A Label-Free Electrochemical Immunosensor for Detection of the Tumor Marker CA242 Based on Reduced Graphene Oxide-Gold-Palladium Nanocomposite
Abstract
:1. Introduction
2. Experimental Methods
2.1. Chemicals and Reagents
2.2. Electrochemical Measurements
2.3. Characterization of the Prepared Nanocomposites
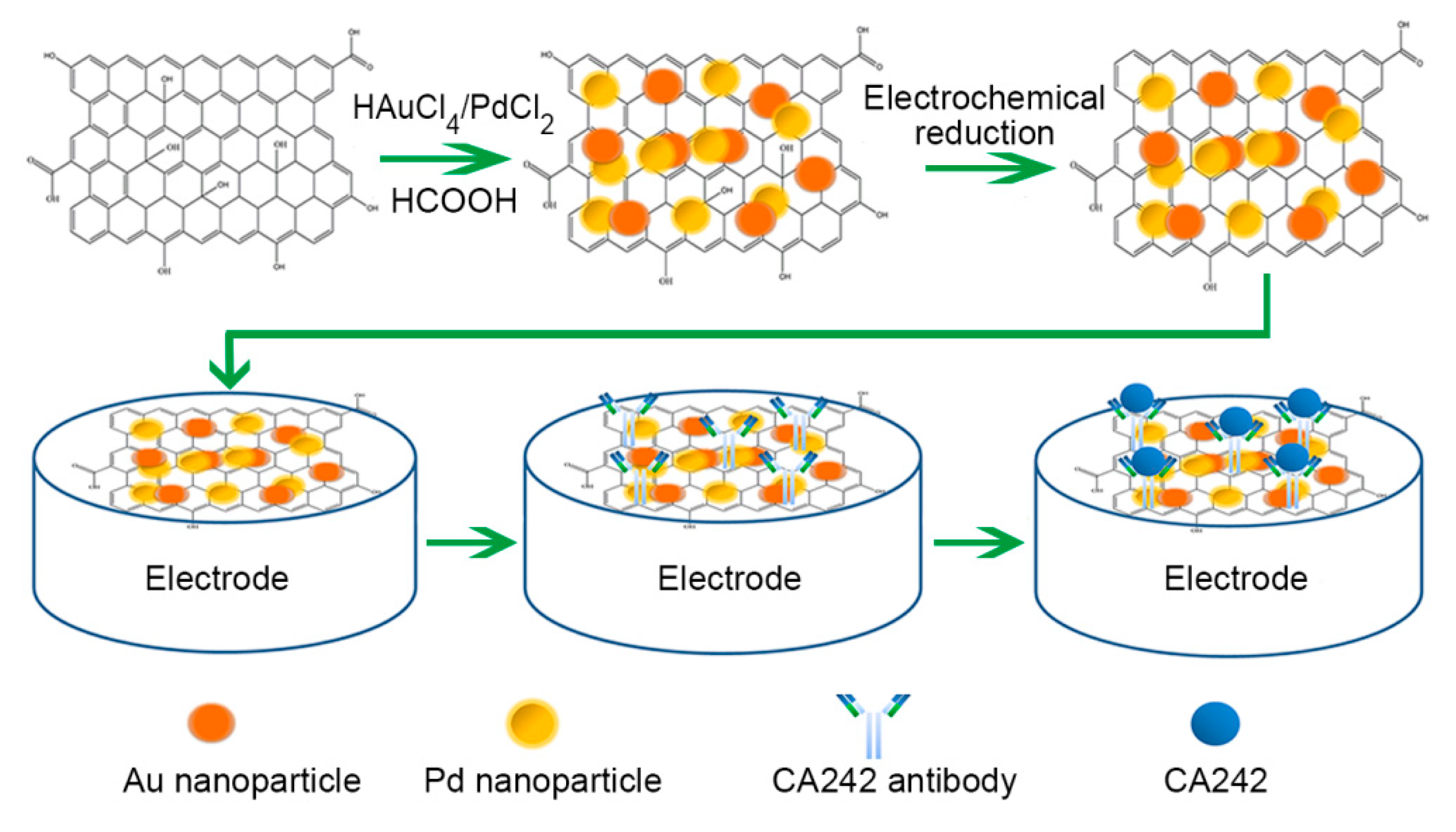
2.4. Fabrication of Homogenous rGO-Au-Pd Composite
2.5. Electrode Modification
2.6. Calculation of the Microscopic Electroactive Areas
3. Results and Discussion
3.1. Characterization of Composite Materials
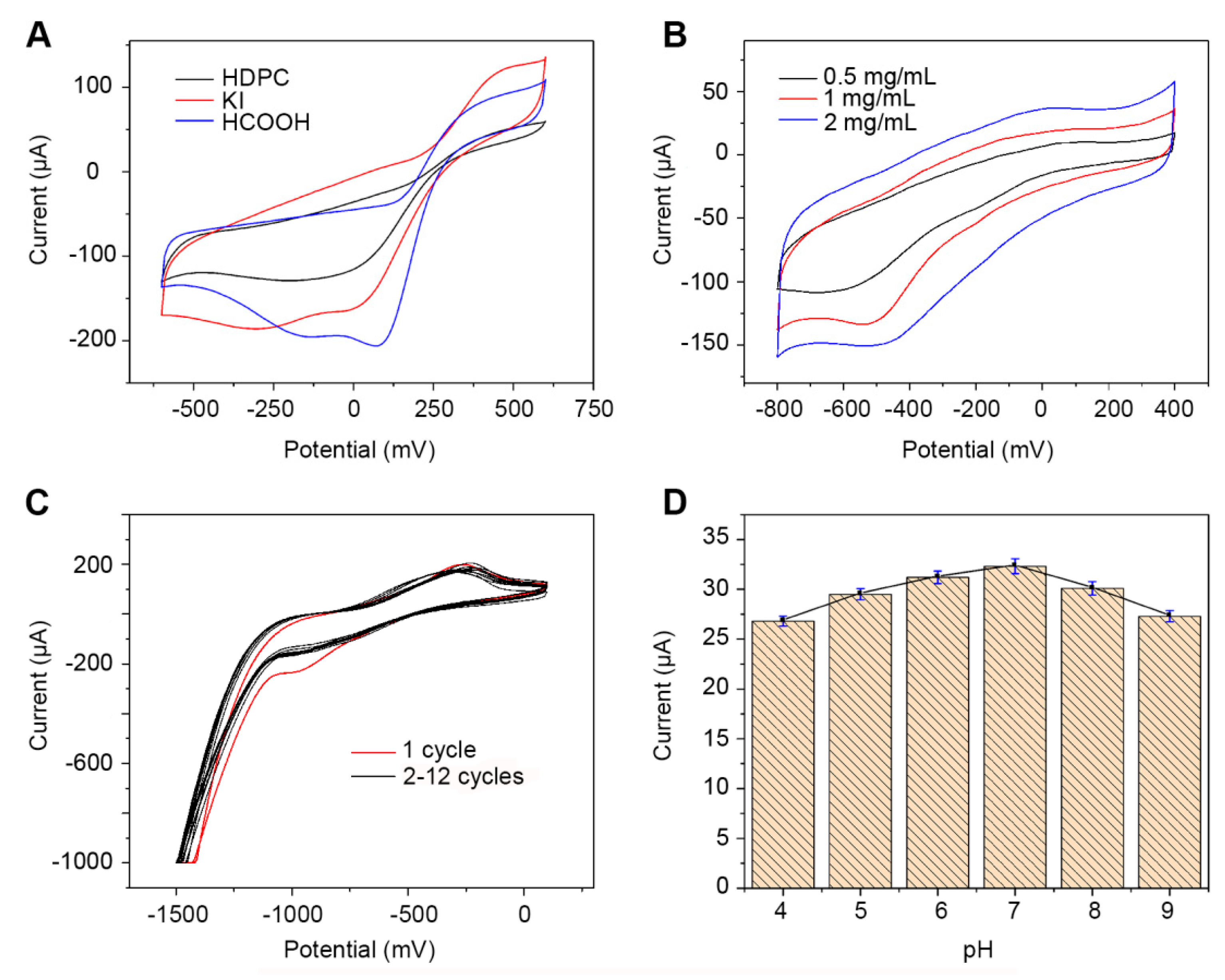
3.2. Characterization of the Electrocatalytic Activities of the Modified Electrodes
3.3. Effect of Experimental Condition on the Performance of the CA242 Immunosensor
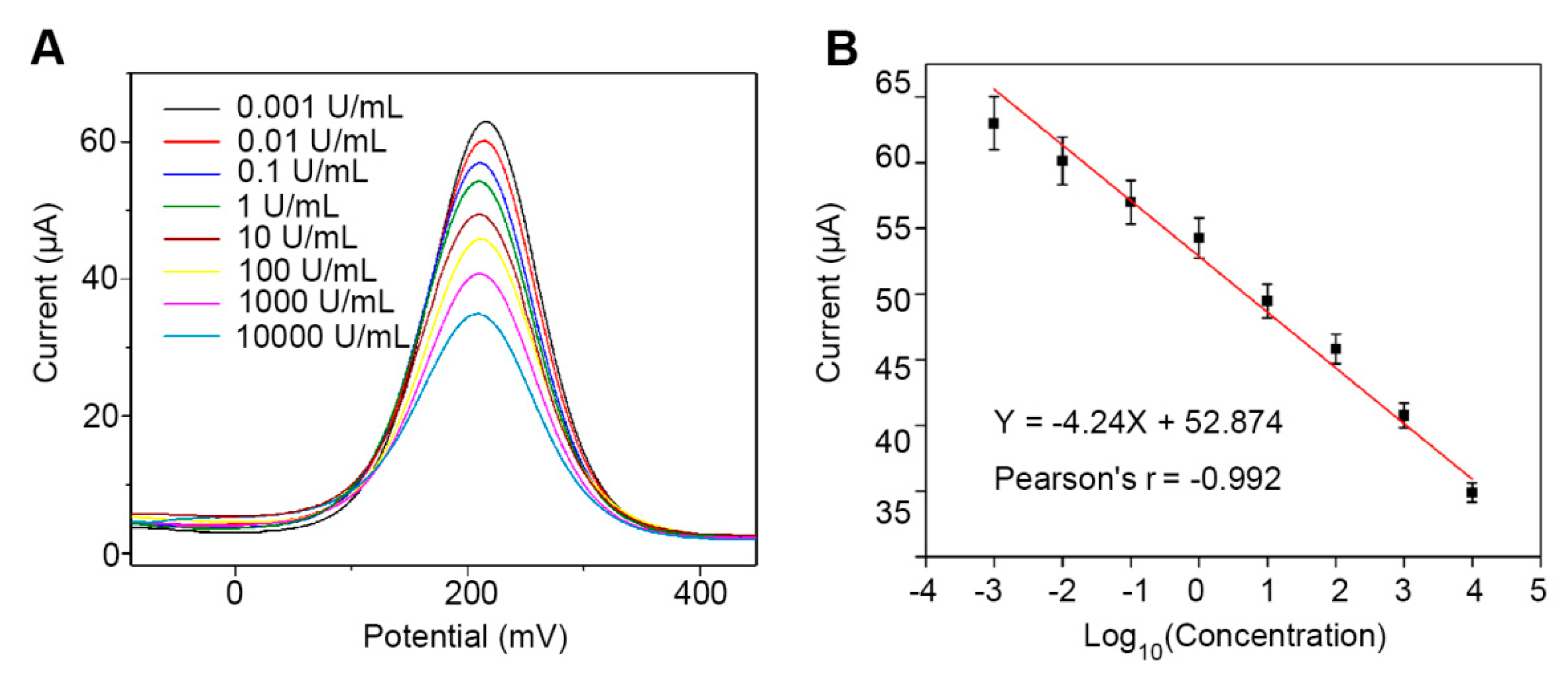
3.4. Characterization of the Analytical Performance of the Immunosensor for Detection of CA242 in PBS
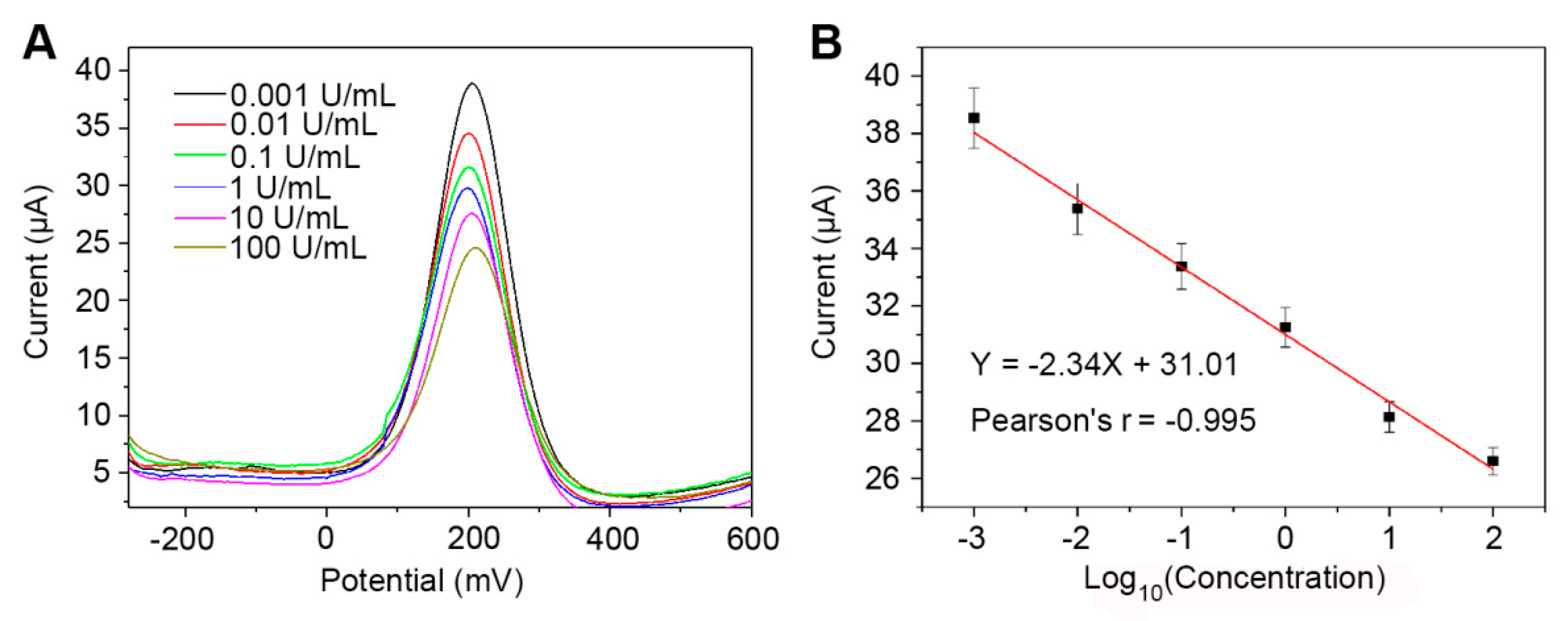
3.5. The CA242 Immunosensor Reliably Detects CA242 in Human Serum
3.6. The CA242 Immunosensor Exhibits High Reproducibility, Selectivity, and Stability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2016. CA Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Tu, C.Y.; Zhu, W.L.; Shao, C.X.; Zhu, J.D.; Tu, J.F.; Zhou, X.M.; Lin, Q.M.; Zhang, K.; Li, Z.K.; Tan, W.; et al. Diagnosis and treatment of heterotopic pancreas coexisting with digestive tract tumor: A report of 26 cases. Int. J. Clin. Exp. Med. 2017, 10, 8535–8544. [Google Scholar]
- Blaker, H. Grading of tumors in the tubular digestive tract. Esophagus, stomach, colon and rectum. Pathologe 2016, 37, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.L.; Yang, G.W.; Pan, J.; Zhang, C. Tumor necrosis factor alpha knockout increases fertility of mice. Theriogenology 2011, 75, 867–876. [Google Scholar] [CrossRef] [PubMed]
