A Label-Free Impedance Immunosensor Using Screen-Printed Interdigitated Electrodes and Magnetic Nanobeads for the Detection of E. coli O157:H7
Abstract
:1. Introduction
2. Experimental Section
2.1. Bacterial Culture
2.2. Biological and Chemical Reagents
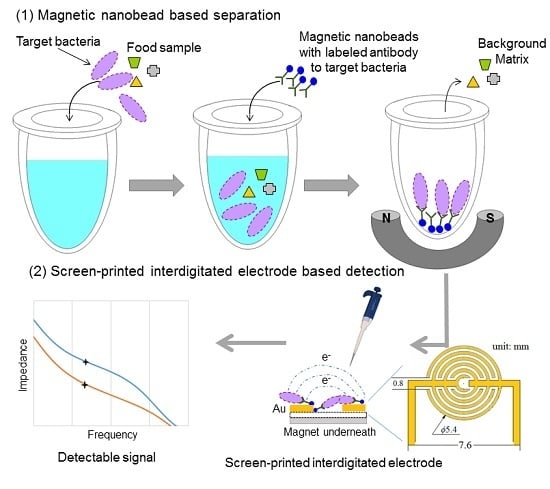
2.3. Screen-Printed Interdigitated Electrodes and Magnetic Nanobeads
2.4. Impedance Measurement
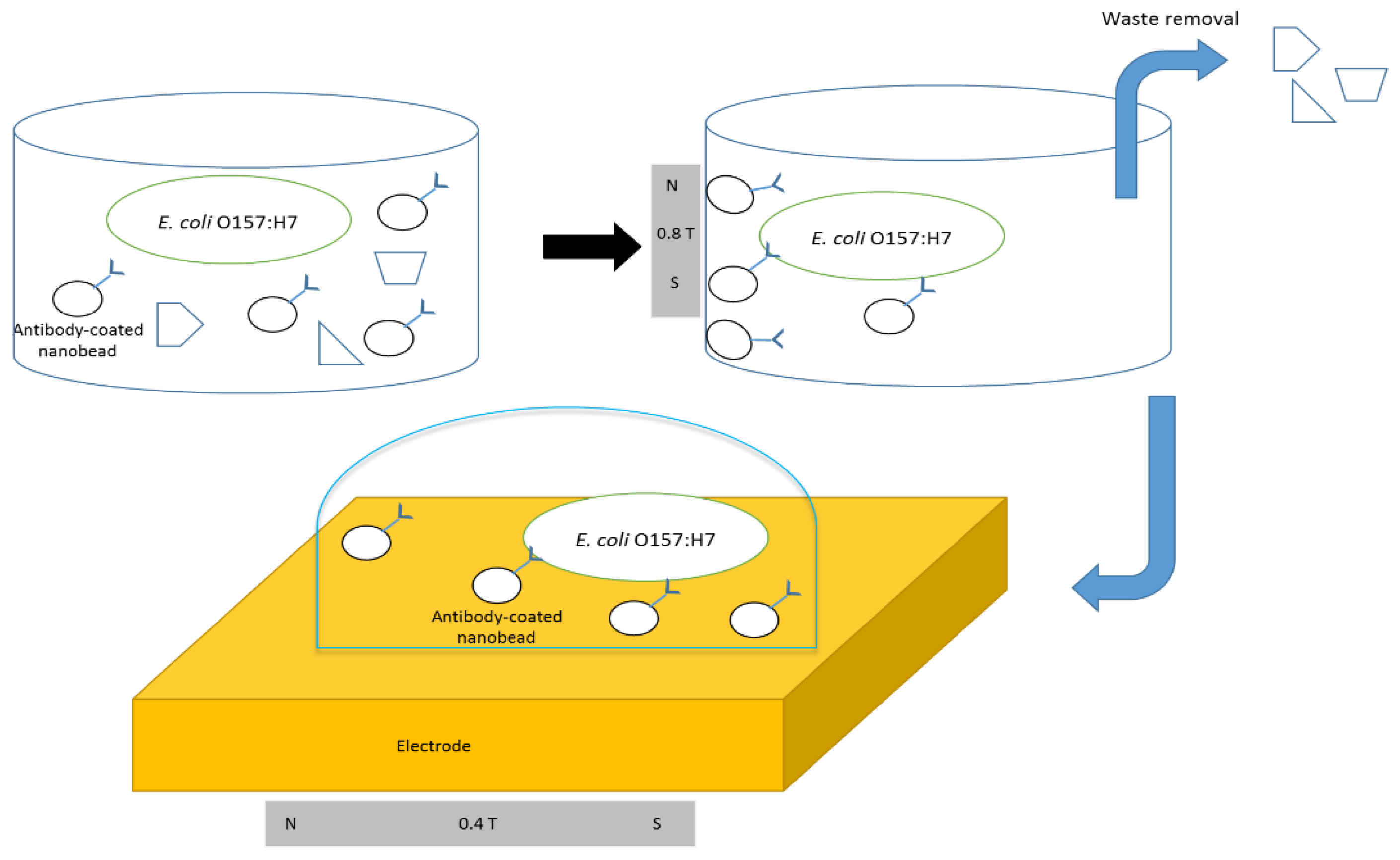
2.5. Immunomagnetic Separation of E. coli O157:H7
2.6. Detection of E. coli O157:H7
2.7. The Preparation, Inoculation and Detection of Ground Beef Samples
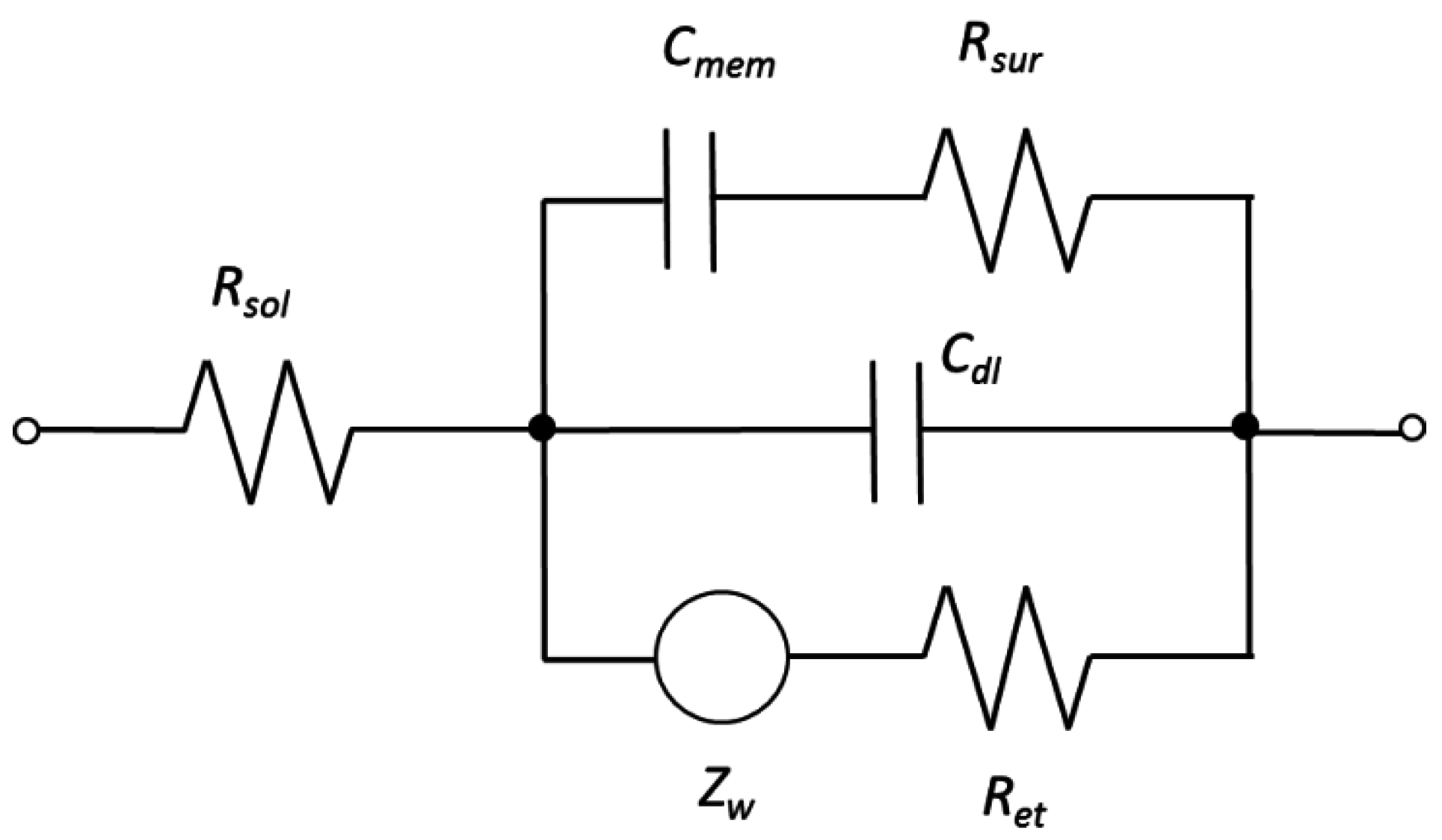
2.8. Equivalent Circuit Modeling and Statistical Analysis
2.9. Electron Microscopy
2.10. Comsol Simulation
3. Results and Discussion
3.1. Characterization of Impedance Spectrum Data
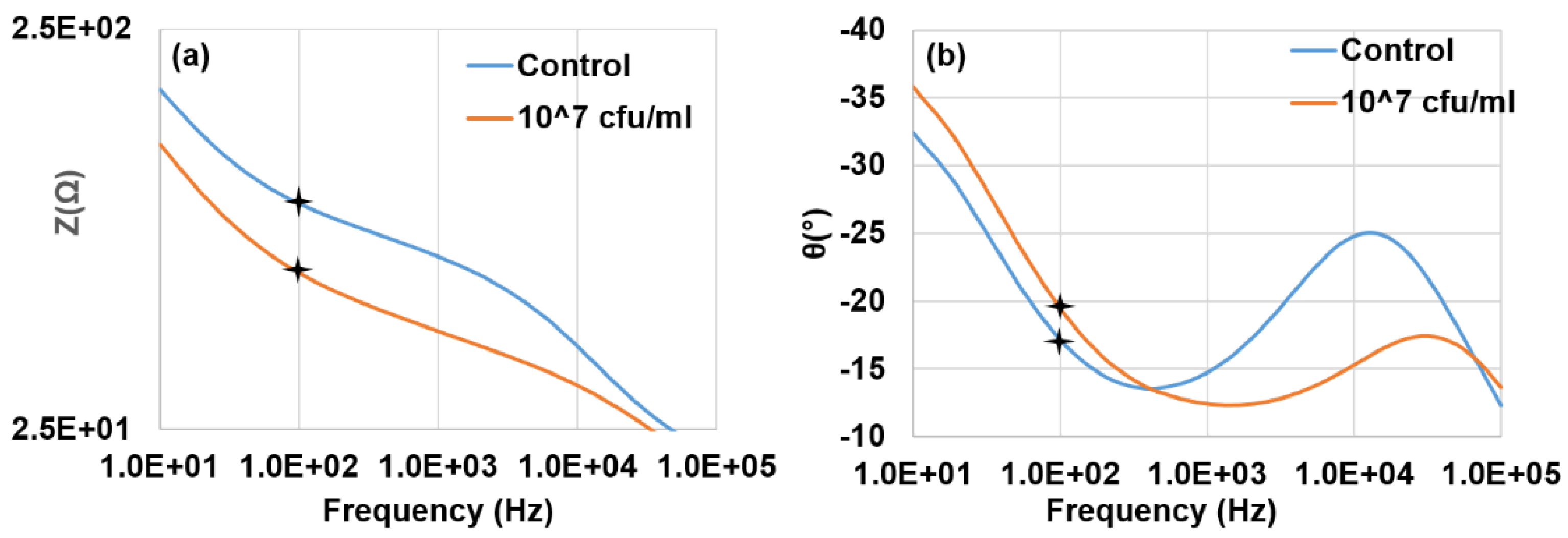
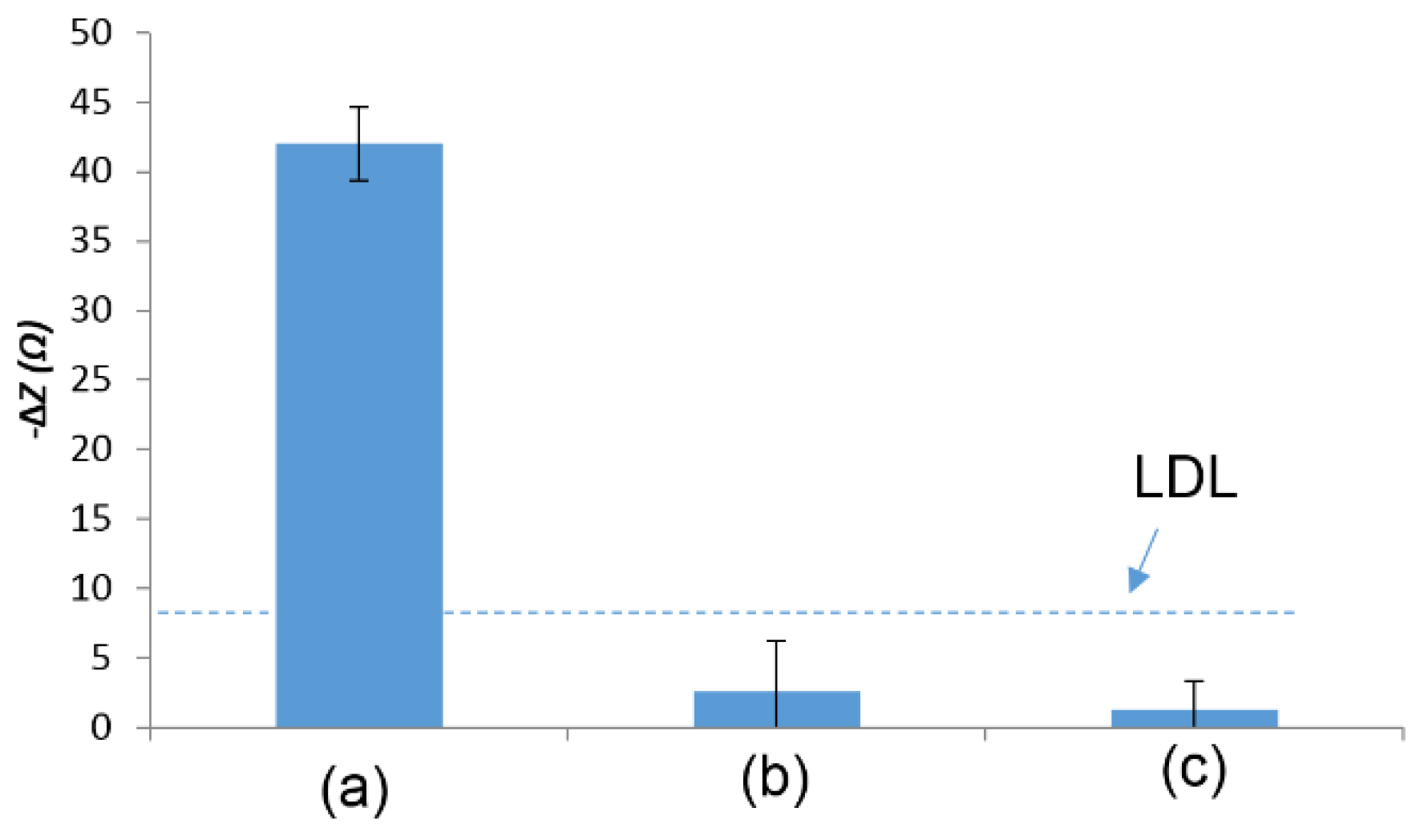
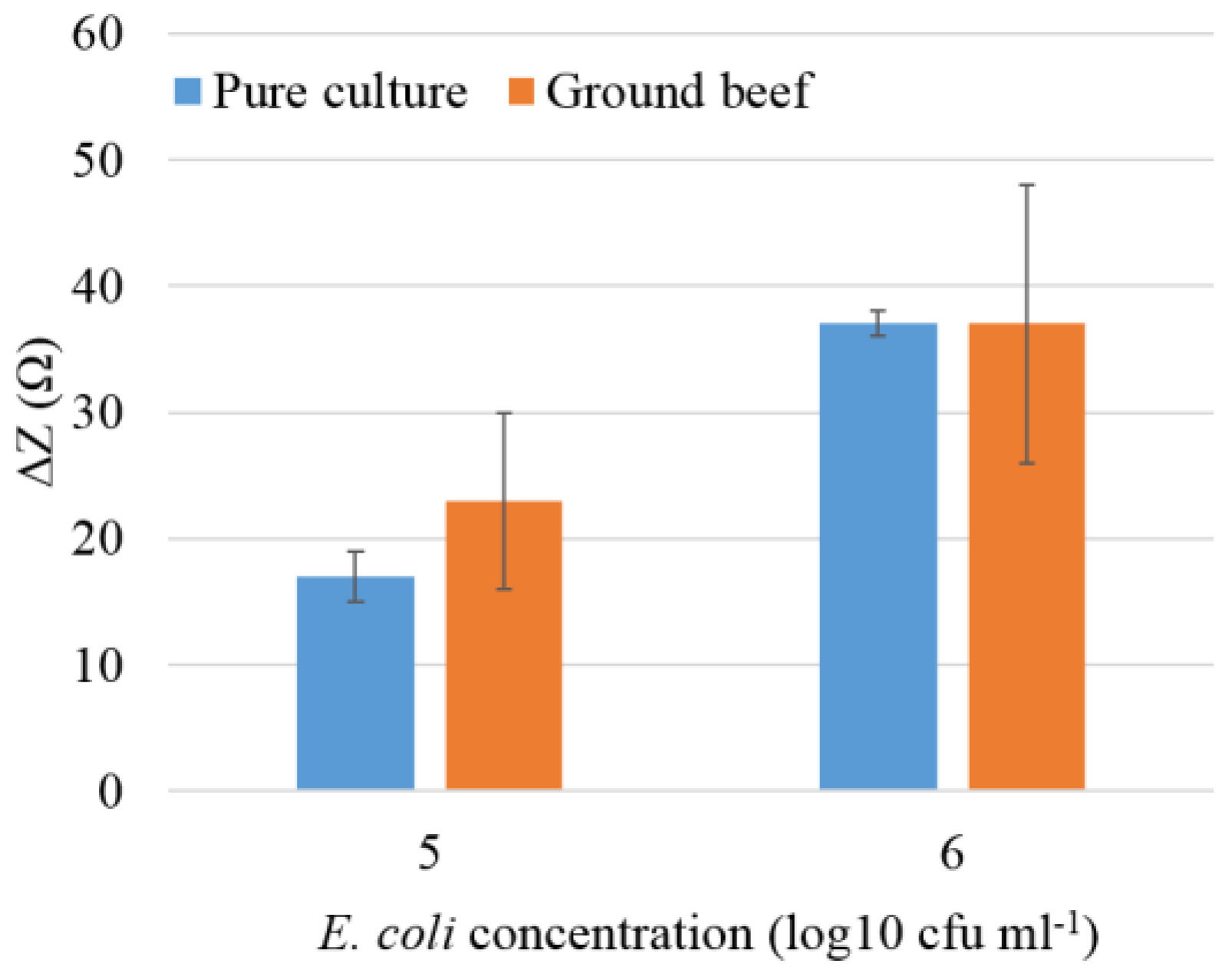
3.2. Detection of E. coli O157:H7
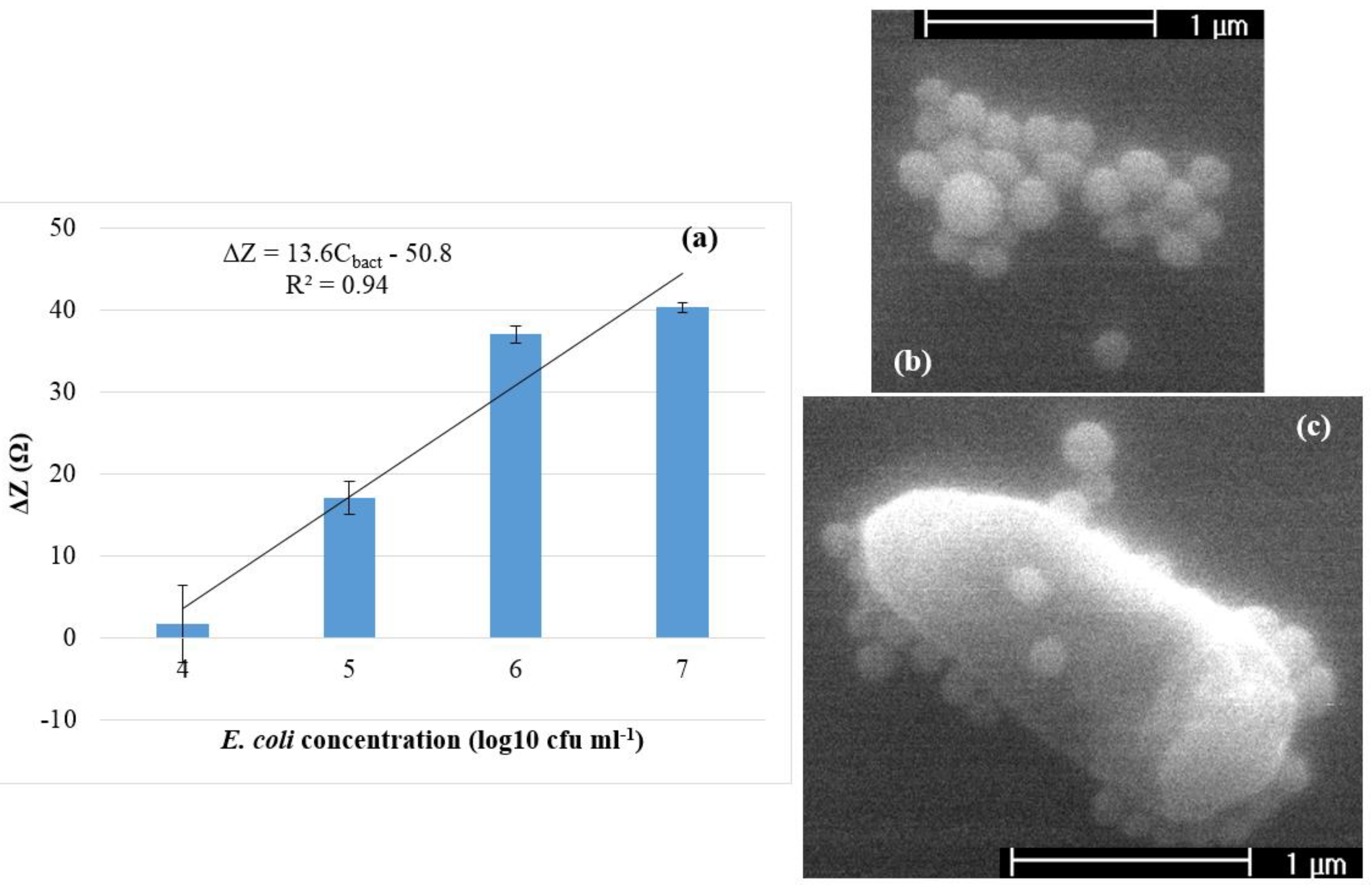
3.3. Simulation of Impedance Change
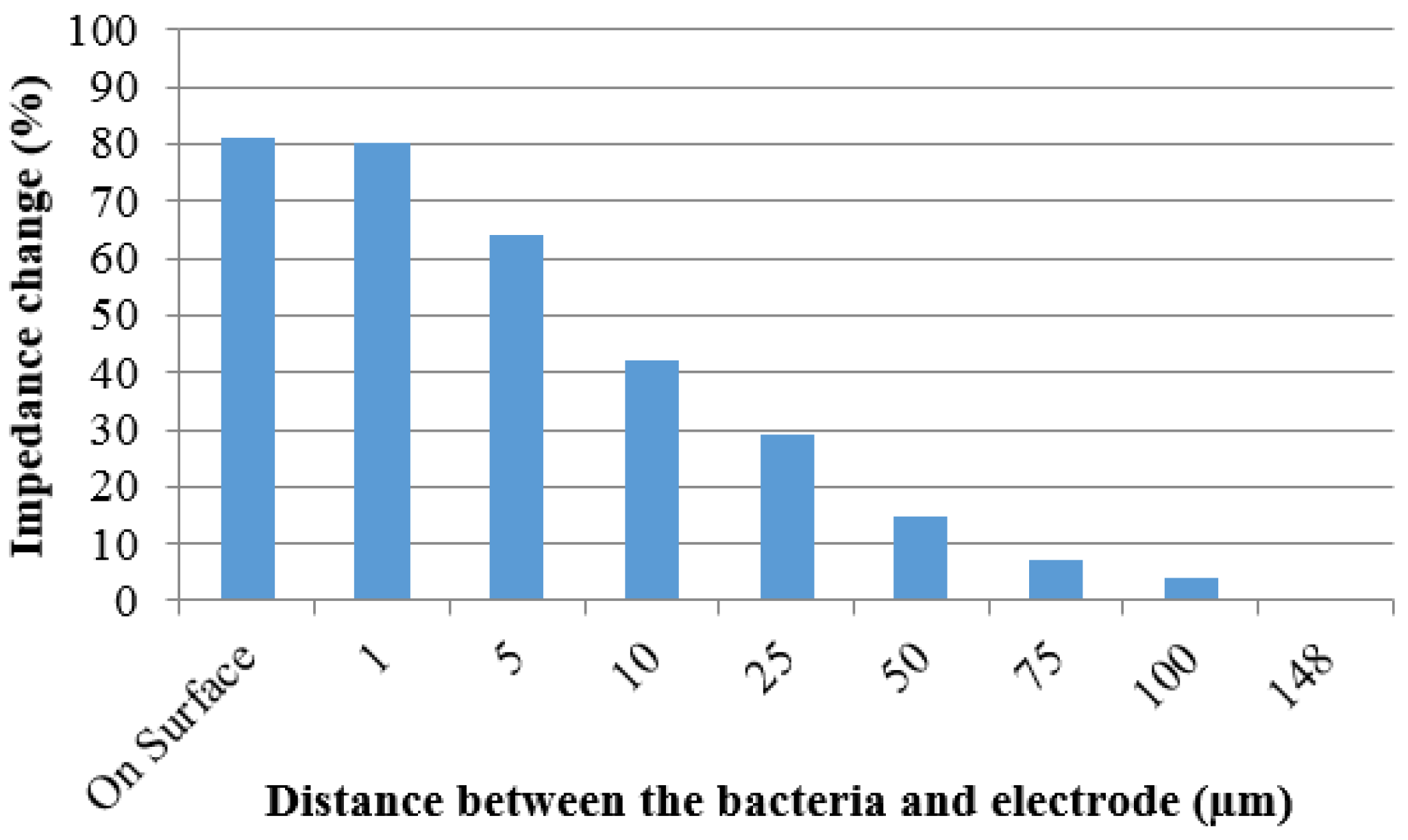
4. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Schmid-Hempel, P.; Frank, S.A. Pathogenesis, Virulence, and Infective Dose. PLoS Pathog. 2007, 3, 1372–1373. [Google Scholar] [CrossRef] [PubMed]
- Scallan, E.; Hoekstra, R.; Angulo, F.; Tauxe, R.; Widdowson, M.; Roy, S.; Jones, J.; Griffin, P. Foodborne Illness Acquired in the United States—Major Pathogens. Emerg. Infect. Dis. 2011, 17, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.D.; Rigdon, C.E.; Ball, J.; Rounds, J.M.; Klos, R.F.; Brennan, B.M.; Arends, K.D.; Kennelly, P.; Hedberg, C.; Smith, K.E. Use of Traceback Methods to Confirm the Source of a Multistate Escherichia coli O157:H7 Outbreak due to In-Shell Hazelnuts. J. Food Prot. 2012, 75, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Rekow, C.L.; Brashears, M.M.; Brooks, J.C.; Loneragan, G.H.; Gragg, S.E.; Miller, M.F. Implementation of Targeted Interventions to Control Escherichia coli O157:H7 in a Commercial Abattoir. Meat Sci. 2011, 87, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Wang, J.; Qiu, Z.; Jin, M.; Wang, X.; Chen, Z.; Li, J.; Cao, F. QCM