Selective Dye Adsorption by Zeolitic Imidazolate Framework-8 Loaded UiO-66-NH2
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of UiO-66-NH2
2.3. Synthesis of ZIF-8-Loaded UiO-66-NH2
2.4. Characterization
2.5. Adsorption Experiments of Dyes
3. Results and Discussion
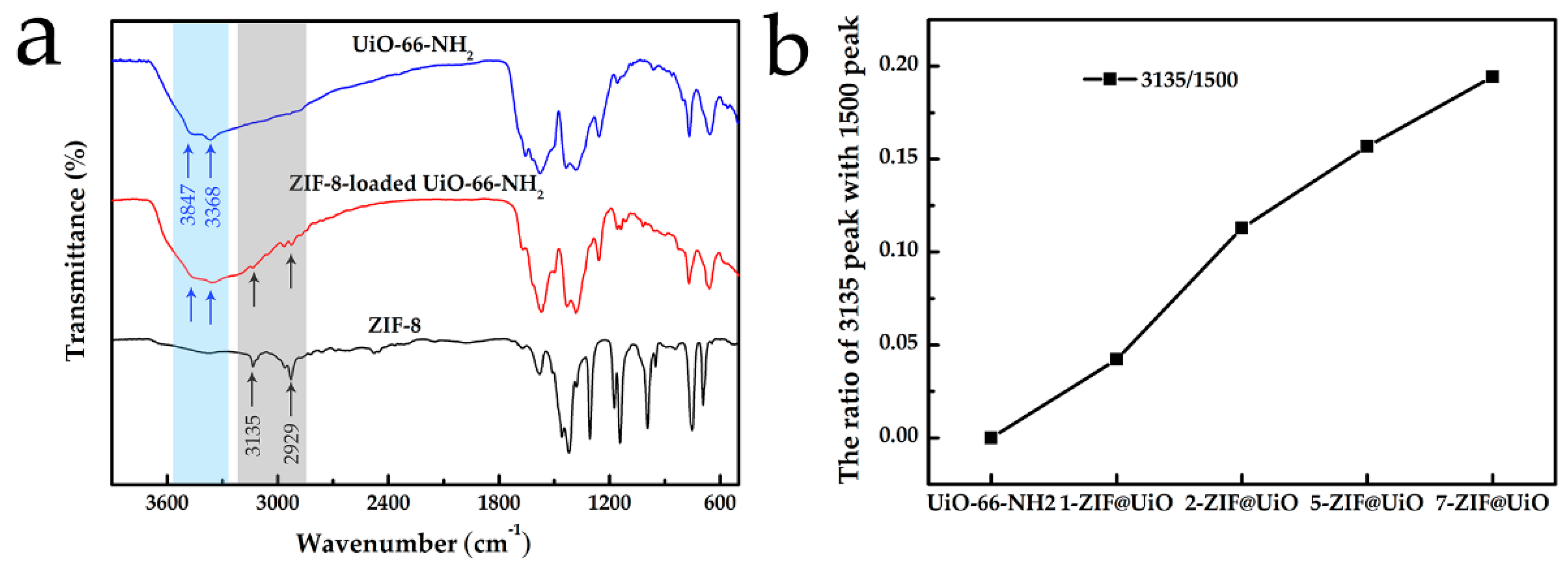
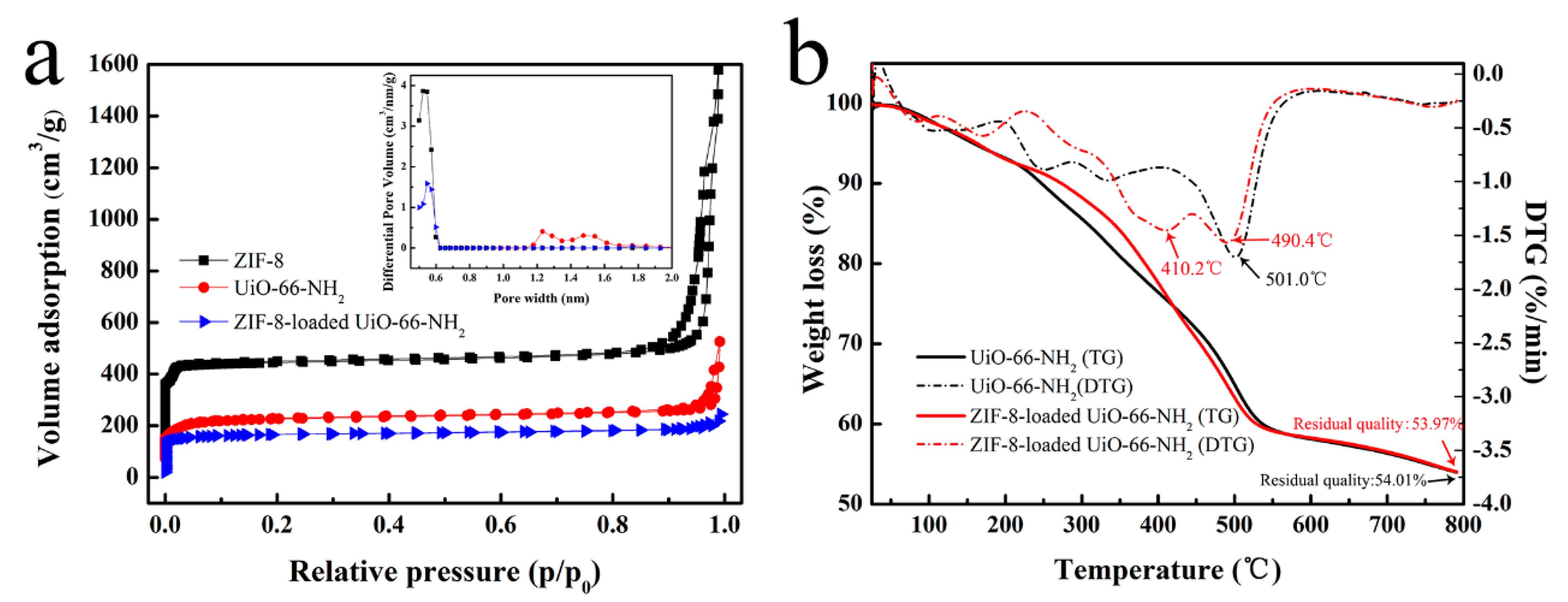
3.1. Adsorbents Characterizations
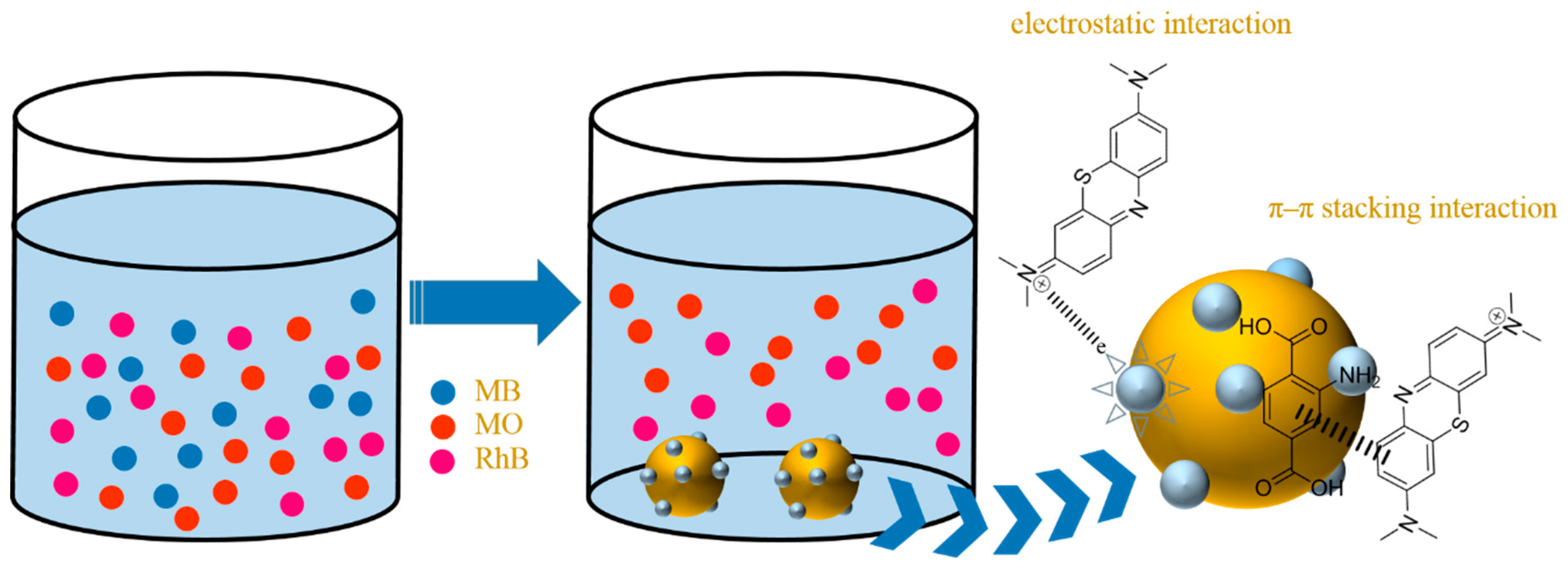
3.2. Separation Selectivity in the Mixed Dye Solution
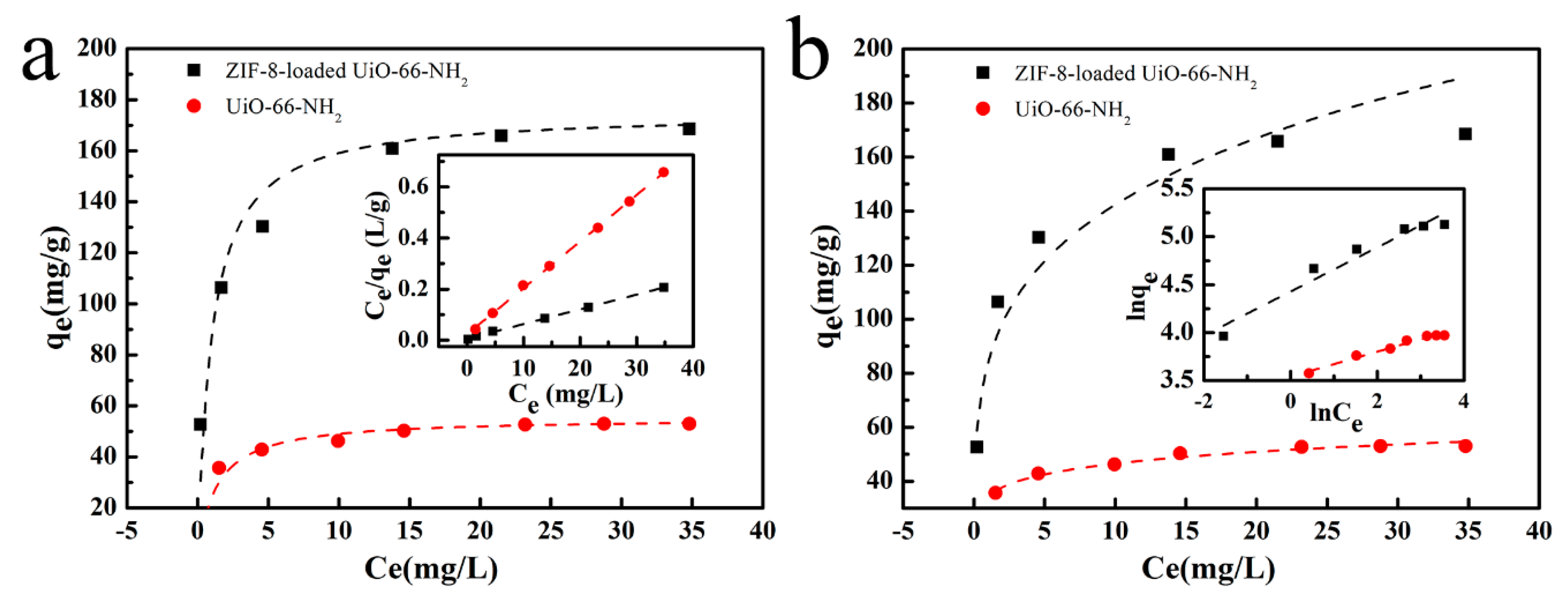
3.3. Adsorption Isotherms
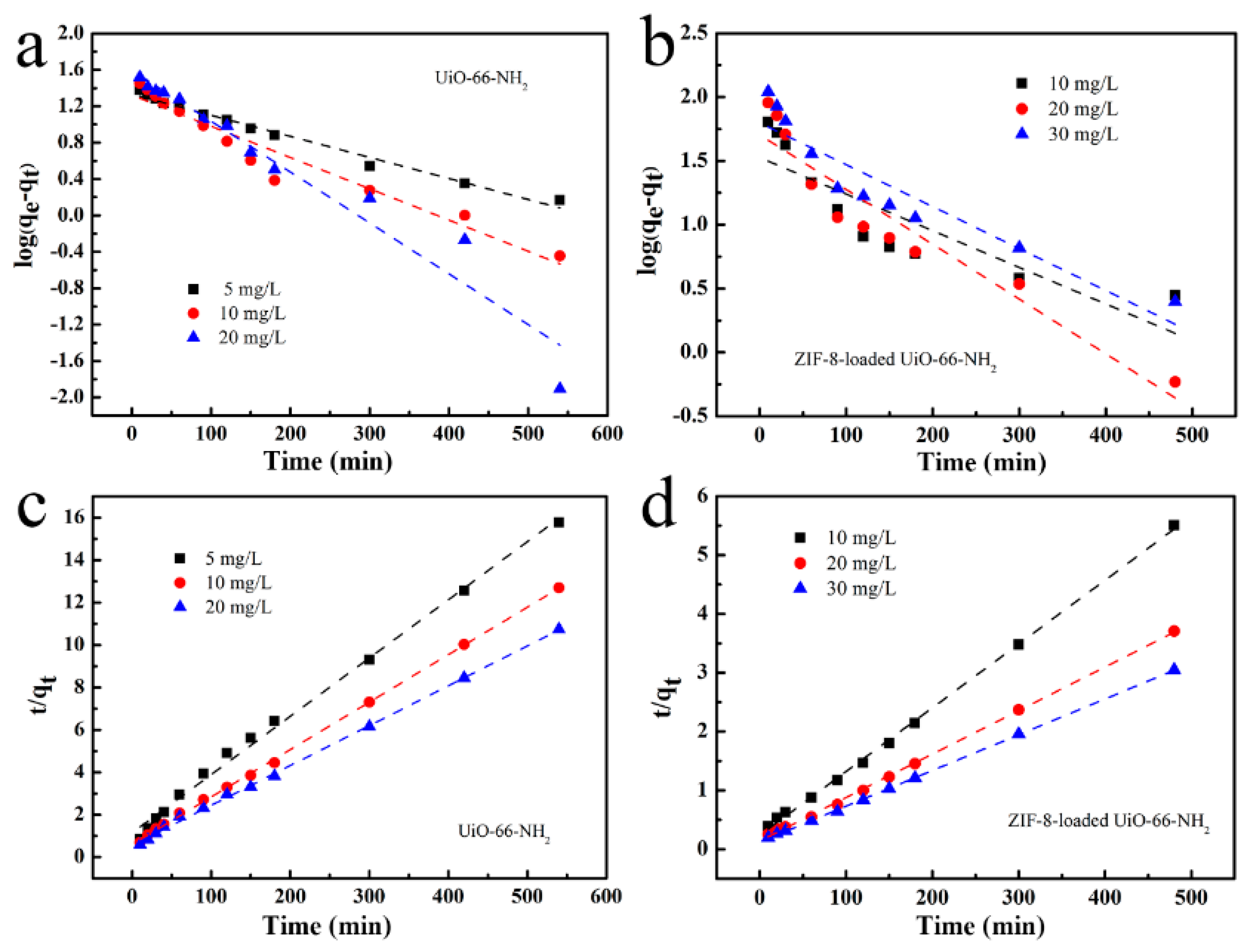
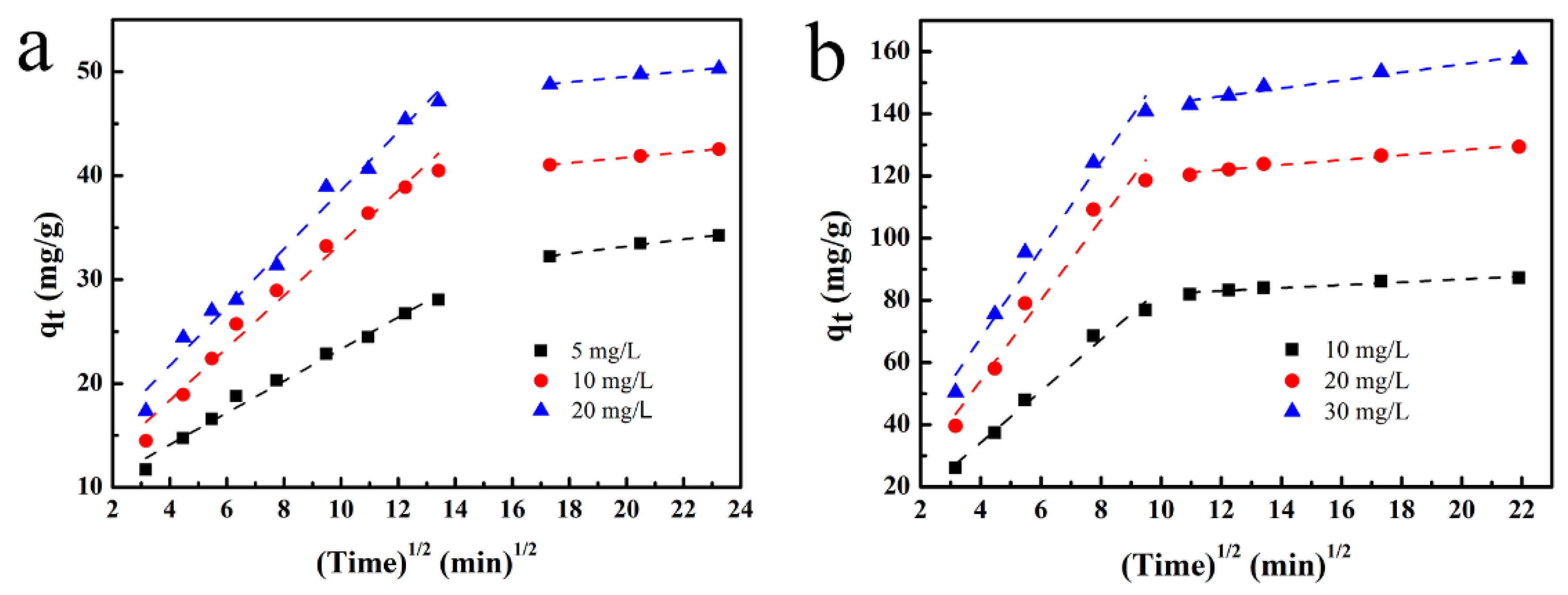
3.4. Kinetic Studies
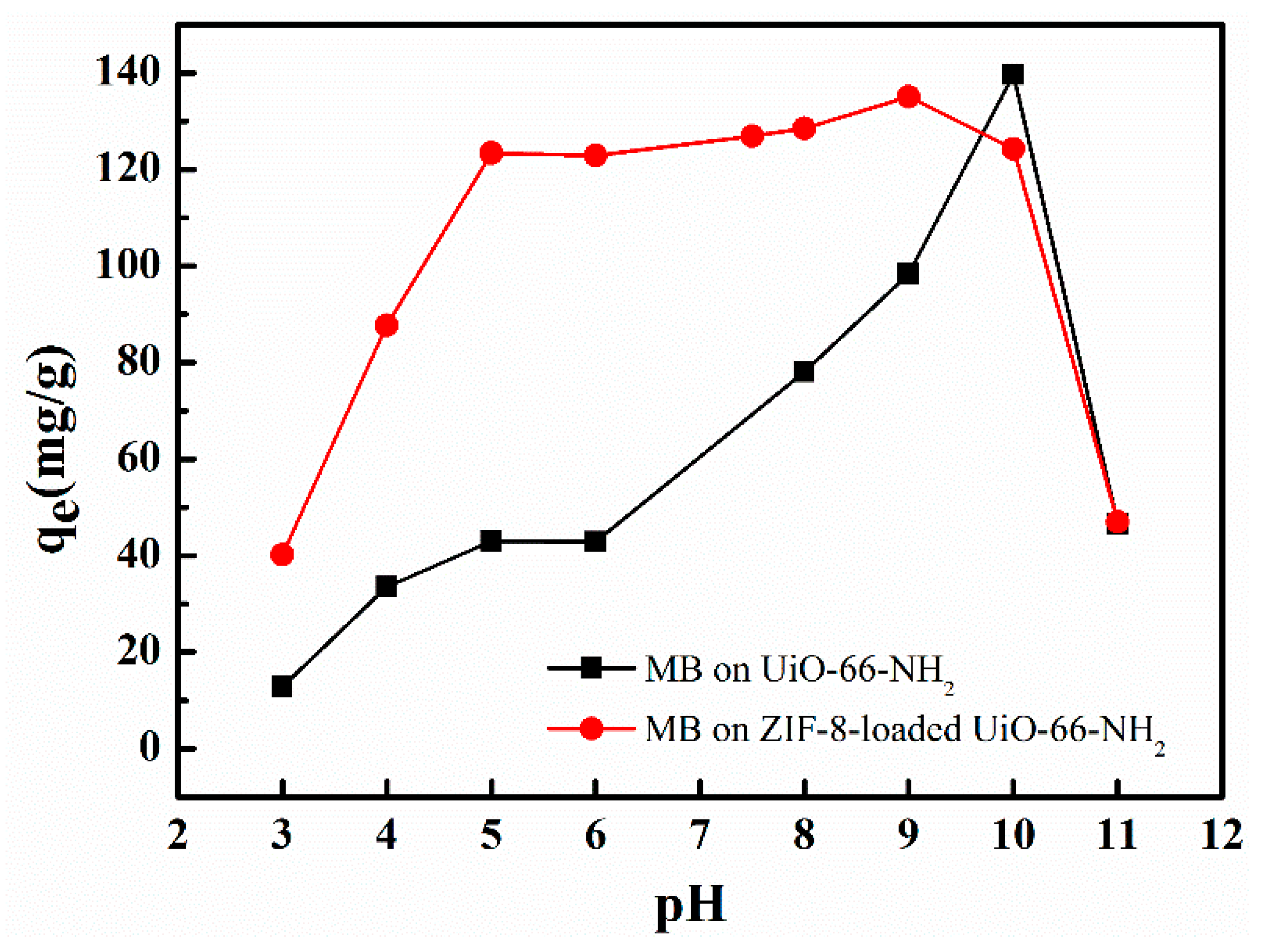
3.5. Effect of pH on Adsorption
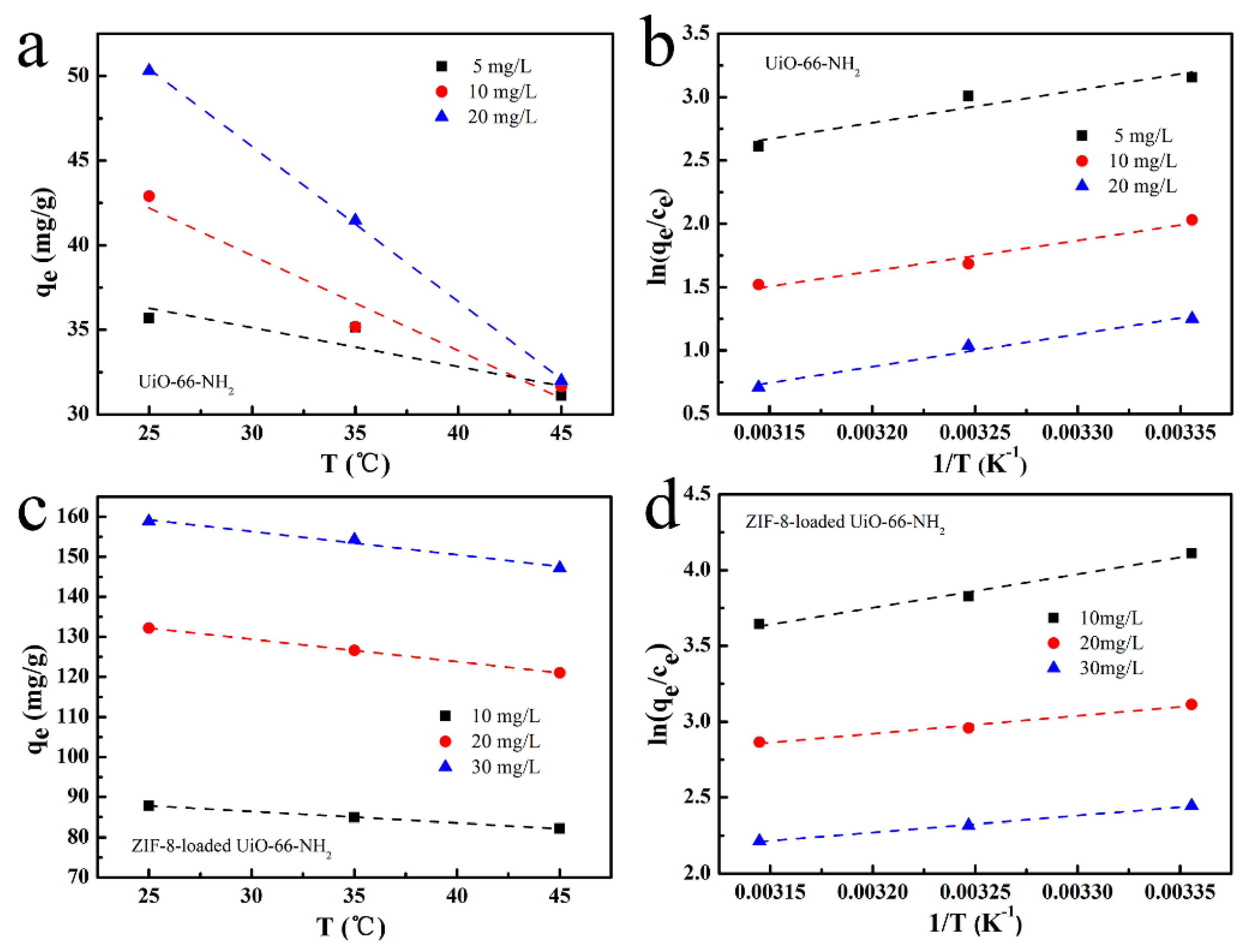
3.6. Adsorption Thermodynamics
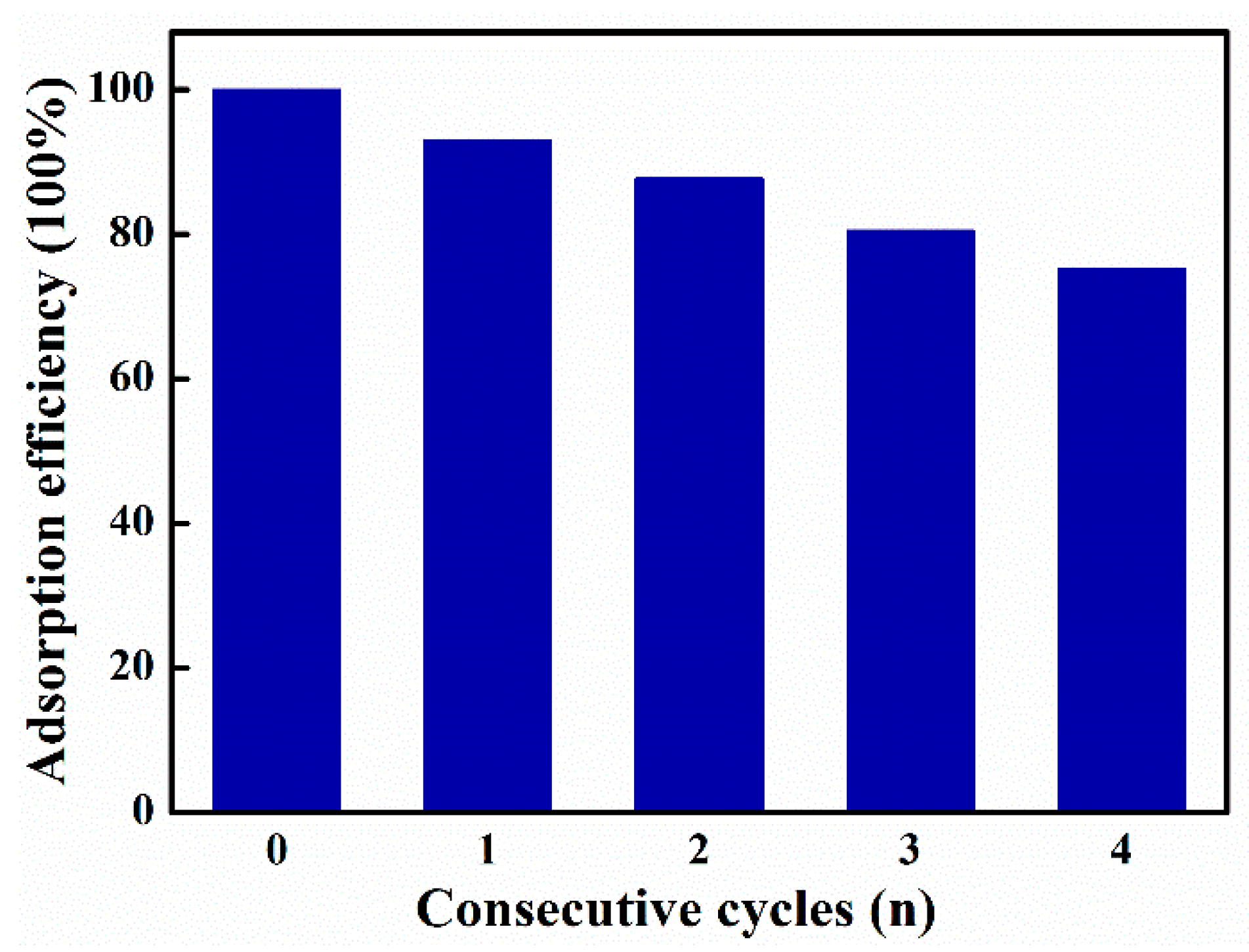
3.7. Reusability of ZIF-8-Loaded UiO-66-NH2
3.8. Adsorption Mechanism
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhou, H.-C.; Long, J.R.; Yaghi, O.M. Introduction to Metal–Organic Frameworks. Chem. Rev. 2012, 112, 673–674. [Google Scholar] [CrossRef] [PubMed]
- Dou, Y.; Rutledge, W.; Bai, Y.; Xie, L.-H.; Li, J.-R.; Zhou, H.-C. Zr-based metal–organic frameworks: Design, synthesis, structure, and applications. Chem. Soc. Rev. 2016, 45, 2327–2367. [Google Scholar]
- Zeng, L.; Xiao, L.; Long, Y.; Shi, X. Trichloroacetic acid-modulated synthesis of polyoxometalate@UiO-66 for selective adsorption of cationic dyes. J. Colloid Interface Sci. 2018, 516, 274–283. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Wang, J.; Chen, Y.; Zhang, J.; Duan, D.; Wang, Y.; Yan, Z. A dye-sensitized Pt@UiO-66(Zr) metal–organic framework for visible-light photocatalytic hydrogen production. Chem. Commun. 