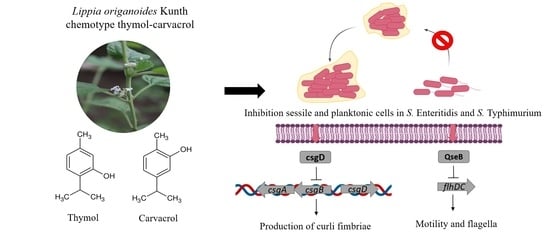
Effect of Essential Oils on the Inhibition of Biofilm and Quorum Sensing in Salmonella enteritidis 13076 and Salmonella typhimurium 14028
Abstract
:1. Introduction
2. Results
2.1. Determination of the In Vitro Antimicrobial Activity of EOs on Planktonic Cells
2.2. In Vitro Inhibition of Biofilm Formation
2.3. Scanning Electron Microscopy
2.4. QS and Biofilm Formation Gene Expression Analysis
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Bacterial Strains and Growth Conditions
4.3. Determination of the In Vitro Antimicrobial Activity of EOs on Planktonic Cells
4.4. In Vitro Inhibition of Biofilm Formation
4.5. Scanning Electron Microscopy
4.6. RNA Extraction and Transcriptional Analysis
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gast, R.K.; Porter, R.E. Salmonella infections. In Diseases of Poultry, 14th ed.; John Wiley and Sons: Hoboken, NJ, USA, 2020; pp. 717–753. [Google Scholar]
- Quesada, A.; Reginatto, G.A.; Español, A.R.; Colantonio, L.D.; Burrone, M.S. Resistencia antimicrobiana de Salmonella spp aislada de alimentos de origen animal para consumo humano. Rev. Peru. Med. Exp. Salud Publica 2016, 33, 32–44. [Google Scholar] [CrossRef]
- Rukambile, E.; Sintchenko, V.; Muscatello, G.; Kock, R.; Alders, R. Infection, colonization and shedding of Campylobacter and Salmonella in animals and their contribution to human disease: A review. Zoonoses Public Health 2019, 66, 562–578. [Google Scholar] [CrossRef] [PubMed]
- Piatek, J.; Sommermeyer, H.; Bernatek, M.; Ciechelska-Rybarczyk, A.; Oleskow, B.; Mikkelsen, L.; Barken, K. Persistent infection by Salmonella enterica servovar Typhimurium: Are synbiotics a therapeutic option?—A case report. Benef. Microbes 2019, 10, 211–217. [Google Scholar] [CrossRef] [PubMed]
- CDC. Antibiotic resistance threats in the United States. Centers Dis. Control Prev. 2019, 114. [Google Scholar]
- WHO. Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Hall, C.W.; Mah, T.F. Molecular mechanisms of biofilm-based antibiotic resistance and tolerance in pathogenic bacteria. FEMS Microbiol. Rev. 2017, 41, 276–301. [Google Scholar] [CrossRef]
- Sultan, I.; Rahman, S.; Jan, A.T.; Siddiqui, M.; Mondal, A.; Haq, Q. Antibiotics, resistome and resistance mechanisms: A bacterial perspective. Front. Microbiol. 2018, 9, 2066. [Google Scholar] [CrossRef] [Green Version]
- Sharma, V.K.; Johnson, N.; Cizmas, L.; Mcdonald, T.J.; Kim, H. A review of the influence of treatment strategies on antibiotic resistant bacteria and antibiotic resistance genes. Chemosphere 2016, 150, 702–714. [Google Scholar] [CrossRef]
- Chylkova, T.; Cadena, M.; Ferreiro, A.; Pitesky, M. Susceptibility of Salmonella biofilm and planktonic bacteria to common disinfectant agents used in poultry processing. J. Food Prot. 2017, 80, 1072–1079. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Ding, L.; Li, K.; Huang, H.; Hu, H.; Geng, J.; Xu, K.; Ren, H. Estimation of spatial distribution of quorum sensing signaling in sequencing batch biofilm reactor (SBBR) biofilms. Sci. Total Environ. 2018, 612, 405–414. [Google Scholar] [CrossRef]
- Eng, S.; Pusparajah, P.; Ab Mutalib, N.; Ser, H.; Chan, K.; Lee, L.H. Salmonella: A review on pathogenesis, epidemiology and antibiotic resistance. Front. Life Sci. 2015, 8, 284–293. [Google Scholar] [CrossRef] [Green Version]