- Hori, S.S.; Gambhir, S.S. A mathematical approach to earlier cancer detection: Using blood biomarker assays to monitor growth of a tumor from a single cell. Cancer Res. 2011, 71. [Google Scholar] [CrossRef]
- Janni, W.; Rack, B.; Haberle, L.; Friedl, T.W.P.; Tesch, H.; Lorenz, R.; Jager, B.; Fehm, T.; Muller, V.; Schneeweiss, A.; et al. Active surveillance with a combination of tumor marker CA27.29 and detection of circulating tumor cells two year after primary diagnosis strongly predicts subsequent prognosis. Cancer Res. 2017, 77. [Google Scholar] [CrossRef]
- Xu, Y.S.; Zhang, X.P.; Luan, C.X.; Wang, H.; Chen, B.A.; Zhao, Y.J. Hybrid hydrogel photonic barcodes for multiplex detection of tumor markers. Biosens. Bioelectron. 2017, 87, 264–270. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, S.; Baine, M.J.; Sasson, A.R.; Batra, S.K. Current status of molecular markers for early detection of sporadic pancreatic cancer. BBA Rev. Cancer 2011, 1815, 44–64. [Google Scholar] [CrossRef] [Green Version]
- Gui, J.C.; Yan, W.L.; Liu, X.D. CA19-9 and CA242 as tumor markers for the diagnosis of pancreatic cancer: A meta-analysis. Clin. Exp. Med. 2014, 14, 225–233. [Google Scholar] [CrossRef]
- Du, X.; Zhou, J. Application of biosensors to detection of epidemic diseases in animals. Res. Vet. Sci. 2018, 118, 444–448. [Google Scholar] [CrossRef]
- Kang, C.C.; Chang, T.C. The mechanism and structure localization relationship studies of a small organic molecule as a fluorescent tumor marker. Cancer Res. 2010, 70. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Zhang, X.J.; Fan, S.J. Meta-analysis of salt-related gene expression profiles identifies common signatures of salt stress responses in Arabidopsis. Plant Syst. Evol. 2017, 303, 757–774. [Google Scholar] [CrossRef]
