Progress and Perspectives of Mid-Infrared Photoacoustic Spectroscopy for Non-Invasive Glucose Detection
Abstract
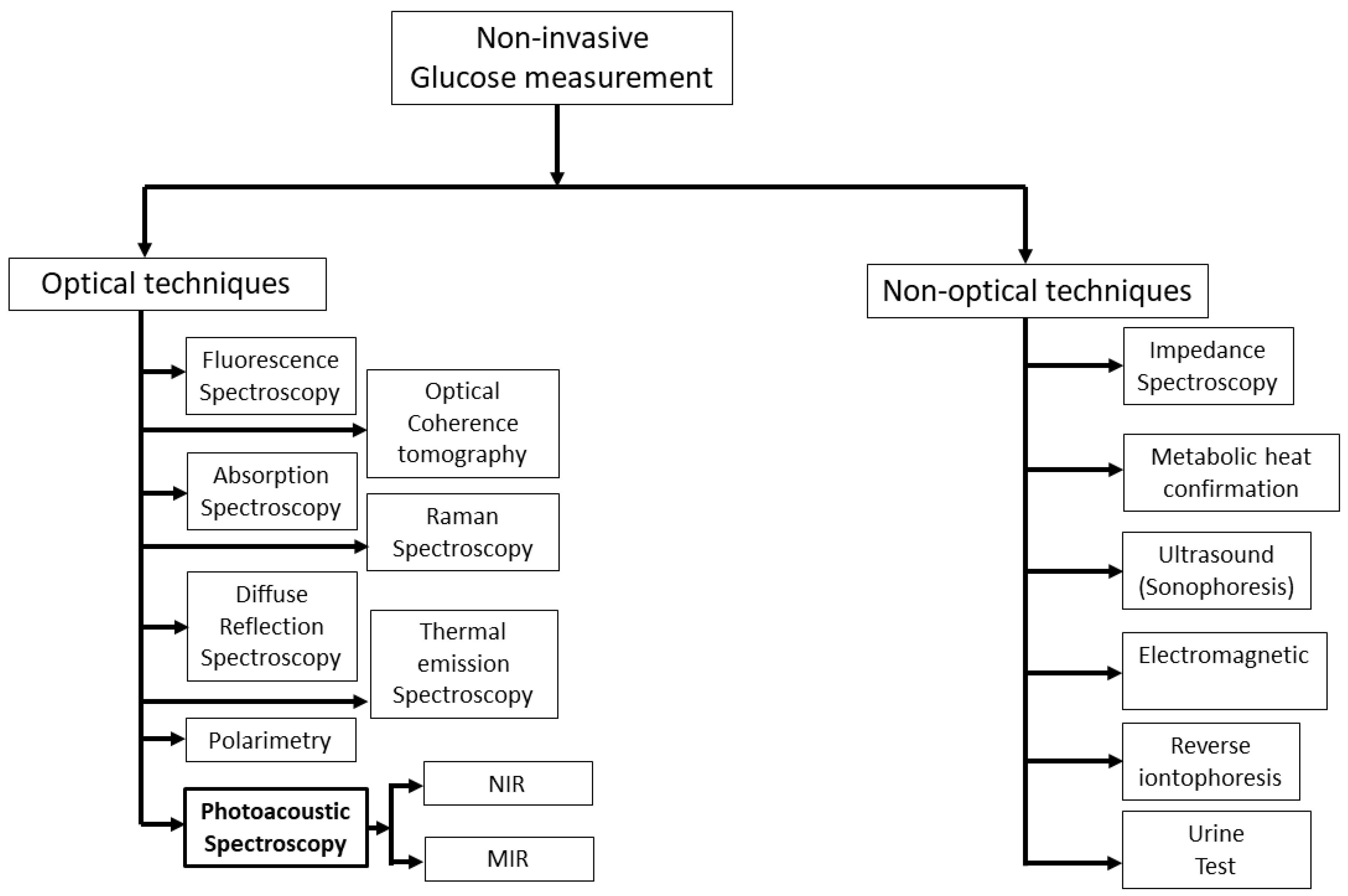
:1. Introduction
2. Basics of Photoacoustic Spectroscopy for Glucose Sensing
2.1. Generation and Behavior of an Acoustic Signal Using MIR Light Source
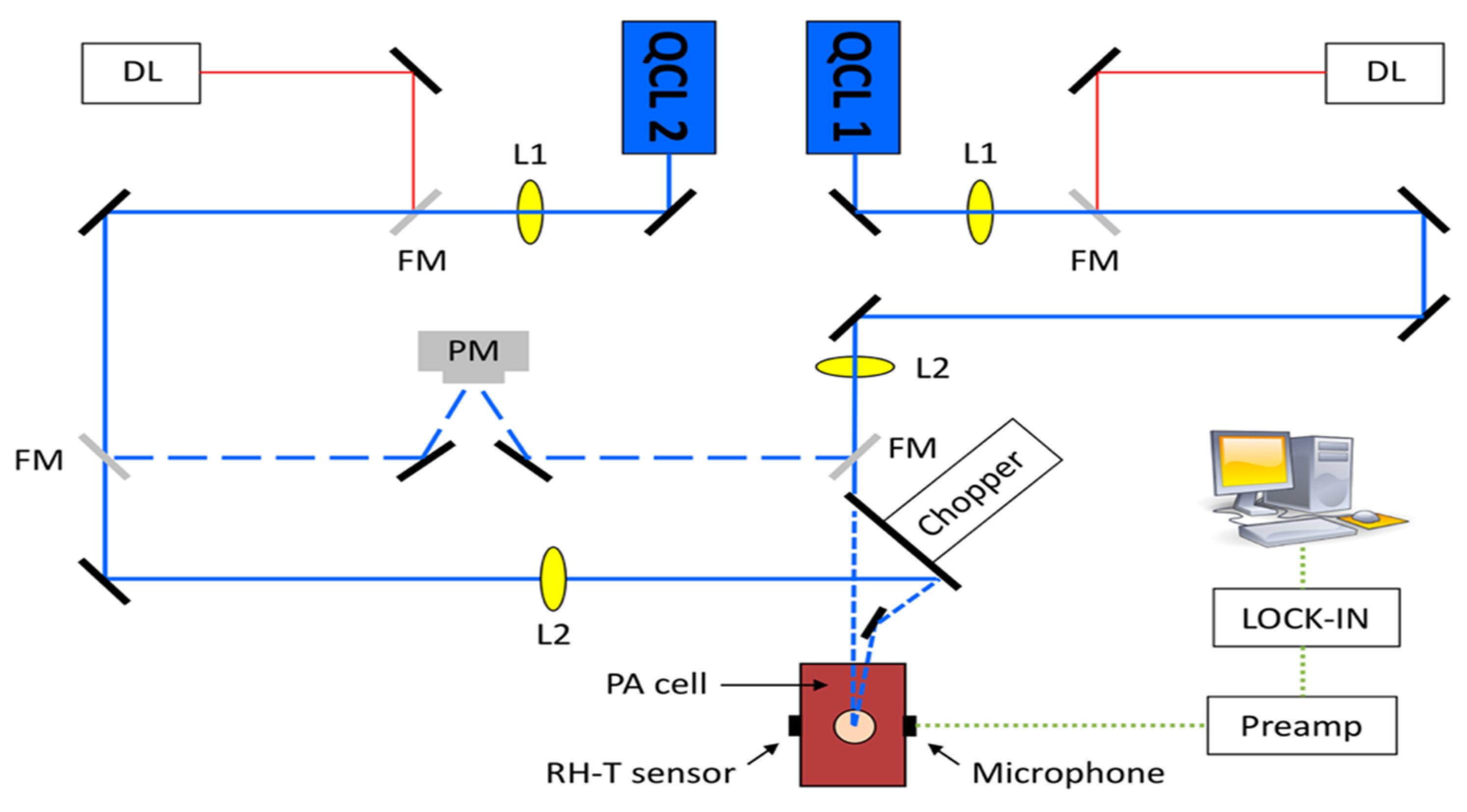
2.2. Required Instrumentation for MIR-Based PAS
2.3. Spectral Response of Glucose in the MIR Region
- (i)
- Greater absorption: Glucose has a relatively weak absorption in the visible and NIR regions, but it has strong absorption in the MIR region. That means the sensitivity of glucose detection in the MIR region can be enhanced.
- (ii)
- High signal-to-noise (from water) ratio: Water has strong absorption in the MIR region in comparison to visible and NIR regions, which can interfere with the detection of glucose. However, as glucose has distinct absorption in the MIR region, the overall signal-to-noise ratio can be improved by selecting an excitation wavelength close to the peak absorption wavelength in the MIR region.
- (iii)
- Ability to detect glucose in complex matrices: The use of MIR PAS can improve the detection of glucose in complex biological matrices, such as blood, by avoiding the interference caused by other molecules present in the matrices.
- (iv)
- Greater specificity: MIR-based PAS can provide greater specificity for glucose detection as it has distinct absorption characteristics in the MIR wavelength region compared to visible and NIR wavelength regions. Thus, MIR-based PAS utilizing multiple wavelengths in the MIR region can be used significantly to improve the specificity of glucose sensing. It should be noted that specificity can be improved by multiple wavelengths in other wavelength regions, where glucose has a good absorption coefficient.
3. Progress of MIR-Based PAS in Glucose Sensing
3.1. Summary of the Chronological Development
3.2. Experimental/Instrumentation Development and Data Analysis
4. Machine Learning in Non-Invasive Glucose Detection
4.1. Classification Methods
4.2. Regression Methods
5. Prospects of MIR-Based PAS
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Report on Diabetes; WHO: Geneva, Switzerland, 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/diabetes (accessed on 26 June 2022).
- American Diabetes Association, “Diabetes Tests”. Available online: https://www.cdc.gov/diabetes/basics/getting-tested.html (accessed on 26 April 2023).