- Verderosa, A.; Totsika, M.; Fairfull-smith, K. Bacterial Biofilm Eradication Agents: A Current Review. Front. Chem. 2019, 7, 824. [Google Scholar] [CrossRef] [Green Version]
- Steenackers, H.; Hermans, K.; Vanderleyden, J.; De Keersmaecker, S. Salmonella biofilms: An overview on occurrence, structure, regulation and eradication. Food Res. Int. 2012, 45, 502–531. [Google Scholar] [CrossRef]
- Singh, S.; Singh, S.K.; Chowdhury, I.; Singh, R. Understanding the Mechanism of Bacterial Biofilms Resistance to Antimicrobial Agents. Open Microbiol. J. 2017, 11, 53–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, J.; Bassler, B. Surviving as a Community: Antibiotic Tolerance and Persistence in Bacterial Biofilms. Cell Host Microbe 2019, 26, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Moraes, J.O.; Cruz, E.A.; Souza, E.G.F.; Oliveira, T.C.M.; Alvarenga, V.O.; Peña, W.E.L.; Sant’Ana, A.S.; Magnani, M. Predicting adhesion and biofilm formation boundaries on stainless steel surfaces by five Salmonella enterica strains belonging to different serovars as a function of pH, temperature and NaCl concentration. Int. J. Food Microbiol. 2018, 281, 90–100. [Google Scholar] [CrossRef]
- Pande, V.; McWhorter, A.; Chousalkar, K. Salmonella enterica isolates from layer farm environments are able to form biofilm on eggshell surfaces. Biofouling 2016, 32, 699–710. [Google Scholar] [CrossRef]
- Alibi, S.; Ben, W.; Ramos-vivas, J.; Ali, M.; Touati, R.; Boukadida, J.; Navas, J.; Ben, H. Anti-oxidant, antibacterial, anti-biofilm, and anti-quorum sensing activities of four essential oils against multidrug-resistant bacterial clinical isolates. Curr. Res. Transl. Med. 2020, 68, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Leal, A.; Braga, A.; de Araujo, B.; Rodriguez, A.; de Carcalho, A.; da Silva, K.; Junio, E.; de Sousa Leite, T.; Amorim, M. Antimicrobial action of essential oil of Lippia origanoides HBK. Clin. Microbiol. Biochem. Technol. 2019, 5, 7–12. [Google Scholar] [CrossRef] [Green Version]
- Čabarkapa, I.; Čolović, R.; Đuragić, O.; Popović, S.; Milanov, D.; Pezo, L. Anti-biofilm activities of essential oils rich in carvacrol and thymol against Salmonella Enteritidis. Biofouling 2019, 35, 361–375. [Google Scholar] [CrossRef]
- Swamy, M.; Akhtar, M.; Sinniah, U. Antimicrobial properties of plant essential oils against human pathogens and their mode of action: An updated review. Evid.-Based Complement. Altern. Med. 2016, 2016. [Google Scholar] [CrossRef]
- Cáceres, M.; Hidalgo, W.; Stashenko, E.; Torres, R.; Ortiz, C. Essential Oils of Aromatic Plants with Antibacterial, Anti-Biofilm and Anti-Quorum Sensing Activities against Pathogenic Bacteria. Antibiotics 2020, 9, 147. [Google Scholar] [CrossRef] [Green Version]
- Lambert, R.J.W.; Skandamis, P.N.; Coote, P.J.; Nychas, G.E. A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. J. Appl. Microbiol. 2001, 91, 453–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouhayoun, J.P. Penetrating the plaque biofilm: Impact of essential oil mouthwash. J. Clin. Periodontol. 2003, 30, 10–12. [Google Scholar] [CrossRef]