- Shishkanova, T.V.; Havlik, M.; Dendisova, M.; Matejka, P.; Kral, V. Synthesis and deposition of a Troger’s base polymer on the electrode surface for potentiometric detection of a neuroblastoma tumor marker metabolite. Chem. Commun. 2016, 52, 11991–11994. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Wu, X.; Shi, J.; Peng, Z.; Gao, M.; Xin, C.; Liu, Y.; Wang, S.; Xu, S.; Han, H.; et al. Rapid specific and visible detection of porcine circovirus type 3 using loop-mediated isothermal amplification (LAMP). Transbound. Emerg. Dis. 2018, 65, 597–601. [Google Scholar] [CrossRef] [PubMed]
- Hou, P.L.; Zhao, G.M.; Wang, H.M.; He, C.Q.; He, H.B. Rapid detection of bovine viral diarrhea virus using recombinase polymerase amplification combined with lateral flow dipstick assays in bulk milk. Vet. Arh. 2018, 88, 627–642. [Google Scholar] [CrossRef]
- Zhang, B.; Ding, C.M. Displacement-type amperometric immunosensing platform for sensitive determination of tumour markers. Biosens. Bioelectron. 2016, 82, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.X.; Ma, Z.F. Multiple functional strategies for amplifying sensitivity of amperometric immunoassay for tumor markers: A review. Biosens. Bioelectron. 2017, 98, 100–112. [Google Scholar] [CrossRef]
- Wang, H.Q.; Ma, Z.F. A cascade reaction signal-amplified amperometric immunosensor platform for ultrasensitive detection of tumour marker. Sens. Actuators B Chem. 2018, 254, 642–647. [Google Scholar] [CrossRef]
