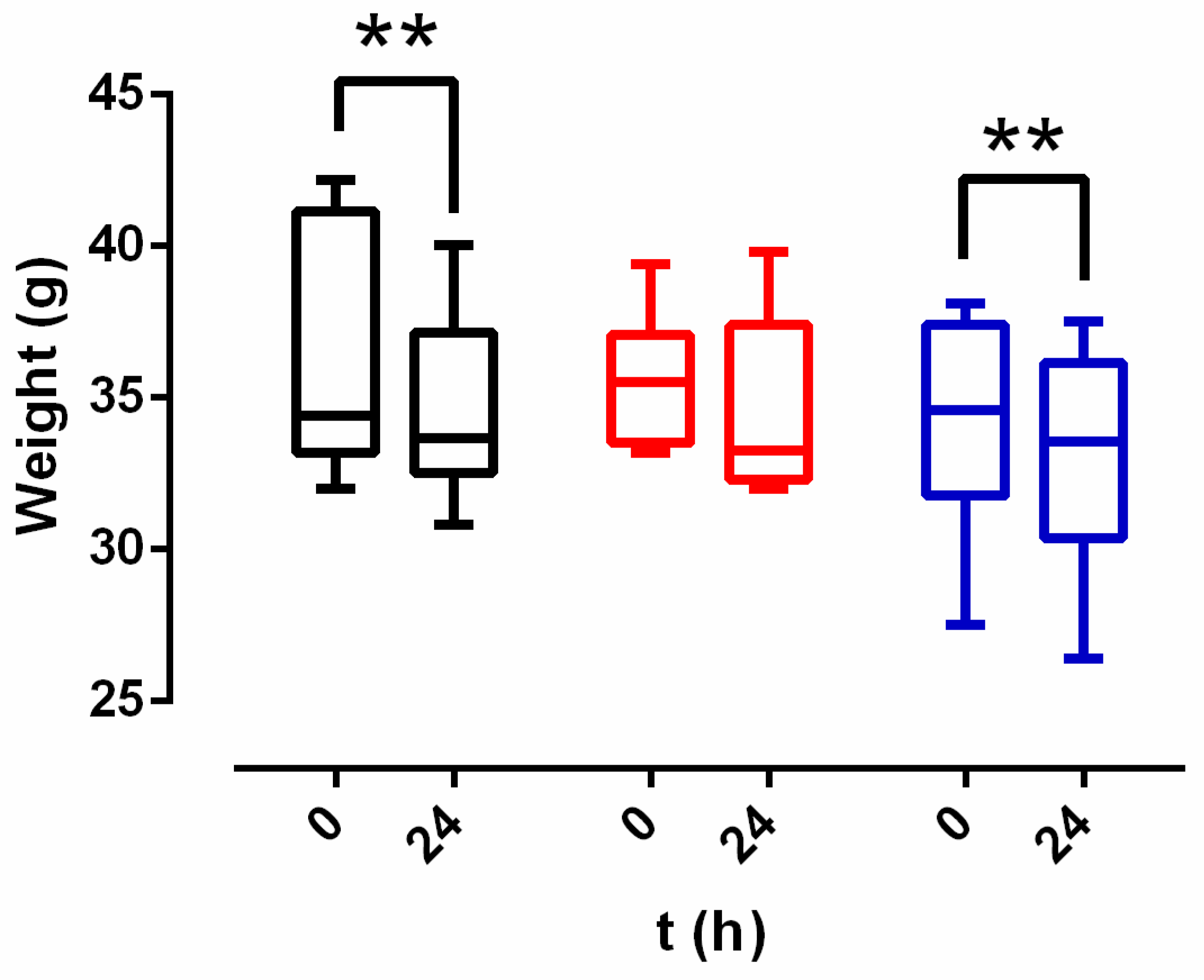
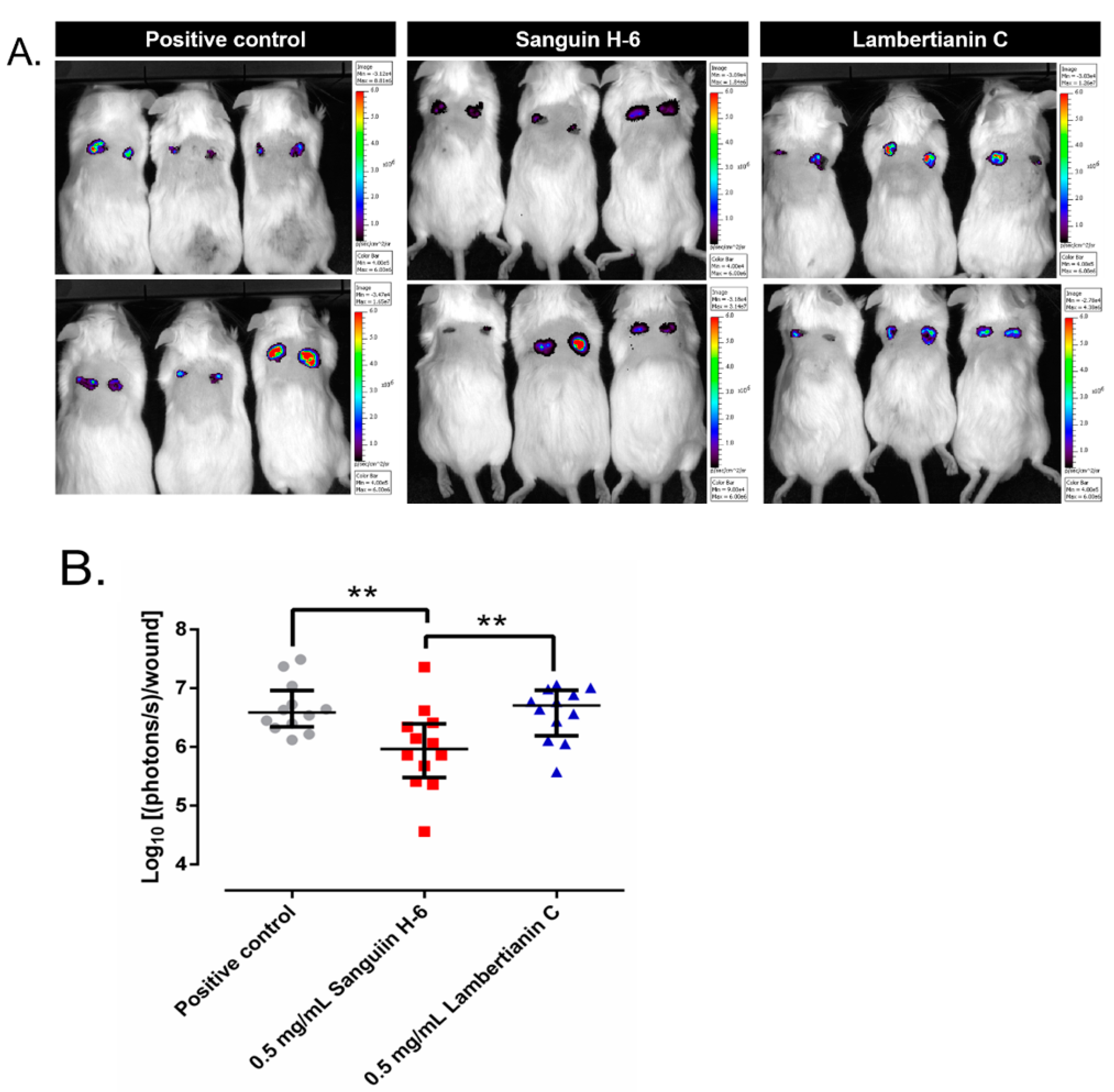
2.6. In Vivo Studies
3. Discussion
4. Materials and Methods
4.1. In Vitro Studies
4.1.1. Ellagitannins Extraction
4.1.2. Bacteria
4.1.3. Minimum Inhibitory Concentration and Minimum Bactericidal Concentration
4.1.4. Effect of Ellagitannins on the MRSA Growth
4.1.5. Effect on Biofilm Development
4.1.6. Biofilm Treatment
4.1.7. Effect on Biofilm Development in Wound-like Medium
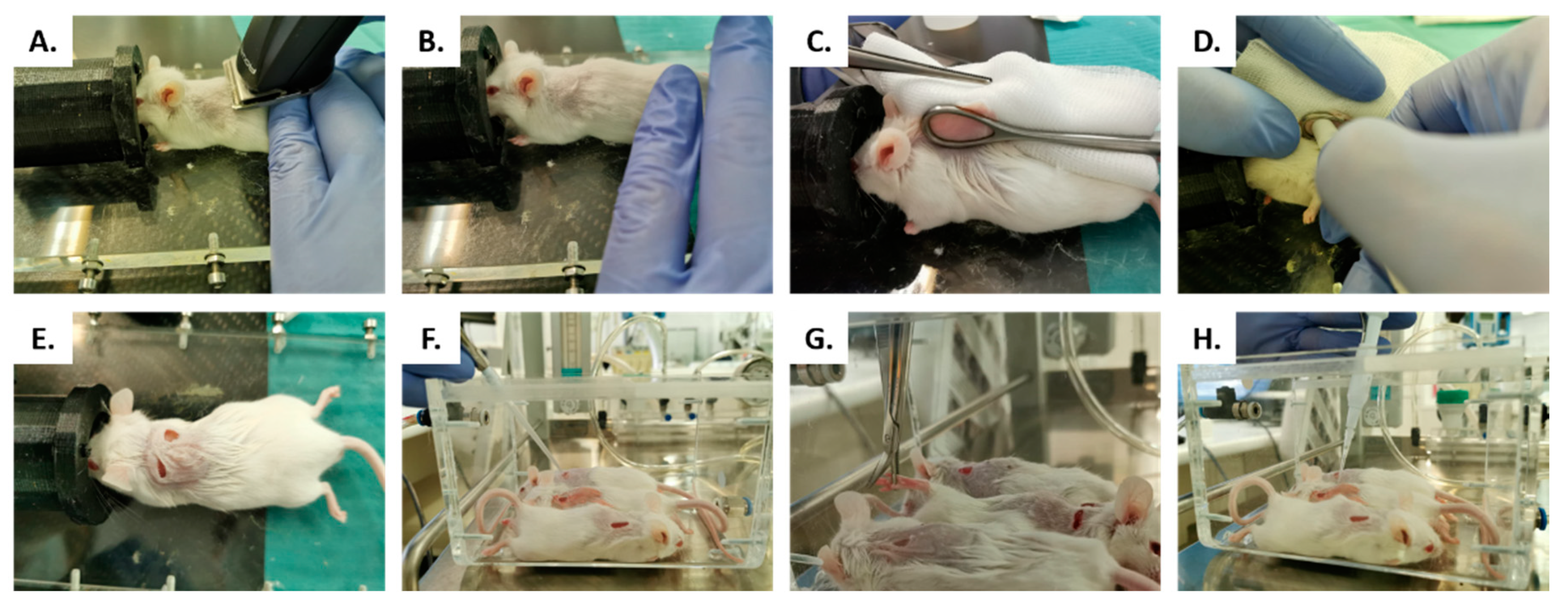
4.2. In Vivo Studies
Statistical Analysis
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weiser, T.G.; Haynes, A.B.; Molina, G.; Lipsitz, S.R.; Esquivel, M.M.; Uribe-Leitz, T.; Fu, R.; Azad, T.; Chao, T.E.; Berry, W.R.; et al. Size and distribution of the global volume of surgery in 2012. Bull. World Health Organ. 2016, 94, 201F–209F. [Google Scholar] [CrossRef]
- Leaper, D.; Ousey, K. Evidence update on prevention of surgical site infection. Curr. Opin. Infect. Dis. 2015, 28, 158–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Awad, S.S. Adherence to surgical care improvement project measures and post-operative surgical site infections. Surg. Infect. 2012, 13, 234–237. [Google Scholar] [CrossRef]
- Zarb, P.; Coignard, B.; Griskeviciene, J.; Muller, A.; Vankerckhoven, V.; Weist, K.; Goossens, M.; Vaerenberg, S.; Hopkins, S.; Catry, B.; et al. Collective National Contact Points for the ECDC pilot point prevalence survey; Hospital Contact Points for the ECDC pilot point prevalence survey Collective. The European Centre for Disease Prevention and Control (ECDC) pilot point prevalence survey of healthcare-associated infections and antimicrobial use. Eurosurveillance 2012, 17, 20316. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Pal, K.; Jain, S.; Chatterjee, S.S.; Konar, J. Surgical Site Infection by Methicillin Resistant Staphylococcus aureus- on Decline? J. Clin. Diagn. Res. 2016, 10, DC32–DC36. [Google Scholar] [CrossRef]
