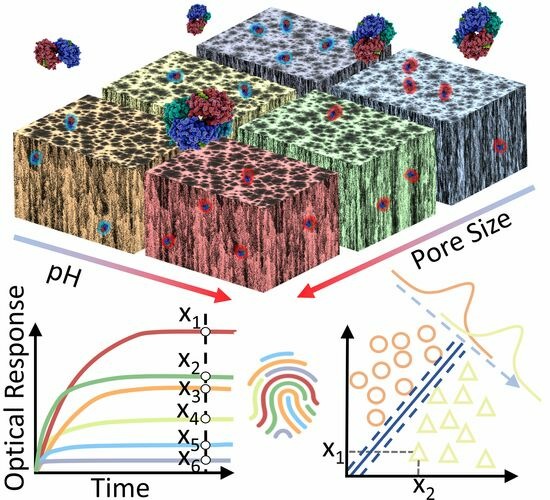
Protein Identification and Quantification Using Porous Silicon Arrays, Optical Measurements, and Machine Learning
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Single Layer PSi
2.2. Material Characterization
2.3. Optical Reflectance Measurements
2.4. Experimental Procedure
2.5. Data Analysis
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Naresh, V.; Lee, N. A review on biosensors and recent development of nanostructured materials-enabled biosensors. Sensors 2021, 21, 1109. [Google Scholar] [CrossRef]
- Velasco-Garcia, M.N.; Mottram, T. Biosensor technology addressing agricultural problems. Biosyst. Eng. 2003, 84, 1–12. [Google Scholar] [CrossRef]
- Griffiths, D.; Hall, G. Biosensors—What real progress is being made? Trends Biotechnol. 1993, 11, 122–130. [Google Scholar] [CrossRef]
- Grieshaber, D.; MacKenzie, R.; Vörös, J.; Reimhult, E. Electrochemical biosensors—Sensor principles and architectures. Sensors 2008, 8, 1400–1458. [Google Scholar] [CrossRef] [PubMed]
- Damborský, P.; Švitel, J.; Katrlík, J. Optical biosensors. Essays Biochem. 2016, 60, 91–100. [Google Scholar] [CrossRef]
- Ramanathan, K.; Danielsson, B. Principles and Applications of Thermal Biosensors. Biosens. Bioelectron. 2001, 16, 417–423. [Google Scholar] [CrossRef]
- Voiculescu, I.; Nordin, A.N. Acoustic wave based MEMS devices for biosensing applications. Biosens. Bioelectron. 2012, 33, 1–9. [Google Scholar] [CrossRef]
- Lin, V.S.Y.; Motesharei, K.; Dancil, K.P.S.; Sailor, M.J.; Ghadiri, M.R. A porous silicon-based optical interferometric biosensor. Science 1997, 278, 840–843. [Google Scholar] [CrossRef]
- Urmann, K.; Tenenbaum, E.; Walter, J.-G.; Segal, E. Porous Silicon Biosensors Employing Emerging Capture Probes. In Electrochemically Engineered Nanoporous Materials; Santos, A., Losic, D., Eds.; Springer: Cham, Switzerland, 2015; pp. 93–116. [Google Scholar]
- Mittal, S.; Kaur, H.; Gautam, N.; Mantha, A.K. Biosensors for breast cancer diagnosis: A review of bioreceptors, biotransducers and signal amplification strategies. Biosens. Bioelectron. 2017, 88, 217–231. [Google Scholar] [CrossRef]
- Wang, W.; Singh, S.; Zeng, D.L.; King, K.; Nema, S. Antibody structure, instability, and formulation. J. Pharm. Sci. 2007, 96, 1–26. [Google Scholar] [CrossRef]
- Wang, W.; Wang, X.; Cheng, N.; Luo, Y.; Lin, Y.; Xu, W.; Du, D. Recent advances in nanomaterials-based electrochemical (bio)sensors for pesticides detection. Trends Anal. Chem. 2020, 132, 116041. [Google Scholar] [CrossRef]
- Arshavsky-Graham, S.; Heuer, C.; Jiang, X.; Segal, E. Aptasensors versus immunosensors—Which will prevail? Eng. Life Sci. 2022, 22, 319–333. [Google Scholar] [CrossRef] [PubMed]
- Sande, M.G.; Rodrigues, J.L.; Ferreira, D.; Silva, C.J.; Rodrigues, L.R. Novel biorecognition elements against pathogens in the design of state-of-the-art diagnostics. Biosensors 2021, 11, 418. [Google Scholar] [CrossRef]
- Kodadek, T. Protein microarrays: Prospects and problems. Chem. Biol. 2001, 8, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Di Natale, C.; Battista, E.; Lettera, V.; Reddy, N.; Pitingolo, G.; Vecchione, R.; Causa, F.; Netti, P.A. Easy Surface Functionalization and Bioconjugation of Peptides as Capture Agents of a Microfluidic Biosensing Platform for Multiplex Assay in Serum. Bioconjug. Chem. 2021, 32, 1593–1601. [Google Scholar] [CrossRef]