- Khoshroo, A.; Mazloum-Ardakani, M.; Forat-Yazdi, M. Enhanced performance of label-free electrochemical immunosensor for carbohydrate antigen 15-3 based on catalytic activity of cobalt sulfide/graphene nanocomposite. Sens. Actuators B Chem. 2018, 255, 580–587. [Google Scholar] [CrossRef]
- Li, Y.; He, J.L.; Chen, J.; Niu, Y.Z.; Zhao, Y.L.; Zhang, Y.C.; Yu, C. A dual-type responsive electrochemical immunosensor for quantitative detection of PCSK9 based on n-C-60-PdPt/N-GNRs and Pt-poly (methylene blue) nanocomposites. Biosens. Bioelectron. 2018, 101, 7–13. [Google Scholar] [CrossRef]
- Liu, C.; Dong, J.; Waterhouse, G.I.N.; Cheng, Z.Q.; Ai, S.Y. Electrochemical immunosensor with nanocellulose-Au composite assisted multiple signal amplification for detection of avian leukosis virus subgroup J. Biosens. Bioelectron. 2018, 101, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, J.; Devarakonda, S.; Kumar, S.; Jang, J. Development of a paper-based electrochemical immunosensor using an antibody-single walled carbon nanotubes bio-conjugate modified electrode for label-free detection of foodborne pathogens. Sens. Actuators B Chem. 2017, 253, 115–123. [Google Scholar] [CrossRef]
- Shore, A.; Mazzochette, Z.; Mugweru, A. Mixed valence Mn,La,Sr-oxide based magnetic nanoparticles coated with silica nanoparticles for use in an electrochemical immunosensor for IgG. Microchim. Acta 2016, 183, 475–483. [Google Scholar] [CrossRef]
- Kong, X.Q.; Wang, T.; Li, W.J.; Tang, W.; Zhang, D.M.; Dong, H.Z. Exogenous nitric oxide delays salt-induced leaf senescence in cotton (Gossypium hirsutum L.). Acta Physiol. Plant. 2016, 38. [Google Scholar] [CrossRef]