2014, 50, 7063–7066. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Yuan, Y.; Chen, C.; Zhang, P.; Wang, J.; Khan, A.; Zhuiykov, S.; Chaemchuen, S.; Verpoort, F. Enhancing catalytic performance via structure core-shell metal-organic frameworks. J. Catal. 2019, 375, 371–379. [Google Scholar] [CrossRef]
- Koh, K.; Wong-Foy, A.G.; Matzger, A.J. MOF@MOF: Microporous core-shell architectures. Chem. Commun. 2009, 6162–6164. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Musyoka, N.M.; Langmi, H.W.; North, B.C.; Mathe, M.; Kang, X. Fabrication of core–shell MIL-101(Cr)@UiO-66(Zr) nanocrystals for hydrogen storage. Int. J. Hydrog. Energy 2014, 39, 14912–14917. [Google Scholar] [CrossRef]
- Zhuang, J.; Chou, L.-Y.; Sneed, B.T.; Cao, Y.; Hu, P.; Feng, L.; Tsung, C.-K. Surfactant-Mediated Conformal Overgrowth of Core-Shell Metal-Organic Framework Materials with Mismatched Topologies. Small 2015, 11, 5551–5555. [Google Scholar] [CrossRef]
- Tambat, S.N.; Sane, P.K.; Suresh, S.; O., N.V.; Pandit, A.B.; Sontakke, S.M.; Varadan, O.N. Hydrothermal synthesis of NH2-UiO-66 and its application for adsorptive removal of dye. Adv. Powder Technol. 2018, 29, 2626–2632. [Google Scholar] [CrossRef]
- Fan, J.; Chen, D.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. Adsorption and biodegradation of dye in wastewater with Fe3O4@MIL-100 (Fe) core–shell bio-nanocomposites. Chemosphere 2018, 191, 315–323. [Google Scholar] [CrossRef]
- Li, H.; Li, Q.; He, X.; Xu, Z.; Wang, Y.; Jia, L. Synthesis of AgBr@MOFs nanocomposite and its photocatalytic activity for dye degradation. Polyhedron 2019, 165, 31–37. [Google Scholar] [CrossRef]