- Byrne, H.J.; Bonnier, F.; McIntyre, J.; Parachalil, D.R. Quantitative Analysis of Human Blood Serum using Vibrational Spectroscopy. Clin. Spectrosc. 2020, 2, 100004. [Google Scholar] [CrossRef]
- Chen, L.; Wang, X.; Lu, W.; Wu, X.; Li, J. Molecular imprinting: Perspectives and applications. Chem. Soc. Rev. 2016, 45, 2137–2211. [Google Scholar] [CrossRef]
- Uzun, L.; Turner, A.P.F. Molecularly-imprinted polymer sensors: Realising their potential. Biosens. Bioelectron. 2016, 76, 131–144. [Google Scholar] [CrossRef]
- Li, Z.; Askim, J.R.; Suslick, K.S. The Optoelectronic Nose: Colorimetric and Fluorometric Sensor Arrays. Chem. Rev. 2019, 119, 231–292. [Google Scholar] [CrossRef]
- Zhang, C.; Suslick, K.S. A Colorimetric Sensor Array for Organics in Water. J. Am. Chem. Soc. 2005, 127, 11548–11549. [Google Scholar] [CrossRef]
- Schackart, K.E.; Yoon, J.Y. Machine Learning Enhances the Performance of Bioreceptor-Free Biosensors. Sensors 2021, 21, 5519. [Google Scholar] [CrossRef] [PubMed]
- De, M.; Rana, S.; Akpinar, H.; Miranda, O.R.; Arvizo, R.R.; Bunz, U.H.F.; Rotello, V.M. Sensing of proteins in human serum using conjugates of nanoparticles and green fluorescent protein. Nat. Chem. 2009, 1, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Cheng, H.; Wang, B.; Braun, M.S.; Fan, X.; Bender, M.; Huang, W.; Domhan, C.; Mier, W.; Lindner, T.; et al. A Polymer/Peptide Complex-Based Sensor Array that Discriminates Bacteria in Urine. Angew. Chem. Int. Ed. 2017, 56, 15246–15251. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Ma, C.; Wang, B.; Bender, M.; Bojanowski, M.; Hergert, M.; Seehafer, K.; Herrmann, A.; Bunz, U.H.F. A Hypothesis-Free Sensor Array Discriminates Whiskies for Brand, Age, and Taste. Chem 2017, 2, 817–824. [Google Scholar] [CrossRef]
- Feng, L.; Li, X.; Li, H.; Yang, W.; Chen, L.; Guan, Y. Enhancement of sensitivity of paper-based sensor array for the identification of heavy-metal ions. Anal. Chim. Acta 2013, 780, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Peveler, W.J.; Roldan, A.; Hollingsworth, N.; Porter, M.J.; Parkin, I.P. Multichannel detection and differentiation of explosives with a quantum dot array. ACS Nano 2016, 10, 1139–1146. [Google Scholar] [CrossRef]
- Sailor, M.J. Porous Silicon in Practice: Preparation, Characterization and Applications, 1st ed.; Wiley-VCH: Weinheim, Germany, 2012; ISBN 9783527313785. [Google Scholar]
- Canham, L.T. Silicon quantum wire array fabrication by electrochemical and chemical dissolution of wafers. Appl. Phys. Lett. 1990, 57, 1046–1048. [Google Scholar] [CrossRef]
- Dian, J.; Macek, A.; Nižňanský, D.; Němec, I.; Vrkoslav, V.; Chvojka, T.; Jelínek, I. SEM and HRTEM study of porous silicon—Relationship between fabrication, morphology and optical properties. Appl. Surf. Sci. 2004, 238, 169–174. [Google Scholar] [CrossRef]
- Herino, R.; Bomchil, G.; Barla, K.; Bertrand, C.; Ginoux, J.L. Porosity and Pore Size Distributions of Porous Silicon Layers. J. Electrochem. Soc. 1987, 134, 1994–2000. [Google Scholar] [CrossRef]
- Granitzer, P.; Rumpf, K. Porous silicon—A versatile host material. Materials 2010, 3, 943–998. [Google Scholar] [CrossRef]