- Geerlings, S.E.; Hoepelman, A.I. Immune dysfunction in patients with diabetes mellitus (DM). FEMS Immunol. Med. Microbiol. 1999, 26, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Strategic Market Research. Blood Glucose Monitoring Devices Market: By Product (Continuous Blood Glucose Monitoring Devices (Sensors, Transmitter & Receiver, Insulin Pumps), Self-Monitoring Devices (Blood Glucose Meter, Testing Strips, Lancets)), Applications (Type 1 Diabetes, Type 2 Diabetes), By End-User (Home Care, Diagnostics Centres, Hospitals), By Geography, Segment Revenue Estimation, Forecast, 2021–2030; Strategic Market Research: New York, NY, USA, 2021. [Google Scholar]
- Alsunaidi, B.; Althobaiti, M.; Tamal, M.; Albaker, W.; Al-Naib, I. A Review of Non-Invasive Optical Systems for Continuous Blood Glucose Monitoring. Sensors 2021, 21, 6820. [Google Scholar] [CrossRef] [PubMed]
- Delbeck, S.; Vahlsing, T.; Leonhardt, S.; Steiner, G.; Heise, H.M. Non-invasive monitoring of blood glucose using optical methods for skin spectroscopy—Opportunities and recent advances. Anal. Bioanal. Chem. 2019, 411, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhang, Y.; Wu, J. Review of non-invasive continuous glucose monitoring based on impedance spectroscopy. Sens. Actuators A Phys. 2020, 311, 112103. [Google Scholar] [CrossRef]
- Tang, L.; Chang, S.J.; Chen, C.-J.; Liu, J.-T. Non-Invasive Blood Glucose Monitoring Technology: A Review. Sensors 2020, 20, 6925. [Google Scholar] [CrossRef]
- Hina, A.; Saadeh, W. Noninvasive Blood Glucose Monitoring Systems Using Near-Infrared Technology—A Review. Sensors 2022, 22, 4855. [Google Scholar] [CrossRef]
- Laha, S.; Rajput, A.; Laha, S.S.; Jadhav, R. A Concise and Systematic Review on Non-Invasive Glucose Monitoring for Potential Diabetes Management. Biosensors 2022, 12, 965. [Google Scholar] [CrossRef]
- Nawaz, A.; Øhlckers, P.; Sælid, S.; Jacobsen, M.; Akram, M.N. Review: Non-Invasive Continuous Blood Glucose Measurement Techniques. J. Bioinform. Diabetes 2016, 1, 1–27. [Google Scholar] [CrossRef] [Green Version]
- Shokrekhodaei, M.; Quinones, S. Review of Non-Invasive Glucose Sensing Techniques: Optical, Electrical and Breath Acetone. Sensors 2020, 20, 1251. [Google Scholar] [CrossRef] [Green Version]
- Buehler, L.A.; Balasubramanian, V.; Baskerville, S.; Bailey, R.; McCarthy, K.; Rippen, M.; Bena, J.F.; Lansang, M.C. Noninvasive Glucose Monitor Using Dielectric Spectroscopy. Endocr. Pr. 2022, 28, 142–147. [Google Scholar] [CrossRef]
- Sieg, A.; Guy, R.H.; Delgado-Charro, M.B. Noninvasive Glucose Monitoring by Reverse Iontophoresis in Vivo: Application of the Internal Standard Concept. Clin. Chem. 2004, 50, 1383–1390. [Google Scholar] [CrossRef]
- Tang, F.; Wang, X.; Wang, D.; Li, J. Non-Invasive Glucose Measurement by Use of Metabolic Heat Conformation Method. Sensors 2008, 8, 3335–3344. [Google Scholar] [CrossRef] [Green Version]
- Kost, J. Ultrasound-Assisted Insulin Delivery and Noninvasive Glucose Sensing. Diabetes Technol. Ther. 2002, 4, 489–497. [Google Scholar] [CrossRef]
- Ballerstadt, R.; Evans, C.; Gowda, A.; McNichols, R. In Vivo Performance Evaluation of a Transdermal Near- Infrared Fluorescence Resonance Energy Transfer Affinity Sensor for Continuous Glucose Monitoring. Diabetes Technol. Ther. 2006, 8, 296–311. [Google Scholar] [CrossRef]
- March, W.; Lazzaro, D.; Rastogi, S. Fluorescent Measurement in the Non-Invasive Contact Lens Glucose Sensor. Diabetes Technol. Ther. 2006, 8, 312–317. [Google Scholar] [CrossRef]
- Esenaliev, R.O.; Larin, K.V.; Larina, I.V.; Motamedi, M. Noninvasive monitoring of glucose concentration with optical coherence tomography. Opt. Lett. 2001, 26, 992–994. [Google Scholar] [CrossRef]