- Wang, K.; Qin, Y.; Quan, S.; Zhang, Y.; Wang, P.; Liang, H.; Ma, J.; Cheng, X.Q. Development of highly permeable polyelectrolytes (PEs)/UiO-66 nanofiltration membranes for dye removal. Chem. Eng. Res. Des. 2019, 147, 222–231. [Google Scholar] [CrossRef]
- Mittal, H.; Maity, A.; Sinha Ray, S. The adsorption of Pb2+ and Cu2+ onto gum ghatti-grafted poly(acrylamide-co-acrylonitrile) biodegradable hydrogel: Isotherms and kinetic models. J. Phys. Chem. B 2015, 119, 2026–2039. [Google Scholar] [CrossRef] [PubMed]
- Kyo, S.P.; Zheng, N.; Adrien, P.C.; Jae, Y.C.; Rudan, H.; Fernando, J.U.; Hee, K.C.; Michael, O.; Omar, M.Y. Exceptional chemical and thermal stability of zeolitic imidazolate frameworks. Proc. Natl. Acad. Sci. USA 2006, 103, 10186–10191. [Google Scholar] [Green Version]
- Li, Y.; Zhou, K.; He, M.; Yao, J. Synthesis of ZIF-8 and ZIF-67 using mixed-base and their dye adsorption. Microporous Mesoporous Mater. 2016, 234, 287–292. [Google Scholar] [CrossRef]
- Yoon, S.; Calvo, J.J.; So, M.C. Removal of Acid Orange 7 from Aqueous Solution by Metal-Organic Frameworks. Crystals 2018, 9, 17. [Google Scholar] [CrossRef]
- Feng, Y.; Li, Y.; Xu, M.; Liu, S.; Yao, J. Fast adsorption of methyl blue on zeolitic imidazolate framework-8 and its adsorption mechanism. RSC Adv. 2016, 6, 109608–109612. [Google Scholar] [CrossRef]
- Thanh, M.T.; Thien, T.V.; Chau, V.T.T.; Du, P.D.; Hung, N.P.; Khieu, D.Q. Synthesis of Iron Doped Zeolite Imidazolate Framework-8 and Its Remazol Deep Black RGB Dye Adsorption Ability. J. Chem. 2017, 2017, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Li, N.; Owens, G.; Chen, Z. Simultaneous removal of mixed contaminants, copper and norfloxacin, from aqueous solution by ZIF-8. Chem. Eng. J. 2019, 362, 628–637. [Google Scholar] [CrossRef]