- Arshavsky-Graham, S.; Massad-Ivanir, N.; Segal, E.; Weiss, S. Porous Silicon-Based Photonic Biosensors: Current Status and Emerging Applications. Anal. Chem. 2019, 91, 441–467. [Google Scholar] [CrossRef] [PubMed]
- Moretta, R.; De Stefano, L.; Terracciano, M.; Rea, I. Porous Silicon Optical Devices: Recent Advances in Biosensing Applications. Sensors 2021, 21, 1336. [Google Scholar] [CrossRef] [PubMed]
- Goodland, F.C. Detection Limits for Protein after Electrophoresis on Cellulose Acetate. Ann. Clin. Biochem. 1982, 19, 117–119. [Google Scholar] [CrossRef] [PubMed]
- Keren, D.F. Proteins in Serum Identified by High-Resolution Electrophoresis. In High-Resolution Electrophoresis and Immunofixation; Elsevier: Amsterdam, The Netherlands, 1994; pp. 41–96. [Google Scholar]
- Tsui, A.K.Y.; Thomas, D.; Hunt, A.; Estey, M.; Christensen, C.L.; Higgins, T.; Sandhu, I.; Rodriguez-Capote, K. Analytical sensitivity and diagnostic performance of serum protein electrophoresis on the HYDRAGEL 30 PROTEIN(E) β1-β2 Sebia Hydrasys system. Clin. Biochem. 2018, 51, 80–84. [Google Scholar] [CrossRef]
- Dai, F.; Zai, J.; Yi, R.; Gordin, M.L.; Sohn, H.; Chen, S.; Wang, D. Bottom-up synthesis of high surface area mesoporous crystalline silicon and evaluation of its hydrogen evolution performance. Nat. Commun. 2014, 5, 3605. [Google Scholar] [CrossRef]
- Weiss Group Pore Size Distribution MATLAB Code. Available online: https://my.vanderbilt.edu/vuphotonics/resources (accessed on 1 September 2023).
- Gaur, G.; Koktysh, D.S.; Weiss, S.M. Immobilization of quantum dots in nanostructured porous silicon films: Characterizations and signal amplification for dual-mode optical biosensing. Adv. Funct. Mater. 2013, 23, 3604–3614. [Google Scholar] [CrossRef]
- Elia, P.; Nativ-Roth, E.; Zeiri, Y.; Porat, Z. Determination of the average pore-size and total porosity in porous silicon layers by image processing of SEM micrographs. Microporous Mesoporous Mater. 2016, 225, 465–471. [Google Scholar] [CrossRef]
- Zuiderveld, K. Contrast Limited Adaptive Histogram Equalization. In Graphics Gems; Heckbert, P.S., Ed.; Morgan Kaufmann: San Diego, CA, USA, 1994; pp. 474–485. [Google Scholar]
- Ward, S.J.; Layouni, R.; Arshavsky-Graham, S.; Segal, E.; Weiss, S.M. Morlet Wavelet Filtering and Phase Analysis to Reduce the Limit of Detection for Thin Film Optical Biosensors. ACS Sensors 2021, 6, 2967–2978. [Google Scholar] [CrossRef]
- Douglas, F.; Pat, L.; Fisher, R. Methods of Conceptual Clustering and their Relation to Numerical Taxonomy. Ann. Eugen. 1985, 7, 179–188. [Google Scholar]
- Chang, C.C.; Lin, C.J. LIBSVM: A Library for support vector machines. ACM Trans. Intell. Syst. Technol. 2011, 2, 27. [Google Scholar] [CrossRef]
- He, Y.; Bourrier, D.; Imbernon, E.; Leichle, T. Lateral porous silicon membranes with size and charge selectivity. In Proceedings of the 2017 IEEE 12th International Conference on Nano/Micro Engineered and Molecular Systems (NEMS), Los Angeles, CA, USA, 9–12 April 2017; pp. 770–773. [Google Scholar] [CrossRef]
- Dubey, R.S.; Gautam, D.K. Fabrication and characterization of porous silicon layers for applications in optoelectronics. Opt. Quantum Electron. 2009, 41, 189–201. [Google Scholar] [CrossRef]
- Smith, R.L.; Collins, S.D. Porous silicon formation mechanisms. J. Appl. Phys. 1992, 71, R1–R22. [Google Scholar] [CrossRef]