- Jonas, K.; Tomenius, H.; Kader, A.; Normark, S.; Römling, U.; Belova, L.; Melefors, Ö. Roles of curli, cellulose and BapA in Salmonella biofilm morphology studied by atomic force microscopy. BMC Microbiol. 2007, 7, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Lasa, I.; Del Pozo, J.L.; Leiva, J.; Penadés, J.R. Bacterial biofilms and infection. An. Sist. Sanit. Navar. 2005, 28, 163–175. [Google Scholar] [CrossRef]
- Crump, J.A.; Heyderman, R.S. A perspective on invasive Salmonella disease in Africa. Clin. Infect. Dis. 2015, 61, S235–S240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial Activity of Some Essential Oils—Present Status and Future Perspectives. Medicines 2017, 4, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kachur, K.; Suntres, Z. The antibacterial properties of phenolic isomers, carvacrol and thymol. Crit. Rev. Food Sci. Nutr. 2020, 60, 3042–3053. [Google Scholar] [CrossRef] [PubMed]
- Gyawali, R.; Ibrahim, S.A. Natural products as antimicrobial agents. Food Control 2014, 46, 412–429. [Google Scholar] [CrossRef]
- Sarrazin, S.L.F.; Da Silva, L.A.; Oliveira, R.B.; Raposo, J.D.A.; Da Silva, J.K.R.; Salimena, F.R.G.; Maia, J.G.S.; Mourão, R.H.V. Antibacterial action against food-borne microorganisms and antioxidant activity of carvacrol-rich oil from Lippia origanoides Kunth. Lipids Health Dis. 2015, 14, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Boskovic, M.; Zdravkovic, N.; Ivanovic, J.; Janjic, J.; Djordjevic, J.; Starcevic, M.; Baltic, M. Antimicrobial Activity of Thyme (Tymus vulgaris) and Oregano (Origanum vulgare) Essential Oils against Some Food-borne Microorganisms. Procedia Food Sci. 2015, 5, 18–21. [Google Scholar] [CrossRef] [Green Version]
- Duarte, M.; Leme, E.; Delarmelina, C.; Soares, A.; Figueira, G.; Sartoratto, A. Activity of essential oils from Brazilian medicinal plants on Escherichia coli. J. Ethnopharmacol. 2007, 111, 197–201. [Google Scholar] [CrossRef]
- Silva, N.; Fernandes, A. Biological properties of medicinal plants: A review of their antimicrobial activity. J. Venom. Anim. Toxins Incl. Trop. Dis. 2010, 16, 402–413. [Google Scholar] [CrossRef]
- Kifer, D.; Mužinić, V.; Klaric, M. Antimicrobial potency of single and combined mupirocin and monoterpenes, thymol, menthol and 1,8-cineole against Staphylococcus aureus planktonic and biofilm growth. J. Antibiot. 2016, 69, 689–696. [Google Scholar] [CrossRef]
- Nostro, A.; Roccaro, A.S.; Bisignano, G.; Marino, A.; Cannatelli, M.A.; Pizzimenti, F.C.; Cioni, P.L.; Procopio, F.; Blanco, A.R. Effects of oregano, carvacrol and thymol on Staphylococcus aureus and Staphylococcus epidermidis biofilms. J. Med. Microbiol. 2007, 56, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Toyofuku, M.; Inaba, T.; Kiyokawa, T.; Obana, N.; Yawata, Y.; Nomura, N. Environmental factors that shape biofilm formation. Biosci. Biotechnol. Biochem. 2016, 80, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Tursi, S.A.; Puligedda, R.D.; Szabo, P.; Nicastro, L.K.; Miller, A.L.; Qiu, C.; Gallucci, S.; Relkin, N.R.; Buttaro, B.A.; Dessain, S.K.; et al. Salmonella Typhimurium biofilm disruption by a human antibody that binds a pan-amyloid epitope on curli. Nat. Commun. 2020, 11, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eran, Z.; Akçelik, M.; Yazıcı, B.C.; Özcengiz, G.; Akçelik, N. Regulation of biofilm formation by marT in Salmonella Typhimurium. Mol. Biol. Rep. 2020, 47, 5041–5050. [Google Scholar] [CrossRef] [PubMed]