- Kumar, N.; Goyal, R.N. Gold-palladium nanoparticles aided electrochemically reduced graphene oxide sensor for the simultaneous estimation of lomefloxacin and amoxicillin. Sens. Actuators B Chem. 2017, 243, 658–668. [Google Scholar] [CrossRef]
- Jiang, J.J.; Du, X.Z. Sensitive electrochemical sensors for simultaneous determination of ascorbic acid, dopamine, and uric acid based on Au@Pd-reduced graphene oxide nanocomposites. Nanoscale 2014, 6, 11303–11309. [Google Scholar] [CrossRef]
- Liu, P.G.; Wu, D.L.; Gao, Y.; Wang, T.; Tan, Y.Y.; Jia, D.Z. Reduced graphene oxide-coated mulberry-shaped α-Fe2O3 nanoparticles composite as high performance electrode material for supercapacitors. J. Alloys Compd. 2018, 738, 89–96. [Google Scholar] [CrossRef]
- Cao, D.R.; Li, H.; Wang, Z.K.; Wei, J.W.; Wang, J.B.; Liu, Q.F. Synthesis, nanostructure and magnetic properties of FeCo-reduced graphene oxide composite films by one-step electrodeposition. Thin Solid Films 2015, 597, 1–6. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Y.J.; Wu, A.G.; Wei, G. Designed graphene-peptide nanocomposites for biosensor applications: A review. Anal. Chim. Acta 2017, 985, 24–40. [Google Scholar] [CrossRef]
- Zhang, T.T.; Gu, Y.; Li, C.; Yan, X.Y.; Lu, N.N.; Liu, H.; Zhang, Z.Q.; Zhang, H. Fabrication of Novel Electrochemical Biosensor Based on Graphene Nanohybrid to Detect H2O2 Released from Living Cells with Ultrahigh Performance. ACS Appl. Mater. Interfaces 2017, 9, 37991–37999. [Google Scholar] [CrossRef]
- Muthusankar, E.; Ponnusamy, V.K.; Ragupathy, D. Electrochemically sandwiched poly(diphenylamine)/phosphotungstic acid/graphene nanohybrid as highly sensitive and selective urea biosensor. Synth. Met. 2019, 254, 134–140. [Google Scholar] [CrossRef]