- AEnejder, A.M.K.; Scecina, T.G.; Oh, J.; Hunter, M.; Shih, W.-C.; Sasic, S.; Horowitz, G.L.; Feld, M.S. Raman spectroscopy for noninvasive glucose measurements. J. Biomed. Opt. 2005, 10, 031114–0311149. [Google Scholar] [CrossRef]
- Lambert, J.L.; Pelletier, C.C.; Borchert, M. Glucose determination in human aqueous humor with Raman spectroscopy. J. Biomed. Opt. 2005, 10, 031110–0311108. [Google Scholar] [CrossRef]
- Malchoff, C.D.; Shoukri, K.; Landau, J.I.; Buchert, J.M. A Novel Noninvasive Blood Glucose Monitor. Diabetes Care 2002, 25, 2268–2275. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.; Mandelis, A.; Matvienko, A.; Sivagurunathan, K.; Zinman, B. Wavelength-modulated differential laser photothermal radiometry for blood glucose measurements. J. Physics Conf. Ser. 2010, 214, 012025. [Google Scholar] [CrossRef]
- Marbach, R.; Koschinsky, T.; Gries, F.A.; Heise, H.M. Noninvasive Blood Glucose Assay by Near-Infrared Diffuse Reflectance Spectroscopy of the Human Inner Lip. Appl. Spectrosc. 1993, 47, 875–881. Available online: http://opg.optica.org/as/abstract.cfm?URI=as-47-7-875 (accessed on 26 April 2023). [CrossRef]
- Maruo, K.; Tsurugi, M.; Tamura, M.; Ozaki, Y. In Vivo Noninvasive Measurement of Blood Glucose by Near-Infrared Diffuse-Reflectance Spectroscopy. Appl. Spectrosc. 2003, 57, 1236–1244. [Google Scholar] [CrossRef] [PubMed]
- BMalik, B.H.; Coté, G.L. Real-time, closed-loop dual-wavelength optical polarimetry for glucose monitoring. J. Biomed. Opt. 2010, 15, 017002. [Google Scholar] [CrossRef]
- Purvinis, G.; Cameron, B.D.; Altrogge, D.M. Noninvasive Polarimetric-Based Glucose Monitoring: An in Vivo Study. J. Diabetes Sci. Technol. 2011, 5, 380–387. [Google Scholar] [CrossRef] [Green Version]
- Vrančić, C.; Fomichova, A.; Gretz, N.; Herrmann, C.; Neudecker, S.; Pucci, A.; Petrich, W. Continuous glucose monitoring by means of mid-infrared transmission laser spectroscopy in vitro. Analyst 2011, 136, 1192–1198. [Google Scholar] [CrossRef]
- Spanner, G. New concept for the non-invasive determination of physiological glucose concentrations using modulated laser diodes. Anal. Bioanal. Chem. 1996, 354, 306–310. [Google Scholar] [CrossRef]
- Kottmann, J.; Rey, J.M.; Sigrist, M.W. New photoacoustic cell design for studying aqueous solutions and gels. Rev. Sci. Instrum. 2011, 82, 84903. [Google Scholar] [CrossRef] [Green Version]
- Spanner, G.; Niessner, R. Noninvasive determination of blood constituents using an array of modulated laser diodes and a photoacoustic sensor head. Anal. Bioanal. Chem. 1996, 355, 327–328. [Google Scholar] [CrossRef]
- Chen, J.; Furukawa, H. Rapid and non-invasive detection of high-thickness glucose solution concentrations using quantum cascade laser-based transmission infrared spectroscopy. Infrared Phys. Technol. 2023, 131, 104717. [Google Scholar] [CrossRef]
- Pai, P.P.; Sanki, P.K.; Banerjee, S. A photoacoustics based continuous non-invasive blood glucose monitoring system. In Proceedings of the 2015 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Turin, Italy, 7–9 May 2015; pp. 106–111. [Google Scholar] [CrossRef]
- Kottmann, J.; Grob, U.; Rey, J.M.; Sigrist, M.W. Mid-Infrared Fiber-Coupled Photoacoustic Sensor for Biomedical Applications. Sensors 2013, 13, 535–549. [Google Scholar] [CrossRef] [Green Version]
- Pleitez, M.A.; Lieblein, T.; Bauer, A.; Hertzberg, O.; von Lilienfeld-Toal, H.; Mäntele, W. In Vivo Noninvasive Monitoring of Glucose Concentration in Human Epidermis by Mid-Infrared Pulsed Photoacoustic Spectroscopy. Anal. Chem. 2012, 85, 1013–1020. [Google Scholar] [CrossRef]