Immunosensor Detection of Escherichia coli O157:H7 Based on Beacon Immunomagnetic Nanoparticles and Catalytic Growth of Colloidal Gold. Biosens. Bioelectron. 2011, 26, 3376–3381. [Google Scholar] [CrossRef] [PubMed]
- Poitras, C.; Tufenkji, N. A QCM-D-Based Biosensor for E. coli O157: H7 Highlighting the Relevance of the Dissipation Slope as a Transduction Signal. Biosens. Bioelectron. 2009, 24, 2137–2142. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Li, Y.; Su, X.; Slavik, M.; Ying, Y.; Wang, J. QCM Immunosensor with Nanoparticle Amplification for Detection of Escherichia coli O157: H7. Sens. Instrum. Food Qual. Saf. 2007, 1, 161–168. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, R.; Wang, Y.; Su, X.; Ying, Y.; Wang, J.; Li, Y. Evaluation of Different Micro/Nanobeads Used as Amplifiers in QCM Immunosensor for More Sensitive Detection of E. coli O157:H7. Biosens. Bioelectron. 2011, 29, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Waswa, J.; Irudayaraj, J.; DebRoy, C. Direct Detection of E. Coli O157:H7 in Selected Food Systems by a Surface Plasmon Resonance Biosensor. LWT Food Sci. Technol. 2007, 40, 187–192. [Google Scholar] [CrossRef]