- Lv, J.J.; Li, S.S.; Wang, A.J.; Mei, L.P.; Chen, J.R.; Feng, J.J. Monodisperse Au-Pd bimetallic alloyed nanoparticles supported on reduced graphene oxide with enhanced electrocatalytic activity towards oxygen reduction reaction. Electrochim. Acta 2014, 136, 521–528. [Google Scholar] [CrossRef]
- Du, X.; Miao, Z.Y.; Zhang, D.; Fang, Y.X.; Ma, M.; Chen, Q. Facile synthesis of β-lactoglobulin-functionalized multi-wall carbon nanotubes and gold nanoparticles on glassy carbon electrode for electrochemical sensing. Biosens. Bioelectron. 2014, 62, 73–78. [Google Scholar] [CrossRef]
- Stobinski, L.; Lesiak, B.; Malolepszy, A.; Mazurkiewicz, M.; Mierzwa, B.; Zemek, J.; Jiricek, P.; Bieloshapka, I. Graphene oxide and reduced graphene oxide studied by the XRD, TEM and electron spectroscopy methods. J. Electron. Spectrosc. 2014, 195, 145–154. [Google Scholar] [CrossRef]
- Dubin, S.; Gilje, S.; Wang, K.; Tung, V.C.; Cha, K.; Hall, A.S.; Farrar, J.; Varshneya, R.; Yang, Y.; Kaner, R.B. A One-Step, Solvothermal Reduction Method for Producing Reduced Graphene Oxide Dispersions in Organic Solvents. ACS Nano 2010, 4, 3845–3852. [Google Scholar] [CrossRef] [Green Version]
- Cybula, A.; Priebe, J.B.; Pohl, M.M.; Sobczak, J.W.; Schneider, M.; Zielhiska-Jurek, A.; Bruckner, A.; Zaleska, A. The effect of calcination temperature on structure and photocatalytic properties of Au/Pd nanoparticles supported on TiO2. Appl. Catal. B Environ. 2014, 152, 202–211. [Google Scholar] [CrossRef]
- Chen, L.Y.; Tang, Y.H.; Wang, K.; Liu, C.B.; Luo, S.L. Direct electrodeposition of reduced graphene oxide on glassy carbon electrode and its electrochemical application. Electrochem. Commun. 2011, 13, 133–137. [Google Scholar] [CrossRef]