- Rosencwaig, A.; Gersho, A. Theory of the photoacoustic effect with solids. J. Appl. Phys. 2008, 47, 64–69. [Google Scholar] [CrossRef]
- Zhang, R.; Luo, Y.; Jin, H.; Gao, F.; Zheng, Y. Time-domain photoacoustic waveform analysis for glucose measurement. Analyst 2020, 145, 7964–7972. [Google Scholar] [CrossRef]
- Hazen, K.H.; Arnold, M.A.; Small, G.W. Measurement of Glucose in Water with First-Overtone Near-Infrared Spectra. Appl. Spectrosc. 1998, 52, 1597–1605. Available online: http://opg.optica.org/as/abstract.cfm?URI=as-52-12-1597 (accessed on 26 April 2023). [CrossRef]
- Olesberg, J.T.; Arnold, M.A.; Mermelstein, C.; Schmitz, J.; Wagner, J. Tunable Laser Diode System for Noninvasive Blood Glucose Measurements. Appl. Spectrosc. 2005, 59, 1480–1484. Available online: http://opg.optica.org/as/abstract.cfm?URI=as-59-12-1480 (accessed on 26 April 2023). [CrossRef]
- Khalil, O.S. Non-Invasive Glucose Measurement Technologies: An Update from 1999 to the Dawn of the New Millennium. Diabetes Technol. Ther. 2004, 6, 660–697. [Google Scholar] [CrossRef]
- Downing, H.D.; Williams, D. Optical constants of water in the infrared. J. Geophys. Res. Atmos. 1975, 80, 1656–1661. [Google Scholar] [CrossRef]
- JKottmann, J.; Rey, J.M.; Luginbühl, J.; Reichmann, E.; Sigrist, M.W. Glucose sensing in human epidermis using mid-infrared photoacoustic detection. Biomed. Opt. Express 2012, 3, 667–680. [Google Scholar] [CrossRef] [Green Version]
- Gebhart, S.; Fowler, R.; Kapsner, C.; Lincoln, D.; McGee, V.; Pasqua, J.; Steed, L.; Wangsness, M.; Xu, F.; Vanstory, M.; et al. Glucose Sensing in Transdermal Body Fluid Collected Under Continuous Vacuum Pressure Via Micropores in the Stratum Corneum. Diabetes Technol. Ther. 2003, 5, 159–166. [Google Scholar] [CrossRef]
- Viengerov, M.L. New method of gas analysis based on tyndall-roentgen optoacoustic effect. Dokl Akad Nauk SSSR 1938, 19, 8. [Google Scholar]
- Kreuzer, L.B. Ultralow Gas Concentration Infrared Absorption Spectroscopy. J. Appl. Phys. 2003, 42, 2934–2943. [Google Scholar] [CrossRef]
- Kottmann, J.; Rey, J.M.; Sigrist, M.W. Mid-Infrared Photoacoustic Detection of Glucose in Human Skin: Towards Non-Invasive Diagnostics. Sensors 2016, 16, 1663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- GChristison, G.B.; MacKenzie, H.A. Laser photoacoustic determination of physiological glucose concentrations in human whole blood. Med. Biol. Eng. Comput. 1993, 31, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Tuchin, V.V. Handbook of Optical Sensing of Glucose in Biological Fluids and Tissues; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Thennadil, S.N.; Rennert, J.L.; Wenzel, B.J.; Hazen, K.H.; Ruchti, T.L.; Block, M.B. Comparison of Glucose Concentration in Interstitial Fluid, and Capillary and Venous Blood During Rapid Changes in Blood Glucose Levels. Diabetes Technol. Ther. 2001, 3, 357–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liakat, S.; Bors, K.A.; Huang, T.-Y.; Michel, A.P.M.; Zanghi, E.; Gmachl, C.F. In vitro measurements of physiological glucose concentrations in biological fluids using mid-infrared light. Biomed. Opt. Express 2013, 4, 1083–1090. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.V.; Wu, H. Biomedical Optics: Principles and Imaging; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Rosencwaig, A. Photoacoustic Spectroscopy of Biological Materials. Science 1973, 181, 657–658. [Google Scholar] [CrossRef]
- Mackenzie, H.A.; Ashton, H.S.; Spiers, S.; Shen, Y.; Freeborn, S.S.; Hannigan, J.; Lindberg, J.; Rae, P. Advances in Photoacoustic Noninvasive Glucose Testing. Clin. Chem. 1999, 45, 1587–1595. [Google Scholar] [CrossRef] [Green Version]