- Inamuco, J.; Veenendaal, A.K.J.; Burt, S.A.; Post, J.A.; Tjeerdsma-van Bokhoven, J.L.M.; Haagsman, H.P.; Veldhuizen, E.J.A. Sub-lethal levels of carvacrol reduce Salmonella Typhimurium motility and invasion of porcine epithelial cells. Vet. Microbiol. 2012, 157, 200–207. [Google Scholar] [CrossRef]
- Alvarez, M.; Ortega, L.; Gutierrez, M.; Berna, A.; Rodriguez, I.; Gonzalez, G.; Ponce, A.; del R. Moreira, M.; Roura, S.; Ayala, J. Oregano essential oil-pectin edible films as anti-quorum sensing and food antimicrobial agents. Front. Microbiol. 2014, 5, 699. [Google Scholar] [CrossRef] [Green Version]
- Tursi, S.A.; Tükel, Ç. Curli-Containing Enteric Biofilms Inside and Out: Matrix Composition, Immune Recognition, and Disease Implications. Microbiol. Mol. Biol. Rev. 2018, 82, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Stashenko, E.E.; Martínez, J.R.; Ruíz, C.A.; Arias, G.; Durán, C.; Salgar, W.; Cala, M. Lippia origanoides chemotype differentiation based on essential oil GC-MS and principal component analysis. J. Sep. Sci. 2010, 33, 93–103. [Google Scholar] [CrossRef]
- O’Toole, G.A. Microtiter Dish Biofilm Formation Assay. J. Vis. Exp. 2011, 353913. [Google Scholar] [CrossRef] [PubMed]
- Molhoek, E.M.; van Dijk, A.; Veldhuizen, E.J.A.; Haagsman, H.P.; Bikker, F.J. A cathelicidin-2-derived peptide effectively impairs Staphylococcus epidermidis biofilms. Int. J. Antimicrob. Agents 2011, 37, 476–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischer, E.R.; Hansen, B.T.; Nair, V.; Hoyt, F.H.; Dorward, D.W. Scanning Electron Microscopy. Curr. Protoc. Microbiol. 2012, 25, 47. [Google Scholar] [CrossRef]
- Untergasser, A.; Cutcutache, I.; Koressaar, T.; Ye, J.; Faircloth, B.C.; Remm, M.; Rozen, S.G. Primer3—new capabilities and interfaces. Nucleic Acids Res. 2012, 40, e115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kibbe, W.A. OligoCalc: An online oligonucleotide properties calculator. Nucleic Acids Res. 2007, 35, W43–W46. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real- Time Quantitative PCR and the 2−ΔΔCt Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Massey, F.J. The Kolmogorov-Smirnov Test for Goodness of Fit. J. Am. Stat. Assoc. 1951, 46, 68–78. [Google Scholar] [CrossRef]
- RStudio Team. RStudio: Integrated Development for R. RStudio; PBC: Boston, MA, USA, 2018. [Google Scholar]



| Essential Oils | Antimicrobial | ||||
|---|---|---|---|---|---|
| S. typhimurium | S. enteritidis | ||||
| Code | Plant | MIC50 | MBC | MIC50 | MBC |
| mg mL−1 | |||||
| LACA | Lippia alba (Mill.) (carvona) | >1.5 | >1.5 | >1.5 | >1.5 |
| LACI | L. alba (citral) | >1.5 | >1.5 | >1.5 | >1.5 |
| CN | Cymbopogon nardus (L.) | >1.5 | >1.5 | >1.5 | >1.5 |
| CM | C. martini (Roxb.) | 0.75 ± 0.07 | 1.5 ± 0.01 | 0.75 ± 0.02 | 1.5 ± 0.04 |
| CF | C. flexuosus (Nees ex Steud.) | >1.5 | >1.5 | >1.5 | >1.5 |
| LTC I | L. origanoides (Kunth) (thymol-carvacrol I) | 0.75 ± 0.16 | 1.5 ± 0.05 | 0.37 ± 0.02 | 0.75 ± 0.03 |
| LTC II | L. origanoides (thymol-carvacrol II) | 0.37 ± 0.08 | 0.75 ± 0.06 | 0.37 ± 0.03 | 0.75 ± 0.02 |
| LOF | L. origanoides (phellandrene) | >1.5 | >1.5 | >1.5 | >1.5 |
| RO | Rosmarinus officinalis (L.) | >1.5 | >1.5 | >1.5 | >1.5 |
| SO | Salvia officinalis (L.) | >1.5 | >1.5 | >1.5 | >1.5 |
| SG | Swinglea glutinosa (Blanco) | >1.5 | >1.5 | >1.5 | >1.5 |
| TL | Tagetes lucida (Cav.) | >1.5 | >1.5 | >1.5 | >1.5 |