- Katz, M.J.; Brown, Z.J.; Colón, Y.J.; Siu, P.W.; Scheidt, K.A.; Snurr, R.Q.; Hupp, J.T.; Farha, O.K. A facile synthesis of UiO-66, UiO-67 and their derivatives. Chem. Commun. 2013, 49, 9449–9451. [Google Scholar] [CrossRef]
- Song, Z.; Qiu, F.; Zaia, E.W.; Wang, Z.; Kunz, M.; Guo, J.; Brady, M.; Mi, B.; Urban, J.J. Dual-Channel, Molecular-Sieving Core/Shell ZIF@MOF Architectures as Engineered Fillers in Hybrid Membranes for Highly Selective CO2 Separation. Nano Lett. 2017, 17, 6752–6758. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.; Bux, H.; Steinbach, F.; Caro, J. Molecular-Sieve Membrane with Hydrogen Permselectivity: ZIF-22 in LTA Topology Prepared with 3-Aminopropyltriethoxysilane as Covalent Linker. Angew. Chem. Int. Ed. 2010, 49, 4958–4961. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Dong, Z.; Li, Q.; Jin, W. Growth of a ZIF-8 membrane on the inner-surface of a ceramic hollow fiber via cycling precursors. Chem. Commun. 2013, 49, 10326. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Tu, H.; Liu, J.; Zhao, C.; Liao, J.; Yang, Y.; Yang, J.; Liu, N. A novel ion-imprinted polymer induced by the glycylglycine modified metal-organic framework for the selective removal of Co(II) from aqueous solutions. Chem. Eng. J. 2018, 333, 280–288. [Google Scholar] [CrossRef]
- Xu, G.; Yao, J.; Wang, K.; He, L.; Webley, P.A.; Chen, C.-S.; Wang, H. Preparation of ZIF-8 membranes supported on ceramic hollow fibers from a concentrated synthesis gel. J. Membr. Sci. 2011, 385, 187–193. [Google Scholar] [CrossRef]