- Rong, Q.F.; Feng, F.; Ma, Z.F. Metal ions doped chitosan-poly(acrylic acid) nanospheres: Synthesis and their application in simultaneously electrochemical detection of four markers of pancreatic cancer. Biosens. Bioelectron. 2016, 75, 148–154. [Google Scholar] [CrossRef]
- Zhu, Q.; Chai, Y.Q.; Yuan, R.; Zhuo, Y. Simultaneous detection of four biomarkers with one sensing surface based on redox probe tagging strategy. Anal. Chim. Acta 2013, 800, 22–28. [Google Scholar] [CrossRef]
- Tang, Z.; Fu, Y.; Ma, Z. Multiple signal amplification strategies for ultrasensitive label-free electrochemical immunoassay for carbohydrate antigen 24-2 based on redox hydrogel. Biosens. Bioelectron. 2017, 91, 299–305. [Google Scholar] [CrossRef]
- Sun, A.L.; Qi, Q.A. Silver-functionalized g-C3N4 nanohybrids as signal-transduction tags for electrochemical immunoassay of human carbohydrate antigen 19-9. Analyst 2016, 141, 4366–4372. [Google Scholar] [CrossRef]
- Wang, R.; Feng, J.J.; Liu, W.D.; Jiang, L.Y.; Wang, A.J. A novel label-free electrochemical immunosensor based on the enhanced catalytic currents of oxygen reduction by AuAg hollow nanocrystals for detecting carbohydrate antigen 199. Biosens. Bioelectron. 2017, 96, 152–158. [Google Scholar] [CrossRef]
- Torati, S.R.; Kasturi, K.C.S.B.; Lim, B.; Kim, C. Hierarchical gold nanostructures modified electrode for electrochemical detection of cancer antigen CA125. Sens. Actuators B Chem. 2017, 243, 64–71. [Google Scholar] [CrossRef]
- Li, C.X.; Qiu, X.Y.; Deng, K.Q.; Hou, Z.H. Electrochemical co-reduction synthesis of Au/ferrocene-graphene nanocomposites and their application in an electrochemical immunosensor of a breast cancer biomarker. Anal. Methods 2014, 6, 9078–9084. [Google Scholar] [CrossRef]