- Sganga, G.; Tascini, C.; Sozio, E.; Carlini, M.; Chirletti, P.; Cortese, F.; Gattuso, R.; Granone, P.; Pempinello, C.; Sartelli, M.; et al. Focus on the prophylaxis, epidemiology and therapy of methicillin-resistant Staphylococcus aureus surgical site infections and a position paper on associated risk factors: The perspective of an Italian group of surgeons. World J. Emerg. Surg. 2016, 11, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engemann, J.J.; Carmeli, Y.; Cosgrove, S.E.; Fowler, V.G.; Bronstein, M.Z.; Trivette, S.L.; Briggs, J.P.; Sexton, D.J.; Kaye, K.S. Adverse clinical and economic outcomes attributable to methicillin resistance among patients with Staphylococcus aureus surgical site infection. Clin. Infect. Dis. 2003, 36, 592–598. [Google Scholar] [CrossRef] [Green Version]
- Rubin, R.J.; Harrington, C.A.; Poon, A.; Dietrich, K.; Greene, J.A.; Moiduddin, A. The economic impact of Staphylococcus aureus infection in New York City hospitals. Emerg. Infect. Dis. 1999, 5, 9–17. [Google Scholar] [CrossRef]
- Omar, A.; Wright, J.B.; Schultz, G.; Burrell, R.; Nadworny, P. Microbial Biofilms and Chronic Wounds. Microorganisms 2017, 5, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Percival, S.L.; McCarty, S.M.; Lipsky, B. Biofilms and Wounds: An Overview of the Evidence. Adv. Wound. Care. 2015, 4, 373–381. [Google Scholar] [CrossRef] [Green Version]
- Neyra, R.C.; Frisancho, J.A.; Rinsky, J.L.; Resnick, C.; Carroll, K.C.; Rule, A.M.; Ross, T.; You, Y.; Price, L.B.; Silbergeld, E.K. Multidrug-resistant and methicillin-resistant Staphylococcus aureus (MRSA) in hog slaughter and processing plant workers and their community in North Carolina (USA). Environ. Health Perspect. 2014, 122, 471–477. [Google Scholar] [CrossRef] [Green Version]
- Appelbaum, P.C. Reduced glycopeptide susceptibility in methicillin-resistant Staphylococcus aureus (MRSA). Int. J. Antimicrob. Agents 2007, 30, 398–408. [Google Scholar] [CrossRef]
- Szymanek-Majchrzak, K.; Mlynarczyk, A.; Mlynarczyk, G. Characteristics of glycopeptide-resistant Staphylococcus aureus strains isolated from inpatients of three teaching hospitals in Warsaw, Poland. Antimicrob. Resist Infect. Control. 2018, 7, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Okwu, M.U.; Olley, M.; Akpoka, A.O.; Izevbuwa, O.E. Methicillin-resistant Staphylococcus aureus (MRSA) and anti-MRSA activities of extracts of some medicinal plants: A brief review. AIMS Microbiol. 2019, 5, 117–137. [Google Scholar] [CrossRef] [PubMed]