- Hugi, A.; Terazzi, R.; Bonetti, Y.; Wittmann, A.; Fischer, M.; Beck, M.; Faist, J.; Gini, E. External cavity quantum cascade laser tunable from 7.6 to 11.4 μm. Appl. Phys. Lett. 2009, 95, 061103. [Google Scholar] [CrossRef]
- Rassel, S.; Xu, C.; Zhang, S.; Ban, D. Noninvasive blood glucose detection using a quantum cascade laser. Analyst 2020, 145, 2441–2456. [Google Scholar] [CrossRef]
- El-Busaidy, S.; Baumann, B.; Wolff, M.; Duggen, L.; Bruhns, H. Experimental and Numerical Investigation of a Photoacoustic Resonator for Solid Samples: Towards a Non-Invasive Glucose Sensor. Sensors 2019, 19, 2889. [Google Scholar] [CrossRef] [Green Version]
- Pleitez, M.A.; Lieblein, T.; Bauer, A.; Hertzberg, O.; Von Lilienfeld-Toal, H.; Mantele, W. Windowless ultrasound photoacoustic cell forin vivomid-IR spectroscopy of human epidermis: Low interference by changes of air pressure, temperature, and humidity caused by skin contact opens the possibility for a non-invasive monitoring of glucose in the interstitial fluid. Rev. Sci. Instrum. 2013, 84, 084901. [Google Scholar] [CrossRef]
- Tanaka, Y.; Tajima, T.; Seyama, M.; Waki, K. Differential Continuous Wave Photoacoustic Spectroscopy for Non-Invasive Glucose Monitoring. IEEE Sens. J. 2020, 20, 4453–4458. [Google Scholar] [CrossRef]
- Guo, X.; Mandelis, A.; Zinman, B. Noninvasive glucose detection in human skin using wavelength modulated differential laser photothermal radiometry. Biomed. Opt. Express 2012, 3, 3012–3021. [Google Scholar] [CrossRef] [Green Version]
- von Lilienfeld-Toal, H.; Weidenmüller, M.; Xhelaj, A.; Mäntele, W. A novel approach to non-invasive glucose measurement by mid-infrared spectroscopy: The combination of quantum cascade lasers (QCL) and photoacoustic detection. Vib. Spectrosc. 2005, 38, 209–215. [Google Scholar] [CrossRef]
- ABauer, A.; Hertzberg, O.; Küderle, A.; Strobel, D.; Pleitez, M.A.; Mäntele, W. IR-spectroscopy of skin in vivo: Optimal skin sites and properties for non-invasive glucose measurement by photoacoustic and photothermal spectroscopy. J. Biophotonics 2018, 11, e201600261. [Google Scholar] [CrossRef]
- JSim, J.Y.; Ahn, C.-G.; Jeong, E.-J.; Kim, B.K. In vivo Microscopic Photoacoustic Spectroscopy for Non-Invasive Glucose Monitoring Invulnerable to Skin Secretion Products. Sci. Rep. 2018, 8, 1059. [Google Scholar] [CrossRef] [Green Version]
- Aloraynan, A.; Rassel, S.; Xu, C.; Ban, D. A Single Wavelength Mid-Infrared Photoacoustic Spectroscopy for Noninvasive Glucose Detection Using Machine Learning. Biosensors 2022, 12, 166. [Google Scholar] [CrossRef]
- Shokrekhodaei, M.; Cistola, D.P.; Roberts, R.C.; Quinones, S. Non-Invasive Glucose Monitoring Using Optical Sensor and Machine Learning Techniques for Diabetes Applications. IEEE Access 2021, 9, 73029–73045. [Google Scholar] [CrossRef]
- RKasahara, R.; Kino, S.; Soyama, S.; Matsuura, Y. Noninvasive glucose monitoring using mid-infrared absorption spectroscopy based on a few wavenumbers. Biomed. Opt. Express 2018, 9, 289–302. [Google Scholar] [CrossRef] [Green Version]
- Sankhala, D.; Sardesai, A.U.; Pali, M.; Lin, K.-C.; Jagannath, B.; Muthukumar, S.; Prasad, S. A machine learning-based on-demand sweat glucose reporting platform. Sci. Rep. 2022, 12, 2442. [Google Scholar] [CrossRef]
- Ho, T.K. The random subspace method for constructing decision forests. IEEE Trans. Pattern Anal. Mach. Intell. 1998, 20, 832–844. [Google Scholar] [CrossRef] [Green Version]