- Hwang, Y.K.; Hong, D.-Y.; Chang, J.-S.; Jhung, S.H.; Seo, Y.-K.; Kim, J.; Vimont, A.; Daturi, M.; Serre, C.; Férey, G. Amine Grafting on Coordinatively Unsaturated Metal Centers of MOFs: Consequences for Catalysis and Metal Encapsulation. Angew. Chem. Int. Ed. 2008, 47, 4144–4148. [Google Scholar] [CrossRef] [PubMed]
- Kandiah, M.; Usseglio, S.; Svelle, S.; Olsbye, U.; Lillerud, K.P.; Tilset, M. Post-synthetic modification of the metal–organic framework compound UiO-66. J. Mater. Chem. C 2010, 20, 9848–9851. [Google Scholar] [CrossRef]
- Beh, J.J.; Lim, J.K.; Ng, E.P.; Ooi, B.S. Synthesis and size control of zeolitic imidazolate framework-8 (ZIF-8): From the perspective of reaction kinetics and thermodynamics of nucleation. Mater. Chem. Phys. 2018, 216, 393–401. [Google Scholar] [CrossRef]
- Qiu, J.; Feng, Y.; Zhang, X.; Jia, M.; Yao, J. Acid-promoted synthesis of UiO-66 for highly selective adsorption of anionic dyes: Adsorption performance and mechanisms. J. Colloid Interface Sci. 2017, 499, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Tuo, L.; Yang, K.; Jeong, H.-K.; Dai, Y.; He, G.; Zhao, W. Simultaneous enhancement of mechanical properties and CO2 selectivity of ZIF-8 mixed matrix membranes: Interfacial toughening effect of ionic liquid. J. Membr. Sci. 2016, 511, 130–142. [Google Scholar] [CrossRef]
- Zhu, B.-J.; Yu, X.-Y.; Jia, Y.; Peng, F.-M.; Sun, B.; Zhang, M.-Y.; Luo, T.; Liu, J.-H.; Huang, X.-J. Iron and 1,3,5-Benzenetricarboxylic Metal–Organic Coordination Polymers Prepared by Solvothermal Method and Their Application in Efficient As(V) Removal from Aqueous Solutions. J. Phys. Chem. C 2012, 116, 8601–8607. [Google Scholar] [CrossRef]