- Sharaf, M.H.; El-Sherbiny, G.M.; Moghannem, S.A.; Abdelmonem, M.; Elsehemy, I.A.; Metwaly, A.M.; Kalaba, M.H. New combination approaches to combat methicillin-resistant Staphylococcus aureus (MRSA). Sci. Rep. 2021, 11, 4240–4244. [Google Scholar] [CrossRef]
- Buzgaia, N.; Awin, T.; Elabbar, F.; Abdusalam, K.; Lee, S.Y.; Rukayadi, Y.; Abas, F.; Shaari, K. Antibacterial Activity of Arbutus pavarii Pamp against Methicillin-Resistant Staphylococcus aureus (MRSA) and UHPLC-MS/MS Profile of the Bioactive Fraction. Plants 2020, 9, 1539. [Google Scholar] [CrossRef] [PubMed]
- Mahadevi, R.; Salmen, S.H.; Alfarraj, S.; Wainwright, M.; Kavitha, R. Screening and characterization of phytochemical content of methanolic extract of Rhizome of Curcuma amada and their antibacterial activity against MRSA. Appl. Nanosci. 2021, 1, 1–11. [Google Scholar] [CrossRef]
- Tayel, A.A.; Shaban, S.M.; Moussa, S.H.; Elguindy, N.M.; Diab, A.M.; Mazrou, K.E.; Ghanem, R.A.; El-Sabbagh, S.M. Bioactivity and application of plant seeds’ extracts to fight resistant strains of Staphylococcus aureus. Ann. Agric. Sci. 2018, 63, 47–53. [Google Scholar] [CrossRef]
- Lipinska, L.; Klewicka, E.; Sojka, M. The structure, occurrence and biological activity of ellagitannins: A general review. Acta Sci. Pol. Technol. Aliment. 2014, 13, 289–299. [Google Scholar] [CrossRef] [Green Version]
- Puupponen-Pimiä, R.; Nohynek, L.; Suvanto, J.; Salminen, J.; Seppänen-Laakso, T.; Tähtiharju, J.; Honkapää, K.; Oksman-Caldentey, K.M. Natural Antimicrobials from Cloudberry (Rubus chamaemorus) Seeds by Sanding and Hydrothermal Extraction. ACS Food Sci. Technol. 2021, 1, 917–927. [Google Scholar] [CrossRef]
- Reddy, M.K.; Gupta, S.K.; Jacob, M.R.; Khan, S.I.; Ferreira, D. Antioxidant, antimalarial and antimicrobial activities of tannin-rich fractions, ellagitannins and phenolic acids from Punica granatum L. Planta Med. 2007, 73, 461–467. [Google Scholar] [CrossRef]