| Material | Target | Linear Range (U/mL) | Detection Limit (U/mL) | Sensitivity (μA (log10 c)−1) | Electrochemical Method | Reference |
|---|---|---|---|---|---|---|
| Chit-Au a/Ab b1/Ag c/Zn-CP d-Ab2 | CA242 | 1–150 U/mL | 0.40 | 5.03 | SWV | [38] |
| Au/PDDA/PTCA/CNTs | CA242 | 0.010 to 10 ng/mL | 3.8 pg/mL | / | DPV | [39] |
| SA-Pb2+-GO hydrogel | CA242 | 0.005–500 | 0.067 | 32.98 | SWV | [40] |
| Chitosan/Ab1/Ag/Ab2-Ag/g-C3N4 e | CA199 | 0.005–50 | 1.2 × 10−3 | 1.46 | DPV | [41] |
| AuAg hollow nanocrystals | CA199 | 1–30 | 0.23 | 0.05 | DPV | [42] |
| Gold nanostructure | CA125 | 10–100 | 5.5 | 0.16 | DPV | [43] |
| Fc–GO f | CA153 | 0.05–20 | 0.015 | 0.34 | DPV | [44] |
| rGO-Au-Pd | CA242 | 0.001–10,000 | 1.54 × 10−3 | 4.24 | DPV | this work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, X.; Zheng, X.; Zhang, Z.; Wu, X.; Sun, L.; Zhou, J.; Liu, M. A Label-Free Electrochemical Immunosensor for Detection of the Tumor Marker CA242 Based on Reduced Graphene Oxide-Gold-Palladium Nanocomposite. Nanomaterials 2019, 9, 1335. https://doi.org/10.3390/nano9091335
Du X, Zheng X, Zhang Z, Wu X, Sun L, Zhou J, Liu M. A Label-Free Electrochemical Immunosensor for Detection of the Tumor Marker CA242 Based on Reduced Graphene Oxide-Gold-Palladium Nanocomposite. Nanomaterials. 2019; 9(9):1335. https://doi.org/10.3390/nano9091335
Chicago/Turabian StyleDu, Xin, Xiaodi Zheng, Zhenhua Zhang, Xiaofan Wu, Lei Sun, Jun Zhou, and Min Liu. 2019. "A Label-Free Electrochemical Immunosensor for Detection of the Tumor Marker CA242 Based on Reduced Graphene Oxide-Gold-Palladium Nanocomposite" Nanomaterials 9, no. 9: 1335. https://doi.org/10.3390/nano9091335