- Dias, E.M.; Petit, C. Towards the use of metal–organic frameworks for water reuse: A review of the recent advances in the field of organic pollutants removal and degradation and the next steps in the field. J. Mater. Chem. A 2015, 3, 22484–22506. [Google Scholar] [CrossRef]
- Molavi, H.; Hakimian, A.; Shojaei, A.; Raeiszadeh, M. Selective dye adsorption by highly water stable metal-organic framework: Long term stability analysis in aqueous media. Appl. Surf. Sci. 2018, 445, 424–436. [Google Scholar] [CrossRef]
- Mohammadi, A.A.; Alinejad, A.; Kamarehie, B.; Javan, S.; Ghaderpoury, A.; Ahmadpour, M.; Ghaderpoori, M. Metal-organic framework Uio-66 for adsorption of methylene blue dye from aqueous solutions. Int. J. Environ. Sci. Technol. 2017, 439, 18–1968. [Google Scholar] [CrossRef]
- Chen, Q.; He, Q.; Lv, M.; Xu, Y.; Yang, H.; Liu, X.; Wei, F. Selective adsorption of cationic dyes by UiO-66-NH2. Appl. Surf. Sci. 2015, 327, 77–85. [Google Scholar] [CrossRef]
- Yang, J.-M. A facile approach to fabricate an immobilized-phosphate zirconium-based metal-organic framework composite (UiO-66-P) and its activity in the adsorption and separation of organic dyes. J. Colloid Interface Sci. 2017, 505, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-M.; Ying, R.-J.; Han, C.-X.; Hu, Q.-T.; Xu, H.-M.; Li, J.-H.; Wang, Q.; Zhang, W. Adsorptive removal of organic dyes from aqueous solution by a Zr-based metal-organic framework: Effects of Ce(iii) doping. Dalton Trans. 2018, 47, 3913–3920. [Google Scholar] [CrossRef]