- Yang, B.; Kortesniemi, M.; Liu, P.; Karonen, M.; Salminen, J.P. Analysis of hydrolyzable tannins and other phenolic compounds in emblic leafflower (Phyllanthus emblica L.) fruits by high performance liquid chromatography-electrospray ionization mass spectrometry. J. Agric. Food Chem. 2012, 60, 8672–8683. [Google Scholar] [CrossRef] [PubMed]
- Mullen, W.; McGinn, J.; Lean, M.E.J.; MacLean, M.R.; Gardner, P.; Duthie, G.G.; Yokota, T.; Crozier, A. Ellagitannins, Flavonoids, and Other Phenolics in Red Raspberries and Their Contribution to Antioxidant Capacity and Vasorelaxation Properties. J. Agric. Food Chem. 2002, 50, 5191–5196. [Google Scholar] [CrossRef]
- Mullen, W.; Yokota, T.; Lean, M.E.; Crozier, A. Analysis of ellagitannins and conjugates of ellagic acid and quercetin in raspberry fruits by LC-MSn. Phytochemistry 2003, 64, 617–624. [Google Scholar] [CrossRef]
- Puupponen-Pimia, R.; Nohynek, L.; Juvonen, R.; Kosso, T.; Truchado, P.; Westerlund-Wikstrom, B.; Leppänen, T.; Moilanen, E.; Oksman-Caldentey, K.M. Fermentation and dry fractionation increase bioactivity of cloudberry (Rubus chamaemorus). Food Chem. 2016, 197, 950–958. [Google Scholar] [CrossRef]
- Krauze-Baranowska, M.; Majdan, M.; Halasa, R.; Glod, D.; Kula, M.; Fecka, I.; Orzel, A. The antimicrobial activity of fruits from some cultivar varieties of Rubus idaeus and Rubus occidentalis. Food Funct. 2014, 5, 2536–2541. [Google Scholar] [CrossRef]
- Waites, K.B.; Bade, D.J.; Bébéar, C.; Brown, S.D.; Davidson, M.K.; Duffy, L.B.; Kenny, G.; Matlow, A.; Shortridge, D.; Talkington, D.; et al. Methods for Antimicrobial Susceptibility Testing for Human Mycoplasmas: Approved Guideline; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2011. [Google Scholar]
- Bastow, K.F.; Bori, I.D.; Fukushima, Y.; Kashiwada, Y.; Tanaka, T.; Nonaka, G.; Nishioka, I.; Lee, K.H. Inhibition of DNA topoisomerases by sanguiin H-6, a cytotoxic dimeric ellagitannin from Sanguisorba officinalis. Planta Med. 1993, 59, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Bax, B.D.; Murshudov, G.; Maxwell, A.; Germe, T. DNA Topoisomerase Inhibitors: Trapping a DNA-Cleaving Machine in Motion. J. Mol. Biol. 2019, 431, 3427–3449. [Google Scholar] [CrossRef]