- Wang, Y.; Ye, Z.; Si, C.; Ying, Y. Subtractive Inhibition Assay for the Detection of E. coli O157:H7 Using Surface Plasmon Resonance. Sensors 2011, 11, 2728–2739. [Google Scholar] [CrossRef] [PubMed]
- Euma, N.; Yeom, S.; Kwon, D.; Kim, H.; Kang, S. Enhancement of Sensitivity Using Gold Nanorods-Antibody Conjugator for Detection of E. coli O157:H7. Sens. Actuators B Chem. 2010, 143, 784–788. [Google Scholar] [CrossRef]
- Subramanian, A.; Irudayaraj, J.; Ryan, T. A Mixed Self-Assembled Monolayer-Based Surface Plasmon Immunosensor for Detection of E. coli O157:H7. Biosens. Bioelectron. 2006, 21, 998–1006. [Google Scholar] [CrossRef] [PubMed]
- Varshney, M.; Li, Y. Interdigitated Array Microelectrode Based Impedance Biosensor Coupled with Magnetic Nanoparticle–Antibody Conjugates for Detection of Escherichia coli O157:H7 in Food Samples. Biosens. Bioelectron. 2007, 22, 2408–2414. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, D.; De, A.; Chaudhuri, C.R.; Bandyopadhyay, K.; Sen, P. Label Free Polyaniline Based Impedimetric Biosensor for Detection of E. coli O157: H7 Bacteria. Sens. Actuators B Chem. 2012, 171–172, 916–923. [Google Scholar] [CrossRef]
- Chan, K.Y.; Ye, W.W.; Zhang, Y.; Xiao, L.D.; Leung, P.H.M.; Li, Y.; Yang, M. Ultrasensitive Detection of E. coli O157: H7 with Biofunctional Magnetic Bead Concentration via Nanoporous Membrane Based Electrochemical Immunosensor. Biosens. Bioelectron. 2013, 41, 532–537. [Google Scholar] [CrossRef] [PubMed]
- Radke, S.M.; Alocilja, E.C. A High Density Microelectrode Array Biosensor for Detection of E. coli O 157:H7. Biosens. Bioelectron. 2005, 20, 1662–1667. [Google Scholar] [CrossRef] [PubMed]
- Varshney, M.; Li, Y.; Srinivasan, B.; Tung, S. A Label-Free, Microfluidics and Interdigitated Array Microelectrode-based Impedance Biosensor in Combination with Nanoparticles Immunoseparation for Detection of Escherichia coli O157:H7 in Food Samples. Sens. Actuators B Chem. 2007, 128, 99–107. [Google Scholar] [CrossRef]