| TV | Thymus vulgaris (L.) | 0.75 ± 0.05 | 1.5 ± 0.03 | 0.75 ± 0.02 | 1.5 ± 0.05 |
| SV | Satureja viminea (L.) | >1.5 | >1.5 | >1.5 | >1.5 |
| CO | Cananga odorata (Lam.) | >1.5 | >1.5 | >1.5 | >1.5 |
| Essential Oils | Anti-Biofilm | ||||
|---|---|---|---|---|---|
| S. typhimurium | S. enteritidis | ||||
| Code | Plant | MICB | Inhibition (%) | MICB | Inhibition (%) |
| mg mL−1 | |||||
| LACA | Lippia alba (Mill.) (carvona) | >1.5 | ND | >1.5 | |
| LACI | L. alba (citral) | >1.5 | ND | >1.5 | ND |
| CN | Cymbopogon nardus (L.) | >1.5 | ND | >1.5 | ND |
| CM | C. martini (Roxb.) | 0.37 ± 0.02 | 50.87 ± 0.04 | 0.37 ± 0.10 | 46.72 ± 0.05 |
| CF | C. flexuosus (Nees ex Steud.) | >1.5 | ND | >1.5 | ND |
| LTC I | L. origanoides (Kunth) (thymol-carvacrol I) | 0.18 ± 0.01 | 62.18 ± 0.06 | 0.18 ± 0.01 | 61.32 ± 0.08 |
| LTC II | L. origanoides (thymol-carvacrol II) | 0.18 ± 0.18 | 66.64 ± 0.08 | 0.18 ± 0.08 | 65.64 ± 0.01 |
| LOF | L. origanoides (phellandrene) | >1.5 | ND | 1.5 ± 0.06 | 64.38 ± 0.03 |
| RO | Rosmarinus officinalis (L.) | >1.5 | ND | >1.5 | ND |
| SO | Salvia officinalis (L.) | >1.5 | ND | >1.5 | ND |
| SG | Swinglea glutinosa (Blanco) | >1.5 | ND | >1.5 | ND |
| TL | Tagetes lucida (Cav.) | 1.5 ± 0.07 | 53.00 ± 0.10 | 1.5 ± 0.02 | 67.02 ± 0.23 |
| TV | Thymus vulgaris (L.) | 0.37 ± 0.7 | 59.33 ± 0.14 | 0.37 ± 0.10 | 68.52 ± 0.21 |
| SV | Satureja viminea (L.) | >1.5 | ND | >1.5 | ND |
| CO | Cananga odorata (Lam.) | >1.5 | ND | >1.5 | ND |
| Code | Plant Species | Identified Metabolites (%) |
|---|---|---|
| CM | C. martini (Roxb.) |  |
| LTC I | L. origanoides (Kunth) (thymol-carvacrol I) |  |
| LTC II | L. origanoides (thymol-carvacrol II) |  |
| TV | Thymus vulgaris (L.) |  |
| Genes | Forward (5′–3′) | Reverse (3′–5′) |
|---|---|---|
| gnd | ACGCAGAAAACGCTGGTATC | CCACTCGGTATGGAAAATGC |
| gst | TGTGGATGAGTCGCTTTCAG | GCAACGGTCGGTCTTTTT |
| sdiA | GTCATCCCGTCCCCTTTAC | GGTTCGGCAACATCACAC |
| luxR | GATTGCTGCCCTCTGTTTTC | CGGCTTCTTCCAGTGAAT |
| luxS | CGACCACCTCAACGGTAA | GCACATCACGCTCCAGAATA |
| QseB | GCGAAAAGGGTAAACAGG | CGCAGTAAGAGTTCCAGCA |
| csgA | ATGCCCGTAAATCTGAAACG | ACCAACCTGACGCACCATTA |
| csgB | CGCATGTCGCTAACAAGGTA | ATTATCCGTGCCGACTTGAC |
| csgD | GATGGAAGCGGATAAGAAGC | GACTCGGTGCTGTTGTAGC |
| fliZ | CGGTTTCAAGCAGTATTTGT | CGGTAAAGGGGGATTTCTG |
| flhD | TTCCGCCTCGGTATCAAC | GCCGTATCGTCCACTTCATT |
| motB | AGTGGAAAAGCAGCCGAATA | GCAACCCCTCCTGAACTAAA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guillín, Y.; Cáceres, M.; Torres, R.; Stashenko, E.; Ortiz, C. Effect of Essential Oils on the Inhibition of Biofilm and Quorum Sensing in Salmonella enteritidis 13076 and Salmonella typhimurium 14028. Antibiotics 2021, 10, 1191. https://doi.org/10.3390/antibiotics10101191
Guillín Y, Cáceres M, Torres R, Stashenko E, Ortiz C. Effect of Essential Oils on the Inhibition of Biofilm and Quorum Sensing in Salmonella enteritidis 13076 and Salmonella typhimurium 14028. Antibiotics. 2021; 10(10):1191. https://doi.org/10.3390/antibiotics10101191
Chicago/Turabian StyleGuillín, Yuliany, Marlon Cáceres, Rodrigo Torres, Elena Stashenko, and Claudia Ortiz. 2021. "Effect of Essential Oils on the Inhibition of Biofilm and Quorum Sensing in Salmonella enteritidis 13076 and Salmonella typhimurium 14028" Antibiotics 10, no. 10: 1191. https://doi.org/10.3390/antibiotics10101191