- Chan, P.F.; Srikannathasan, V.; Huang, J.; Cui, H.; Fosberry, A.P.; Gu, M.; Hann, M.M.; Hibbs, M.; Homes, P.; Ingraham, K.; et al. Structural basis of DNA gyrase inhibition by antibacterial QPT-1, anticancer drug etoposide and moxifloxacin. Nat. Commun. 2015, 6, 10048. [Google Scholar] [CrossRef]
- Schacter, L. Etoposide phosphate: What, why, where, and how? Semin. Oncol. 1996, 23, 1–7. [Google Scholar]
- Heide, L. New aminocoumarin antibiotics as gyrase inhibitors. Int. J. Med. Microbiol. 2014, 304, 31–36. [Google Scholar] [CrossRef]
- Sun, Y.; Dowd, S.E.; Smith, E.; Rhoads, D.D.; Wolcott, R.D. In vitro multispecies Lubbock chronic wound biofilm model. Wound Repair Regen. 2008, 16, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.G.; McAdow, M.; Kim, H.K.; Bae, T.; Missiakas, D.M.; Schneewind, O. Contribution of coagulases towards Staphylococcus aureus disease and protective immunity. PLoS Pathog. 2010, 6, e1001036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.Q.; Yang, Y.X.; Qv, J.W.; Hu, G.; Hu, Y.J.; Xia, Z.N.; Yang, F.Q. Investigation of Interactions between Thrombin and Ten Phenolic Compounds by Affinity Capillary Electrophoresis and Molecular Docking. J. Anal. Methods Chem. 2018, 2018, 4707609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Heeckeren, A.M.; Tscheikuna, J.; Walenga, R.W.; Konstan, M.W.; Davis, P.B.; Erokwu, B.; Haxhiu, M.A.; Ferkol, T.W. Effect of Pseudomonas infection on weight loss, lung mechanics, and cytokines in mice. Am. J. Respir. Crit. Care Med. 2000, 161, 271–279. [Google Scholar] [CrossRef]
- Chew, J.; Peh, S.; Sin Yeang, T. Non-microbial Natural Products That Inhibit Drug-Resistant Staphylococcus aureus. Staphylococcus Aureus 2019. [Google Scholar] [CrossRef] [Green Version]
- Abreu, A.C.; Coqueiro, A.; Sultan, A.R.; Lemmens, N.; Kim, H.K.; Verpoorte, R.; van Wamel, W.J.B.; Simoes, M.; Choi, Y.H. Looking to nature for a new concept in antimicrobial treatments: Isoflavonoids from Cytisus striatus as antibiotic adjuvants against MRSA. Sci. Rep. 2017, 7, 1–16. [Google Scholar] [CrossRef]