- Varshney, M.; Li, Y. Double Interdigitated Array Microelectrode-Based Impedance Biosensor for Detection of Viable Escherichia coli O157: H7 in Growth Medium. Talanta 2008, 74, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Chen, S.; Chuang, Y.; Lu, Y.; Shen, T.Y.; Chang, C.A.; Lin, C. Disposable Amperometric Immunosensing Strips Fabricated by Au Nanoparticles-Modified Screen-Printed Carbon Electrodes for the Detection of Foodborne Pathogen Escherichia coli O157:H7. Biosens. Bioelectron. 2008, 23, 1832–1837. [Google Scholar] [CrossRef] [PubMed]
- Setterington, E.B.; Alocilja, E.C. Rapid Electrochemical Detection of Polyaniline-labeled Escherichia coli O157: H7. Biosens. Bioelectron. 2011, 26, 2208–2214. [Google Scholar] [CrossRef] [PubMed]
- Barreiros dos Santos, M.; Agusil, J.P.; Prieto-Simón, B.; Sporer, C.; Teixeira, V.; Samitier, J. Highly Sensitive Detection of Pathogen Escherichia coli O157:H7 by Electrochemical Impedance Spectroscopy. Biosens. Bioelectron. 2013, 45, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Gehring, G.; Albin, D.M.; Bhunia, A.K.; Reed, S.A.; Tu, S.I.; Uknalis, J. Antibody Microarray Detection of Escherichia coli O157: H7: Quantification, Assay Limitations, and Capture Efficiency. Anal. Chem. 2006, 78, 6601–6607. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Callaway, Z.; Lum, J.; Wang, R.; Lin, J.; Li, Y. Exploiting Enzyme Catalysis in Ultra-Low Ion Strength Media for Impedance Biosensing of Avian Influenza Virus Using a Bare Interdigitated Electrode. Anal. Chem. 2014, 86, 1965–1971. [Google Scholar] [CrossRef] [PubMed]
- Asami, K.; Hanai, T.; Koizumi, N. Dielectric Analysis of Escherichia coli Suspensions in the Light of the Theory of Interfacial Polarization. Biophys. J. 1980, 31, 215–228. [Google Scholar] [CrossRef]
- Gielen, F.; Pereira, F.; Demello, A.; Edel, J. High-Resolution Local Imaging of Temperature in Dielectrophoretic Platforms. Anal. Chem. 2010, 82, 7509–7514. [Google Scholar] [CrossRef] [PubMed]
- Santos-Sacchi, J. Determination of Cell Capacitance Using the Exact Empirical Solution of dY/dCm and Its Phase Angle. Biophys. J. 2004, 87, 714–727. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Ruan, C.; Lin, J.; Li, M.; Cooney, L.; Oliver, W.; Li, Y.; Wang, A. Magnetic Nanoparticle Based Magnetophoresis for Efficient Separation of E. coli O157:H7. Trans. ASABE, 2011, 54, 1015–1024. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, R.; Lum, J.; Callaway, Z.; Lin, J.; Bottje, W.; Li, Y. A Label-Free Impedance Immunosensor Using Screen-Printed Interdigitated Electrodes and Magnetic Nanobeads for the Detection of E. coli O157:H7. Biosensors 2015, 5, 791-803. https://doi.org/10.3390/bios5040791
Wang R, Lum J, Callaway Z, Lin J, Bottje W, Li Y. A Label-Free Impedance Immunosensor Using Screen-Printed Interdigitated Electrodes and Magnetic Nanobeads for the Detection of E. coli O157:H7. Biosensors. 2015; 5(4):791-803. https://doi.org/10.3390/bios5040791
Chicago/Turabian StyleWang, Ronghui, Jacob Lum, Zach Callaway, Jianhan Lin, Walter Bottje, and Yanbin Li. 2015. "A Label-Free Impedance Immunosensor Using Screen-Printed Interdigitated Electrodes and Magnetic Nanobeads for the Detection of E. coli O157:H7" Biosensors 5, no. 4: 791-803. https://doi.org/10.3390/bios5040791