- Zech, J.R.; Badgeley, M.A.; Liu, M.; Costa, A.B.; Titano, J.J.; Oermann, E.K. Variable generalization performance of a deep learning model to detect pneumonia in chest radiographs: A cross-sectional study. PLoS Med. 2018, 15, e1002683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez-Rodríguez, I.; Chatzigiannakis, I.; Rodríguez, J.-V.; Maranghi, M.; Gentili, M.; Zamora-Izquierdo, M.-Á. Utility of Big Data in Predicting Short-Term Blood Glucose Levels in Type 1 Diabetes Mellitus Through Machine Learning Techniques. Sensors 2019, 19, 4482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klonoff, D.C.; Nguyen, K.T.; Xu, N.Y.; Arnold, M.A. Noninvasive Glucose Monitoring: In God We Trust—All Others Bring Data. J. Diabetes Sci. Technol. 2021, 15, 1211–1215. [Google Scholar] [CrossRef]
- Aloraynan, A.; Rassel, S.; Kaysir, R.; Ban, D. Dual quantum cascade lasers for noninvasive glucose detection using photoacoustic spectroscopy. Sci. Rep. 2023, 13, 7927. [Google Scholar] [CrossRef]







| Condition \Results | Fasting Blood Glucose Test (mg/dL) | Glucose Tolerance Test (mg/dL) | A1C Test (%) |
|---|---|---|---|
| Normal Pre-diabetic Diabetic | ≤99 | ≤140 | <5.7 |
| 100–125 | 141–199 | 5.7–6.4 | |
| ≥126 | ≥200 | ≥6.5 |
| Reference Year | MIR Sources | What Was Examined | PAC Used | Results |
|---|---|---|---|---|
| 2005 Lilienfeld-Toal et al. [60] | Two QCLs—1080 cm−1 (9.26 µm) and 1066 cm−1 (9.38 µm) | The skin of the forearm | PAC with a twin measuring chamber, one for photoacoustic signal and another for background signal | The relationship between the PA signal produced and blood glucose levels are not likely to be very strong. However, the correlation was most significant when a 10 min time lag in blood glucose levels was considered. |
| 2012 Kottmann et al. [42] | Tunable laser—1010–1095 cm−1 (i.e., 9.9–9.13 µm) | Human epidermal skin samples in contact with aqueous glucose solution | N2 ventilated PAC (77 mm3 volume) with gold coated on the inner surface and sealed with a diamond window | The PA signal is directly proportional to the concentration of glucose, both in a broad range of 0 to 10 g/dL and in a narrower range of 0 to 2000 mg/dL, compensating PA signal changes due to a variation of relative humidity and temperature. Detection limit—100 mg/dL. |
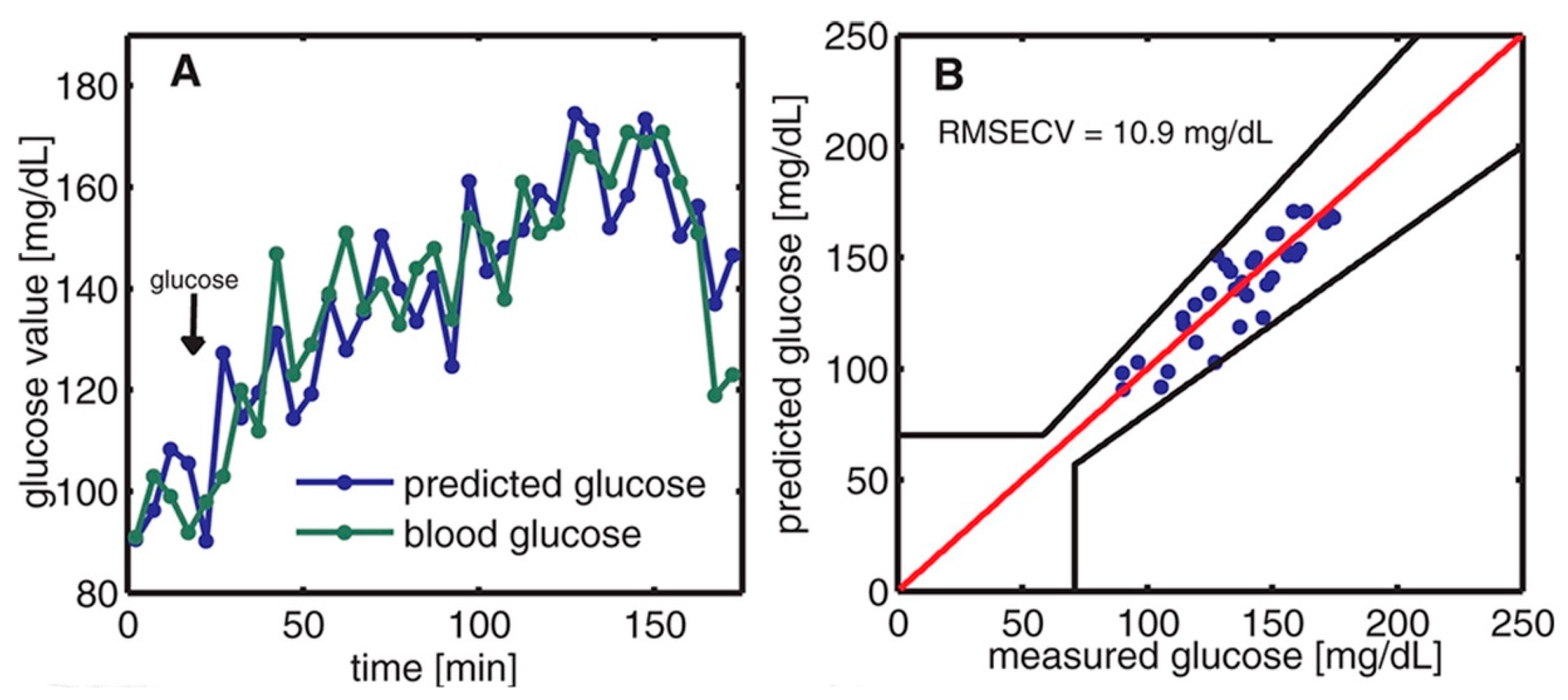
| 2012 Pleitez et al. [35] | EC- tunable QCL—1000–1220 cm−1 (i.e., 10–8.2 µm) | An interstitial layer of the human skin | T-shaped PAC | Successfully measured in vivo glucose concentration from 50 mg/dL to 300 mg/dL and the measurement followed to the real blood glucose level without significant delay (<10 min). |
| 2013 Pleitez et al. [57] | EC- tunable QCL—1000–1245 cm−1 (i.e., 10–8.03 µm) | Glucose in the human epidermis in the fingerprint region | Windowless PAC with two cylindrical cavities connected perpendicularly | The prediction value correlates well with the glucose concentration profile. The highest SNR of 72 was achieved at a resonance frequency of 51.7 kHz. |
| 2013 Kottmann et al. [34] | Tunable QCL—1010–1095 cm−1 (i.e., 9.9–9.13 µm) | Glucose solutions with concentrations ranging from 0 to 5 g/dL; human skin at the fingertip and the forearm | Fiber-coupled PAC with a conically shaped PAC chamber and ventilated with N2. | In the entire range of 0–5 g/dL studied, the PA signal recorded showed a linear increase in glucose concentration (R2 = 0.993). An SNR of 1 was achieved with a detection limit of 57 mg/dL during in vitro testing. The signal generated at the fingertip was significantly stronger than that recorded at the forearm. However, the sensitivity obtained was not adequate for practical in vivo glucose detection. |