- Puupponen-Pimiä, R.; Kössö, T.; Nohynek, L.; Mokkila, M. Process for Converting Berry and Fruit Material to Antimicrobially Active Fractions. U.S. Patent Application No. 15/537,349, 29 June 2021. [Google Scholar]
- Plaut, R.D.; Mocca, C.P.; Prabhakara, R.; Merkel, T.J.; Stibitz, S. Stably luminescent Staphylococcus aureus clinical strains for use in bioluminescent imaging. PLoS ONE 2013, 8, e59232. [Google Scholar] [CrossRef]
- Elshikh, M.; Ahmed, S.; Funston, S.; Dunlop, P.; McGaw, M.; Marchant, R.; Banat, I.M. Resazurin-based 96-well plate microdilution method for the determination of minimum inhibitory concentration of biosurfactants. Biotechnol. Lett. 2016, 38, 1015–1019. [Google Scholar] [CrossRef] [Green Version]
- Pettit, R.K.; Weber, C.A.; Pettit, G.R. Application of a high throughput Alamar blue biofilm susceptibility assay to Staphylococcus aureus biofilms. Ann. Clin. Microbiol. Antimicrob. 2009, 8, 28. [Google Scholar] [CrossRef]
- Peeters, E.; Nelis, H.J.; Coenye, T. Comparison of multiple methods for quantification of microbial biofilms grown in microtiter plates. J. Microbiol. Methods 2008, 72, 157–165. [Google Scholar] [CrossRef] [Green Version]
- Hernandes, C.; Coppede, J.d.S.; Bertoni, B.W.; França, S.d.C.; Pereira, A.M.S. Flash microbiocide: A Rapid and Economic Method for Determination of MBC and MFC. Am. J. Plant Sci. 2013, 4, 850–852. [Google Scholar] [CrossRef]
- Yang, H.; Abouelhassan, Y.; Burch, G.M.; Kallifidas, D.; Huang, G.; Yousaf, H.; Jin, S.; Luesch, H.; Huigens, R.W. A Highly Potent Class of Halogenated Phenazine Antibacterial and Biofilm-Eradicating Agents Accessed Through a Modular Wohl-Aue Synthesis. Sci. Rep. 2017, 7, 1–16. [Google Scholar] [CrossRef] [Green Version]
- DeLeon, S.; Clinton, A.; Fowler, H.; Everett, J.; Horswill, A.R.; Rumbaugh, K.P. Synergistic interactions of Pseudomonas aeruginosa and Staphylococcus aureus in an in vitro wound model. Infect. Immun. 2014, 82, 4718–4728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.C.; Yang, P.W.; Yang, S.F.; Hsieh, K.P.; Tseng, S.P.; Lin, Y.C. Topical simvastatin promotes healing of Staphylococcus aureus-contaminated cutaneous wounds. Int. Wound. J. 2016, 13, 1150–1157. [Google Scholar] [CrossRef]