- Zhao, Y.; Gaur, G.; Mernaugh, R.L.; Laibinis, P.E.; Weiss, S.M. Comparative Kinetic Analysis of Closed-Ended and Open-Ended Porous Sensors. Nanoscale Res. Lett. 2016, 11, 395. [Google Scholar] [CrossRef] [PubMed]
- Redlich, O.; Peterson, D.L. A Useful Adsorption Isotherm. J. Phys. Chem. 1959, 63, 1024. [Google Scholar] [CrossRef]
- Nadarassan, D. Biomolecule Adsorption and Release from Porous Silicon. In Handbook of Porous Silicon; Canham, L., Ed.; Springer: Cham, Switzerland, 2016; pp. 1–18. [Google Scholar]
- Chen, M.Y.; Sailor, M.J. Charge-gated transport of proteins in nanostructured optical films of mesoporous silica. Anal. Chem. 2011, 83, 7186–7193. [Google Scholar] [CrossRef] [PubMed]
- Parks, G.A. The Isoelectric Points of Solid Oxides, Solid Hydroxides, and Aqueous Hydroxo Complex Systems. Chem. Rev. 1965, 65, 177–198. [Google Scholar] [CrossRef]
- Wada, A.; Nakamura, H. Nature of the Charge Distribution in Proteins. Nature 1981, 293, 757–758. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, M.; Jain, N.; Bhasne, K.; Kumari, V.; Mukhopadhyay, S. pH-induced Conformational Isomerization of Bovine Serum Albumin Studied by Extrinsic and Intrinsic Protein Fluorescence. J. Fluoresc. 2011, 21, 1083–1090. [Google Scholar] [CrossRef]
- Tsang, C.K.; Kelly, T.L.; Sailor, M.J.; Li, Y.Y. Highly stable porous silicon-carbon composites as label-free optical biosensors. ACS Nano 2012, 6, 10546–10554. [Google Scholar] [CrossRef]
- Cao, T.; Zhao, Y.; Nattoo, C.A.; Layouni, R.; Weiss, S.M. A smartphone biosensor based on analysing structural colour of porous silicon. Analyst 2019, 144, 3942–3948. [Google Scholar] [CrossRef]



| Etching Current Density | 25 mA cm−2 | 40 mA cm−2 | 55 mA cm−2 |
|---|---|---|---|
| Mean Pore Diameter (nm) | 12.0 ± 0.2 | 15.1 ± 0.2 | 17.3 ± 0.2 |
| Pore Diameter Standard Deviation (nm) | 6.0 ± 0.2 | 6.7 ± 0.3 | 7.9 ± 0.3 |
| Thickness (μm) | 1.78 ± 0.01 | 1.94 ± 0.01 | 2.04 ± 0.01 |
| Mean Effective Optical Thickness in Air (μm) | 6.91 | 6.34 | 5.82 |
| Mean Effective Refractive Index in Air | 1.94 ± 0.02 | 1.63 ± 0.01 | 1.43 ± 0.01 |
| % Porosity | 53 ± 1 | 61 ± 1 | 66 ± 1 |
| Fraction of Pores > 30 nm | 0.2 ± 0.1% | 2.0 ± 0.4% | 5.8 ± 1.3% |
| Test Procedure | Prediction Target | Accuracy |
|---|---|---|
| Leave-one-out cross validation (previously seen concentrations) | Protein type and concentration | 100% |
| Independent Test Set (previously unseen concentration) | Protein type | 87.5% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ward, S.J.; Cao, T.; Zhou, X.; Chang, C.; Weiss, S.M. Protein Identification and Quantification Using Porous Silicon Arrays, Optical Measurements, and Machine Learning. Biosensors 2023, 13, 879. https://doi.org/10.3390/bios13090879
Ward SJ, Cao T, Zhou X, Chang C, Weiss SM. Protein Identification and Quantification Using Porous Silicon Arrays, Optical Measurements, and Machine Learning. Biosensors. 2023; 13(9):879. https://doi.org/10.3390/bios13090879
Chicago/Turabian StyleWard, Simon J., Tengfei Cao, Xiang Zhou, Catie Chang, and Sharon M. Weiss. 2023. "Protein Identification and Quantification Using Porous Silicon Arrays, Optical Measurements, and Machine Learning" Biosensors 13, no. 9: 879. https://doi.org/10.3390/bios13090879