| 2016 Kottmann et al. [46] | Two setups—(i) tunable QCLs, 1005–1100 cm−1 (i.e., 9.95–9.09 µm), and (ii) two lasers, 1080 cm−1 and 1180 cm−1 (i.e., 9.26 and 8.47 µm) | The skin of the human forearm | Fiber-coupled PAC | By using the dual-wavelength method, the stability of results was significantly enhanced, and the uncertainty in blood glucose concentration level was only 30 mg/dL at a 90% confidence level. |
| 2018 Bauer et al. [61] | Tunable QCLs— 980–1245 cm−1 (i.e., 10.20–8.03 µm) | Index fingertip, thumb finger, hypothenar, and forearm | T-shaped PAC | PA detection errors were found to be higher than photothermal detection due to acoustic impedance mismatch. The index finger and thumb are the most suitable choices for glucose measurements because they have a dense network of capillaries, which facilitates the transfer of glucose from the blood to the ISF, and also because they have less fatty tissue. |
| 2018 Sim et al. [62] | Tunable QCL—950–1240 cm−1 (i.e., 10.53–8.06 µm) | 2D position scanning for the image at the fingertips | T-shaped PAC | Following careful hand washing, the valley between the ridges of the skin was found to be free from skin secretions, making it more resilient than the area where the eccrine sweat pores are situated on the top of the ridges. This discovery presents an encouraging prospect for achieving consistent results over consecutive days, as the skin’s exocrine activity and condition can be managed. |
| 2022 Aloraynan et al. [63] | Single QCL—1080 cm−1 (9.26 µm) | Skin samples with different glucose concentrations | T-shaped PAC | The sensitivity of detection was improved to 25 mg/dL by employing a single wavelength QCL. The models created using unprocessed and processed data sets demonstrated a prediction accuracy of 86.7% and 90.4%, respectively. |
| Date Reference | Selected Features | Model Used | Results |
|---|---|---|---|
| 2013 Pleitez et al. [57] | 1000 cm−1 to 1220 cm−1 MIR-PA signal | PCR, PLSR | The mean prediction error was found to be approximately 11 mg/dL. |
| 2017 Kasahara et al. [65] | MIR absorption spectroscopy | MLR, PLSR | The maximum correlation coefficient of 0.49 was achieved. |
| 2018 Sim et al. [62] | PA signal in the wavelength spectrum | PLSR, PCR | PCR and PLSR resulted in a Mean Absolute Relative Deviation of 8.95 and 8.67, respectively. |
| 2018 Bauer et al. [61] | PA signal in the wavelength spectrum | PLSR | RMSE was cross-validated and standard deviations were obtained in the four different skin locations. |
| 2021 Shokrekhodaei et al. [64] | Four wavelengths in the optical sensor | KNN, DT, SVM; MLR, FFNN | The FFNN model exhibits the smallest RMSE value of 11.1 mg/dL, whereas the SVM model demonstrates the highest F1 score of 0.99. |
| 2022 Aloraynan et al. [63] | MIR-PA signal in 10–30 kHz | Ensemble Classification Model | Unprocessed and processed datasets achieved 86.7% and 90.4% prediction accuracy, respectively. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaysir, M.R.; Song, J.; Rassel, S.; Aloraynan, A.; Ban, D. Progress and Perspectives of Mid-Infrared Photoacoustic Spectroscopy for Non-Invasive Glucose Detection. Biosensors 2023, 13, 716. https://doi.org/10.3390/bios13070716
Kaysir MR, Song J, Rassel S, Aloraynan A, Ban D. Progress and Perspectives of Mid-Infrared Photoacoustic Spectroscopy for Non-Invasive Glucose Detection. Biosensors. 2023; 13(7):716. https://doi.org/10.3390/bios13070716
Chicago/Turabian StyleKaysir, Md Rejvi, Jiaqi Song, Shazzad Rassel, Abdulrahman Aloraynan, and Dayan Ban. 2023. "Progress and Perspectives of Mid-Infrared Photoacoustic Spectroscopy for Non-Invasive Glucose Detection" Biosensors 13, no. 7: 716. https://doi.org/10.3390/bios13070716