- Xiong, Y.Q.; Willard, J.; Kadurugamuwa, J.L.; Yu, J.; Francis, K.P.; Bayer, A.S. Real-Time In Vivo Bioluminescent Imaging for Evaluating the Efficacy of Antibiotics in a Rat Staphylococcus aureus Endocarditis Model. Antimicrob. Agents Chemother. 2005, 49, 380–387. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguilera-Correa, J.J.; Fernández-López, S.; Cuñas-Figueroa, I.D.; Pérez-Rial, S.; Alakomi, H.-L.; Nohynek, L.; Oksman-Caldentey, K.-M.; Salminen, J.-P.; Esteban, J.; Cuadros, J.; et al. Sanguiin H-6 Fractionated from Cloudberry (Rubus chamaemorus) Seeds Can Prevent the Methicillin-Resistant Staphylococcus aureus Biofilm Development during Wound Infection. Antibiotics 2021, 10, 1481. https://doi.org/10.3390/antibiotics10121481
Aguilera-Correa JJ, Fernández-López S, Cuñas-Figueroa ID, Pérez-Rial S, Alakomi H-L, Nohynek L, Oksman-Caldentey K-M, Salminen J-P, Esteban J, Cuadros J, et al. Sanguiin H-6 Fractionated from Cloudberry (Rubus chamaemorus) Seeds Can Prevent the Methicillin-Resistant Staphylococcus aureus Biofilm Development during Wound Infection. Antibiotics. 2021; 10(12):1481. https://doi.org/10.3390/antibiotics10121481
Chicago/Turabian StyleAguilera-Correa, John Jairo, Sara Fernández-López, Iskra Dennisse Cuñas-Figueroa, Sandra Pérez-Rial, Hanna-Leena Alakomi, Liisa Nohynek, Kirsi-Marja Oksman-Caldentey, Juha-Pekka Salminen, Jaime Esteban, Juan Cuadros, and et al. 2021. "Sanguiin H-6 Fractionated from Cloudberry (Rubus chamaemorus) Seeds Can Prevent the Methicillin-Resistant Staphylococcus aureus Biofilm Development during Wound Infection" Antibiotics 10, no. 12: 1481. https://doi.org/10.3390/antibiotics10121481
APA StyleAguilera-Correa, J. J., Fernández-López, S., Cuñas-Figueroa, I. D., Pérez-Rial, S., Alakomi, H.-L., Nohynek, L., Oksman-Caldentey, K.-M., Salminen, J.-P., Esteban, J., Cuadros, J., Puupponen-Pimiä, R., Perez-Tanoira, R., & Kinnari, T. J. (2021). Sanguiin H-6 Fractionated from Cloudberry (Rubus chamaemorus) Seeds Can Prevent the Methicillin-Resistant Staphylococcus aureus Biofilm Development during Wound Infection. Antibiotics, 10(12), 1481. https://doi.org/10.3390/antibiotics10121481