- Mahdavi, H.; Ahmadian-Alam, L.; Molavi, H.; Ahmadian-Alam, L. Grafting of sulfonated monomer onto an amino-silane functionalized 2-aminoterephthalate metal—Organic framework via surface-initiated redox polymerization: Proton-conducting solid electrolytes. Polym. Int. 2015, 64, 1578–1584. [Google Scholar] [CrossRef]
- Aghajanzadeh, M.; Zamani, M.; Molavi, H.; Manjili, H.K.; Danafar, H.; Shojaei, A. Preparation of Metal–Organic Frameworks UiO-66 for Adsorptive Removal of Methotrexate from Aqueous Solution. J. Inorg. Organomet. Polym. Mater. 2017, 28, 177–186. [Google Scholar] [CrossRef]
- Molavi, H.; Zamani, M.; Aghajanzadeh, M.; Manjili, H.K.; Danafar, H.; Shojaei, A. Evaluation of UiO-66 metal organic framework as an effective sorbent for Curcumin’s overdose. Appl. Organomet. Chem. 2018, 32, e4221. [Google Scholar] [CrossRef]
- Fisal, A.; Daud, W.M.A.W.; Ahmad, M.A.; Radzi, R. Using cocoa (Theobroma cacao) shell-based activated carbon to remove 4-nitrophenol from aqueous solution: Kinetics and equilibrium studies. Chem. Eng. J. 2011, 178, 461–467. [Google Scholar] [CrossRef]
- Darwish, A.; Rashad, M.; Al-Aoh, H.A. Methyl orange adsorption comparison on nanoparticles: Isotherm, kinetics, and thermodynamic studies. Dye. Pigment. 2019, 160, 563–571. [Google Scholar] [CrossRef]
- Lafi, R.; Hafiane, A. Removal of methyl orange (MO) from aqueous solution using cationic surfactants modified coffee waste (MCWs). J. Taiwan Inst. Chem. Eng. 2016, 58, 424–433. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, Y.; Wang, J.; Liu, F.; Hu, N.; Pei, H.; Yang, W.; Li, Z.; Suo, Y.; Wang, J. High effective adsorption/removal of illegal food dyes from contaminated aqueous solution by Zr-MOFs (UiO-67). Food Chem. 2018, 254, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Jian, M.; Liu, B.; Zhang, G.; Liu, R.; Zhang, X. Adsorptive removal of arsenic from aqueous solution by zeolitic imidazolate framework-8 (ZIF-8) nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2015, 465, 67–76. [Google Scholar] [CrossRef]
- Lin, S.; Song, Z.; Che, G.; Ren, A.; Li, P.; Liu, C.; Zhang, J. Adsorption behavior of metal–organic frameworks for methylene blue from aqueous solution. Microporous Mesoporous Mater. 2014, 193, 27–34. [Google Scholar] [CrossRef]
- Qiu, T.; Zeng, Y.; Ye, C.; Tian, H. Adsorption Thermodynamics and Kinetics of p-Xylene on Activated Carbon. J. Chem. Eng. Data 2012, 57, 1551–1556. [Google Scholar] [CrossRef]
- Kuo, C.-Y.; Wu, C.-H.; Wu, J.-Y. Adsorption of direct dyes from aqueous solutions by carbon nanotubes: Determination of equilibrium, kinetics and thermodynamics parameters. J. Colloid Interface Sci. 2008, 327, 308–315. [Google Scholar] [CrossRef]
- Zhu, X.; Li, B.; Yang, J.; Li, Y.; Zhao, W.; Shi, J.; Gu, J. Effective adsorption and enhanced removal of organophosphorus pesticides from aqueous solution by Zr-based MOFs of UiO-67. ACS Appl. Mater. Interfaces 2015, 7, 223–231. [Google Scholar] [CrossRef]
- Cavka, J.H.; Jakobsen, S.; Olsbye, U.; Guillou, N.; Lamberti, C.; Bordiga, S.; Lillerud, K.P. A New Zirconium Inorganic Building Brick Forming Metal Organic Frameworks with Exceptional Stability. J. Am. Chem. Soc. 2008, 130, 13850–13851. [Google Scholar] [CrossRef]
- Ai, L.; Zhang, C.; Liao, F.; Wang, Y.; Li, M.; Meng, L.; Jiang, J. Removal of methylene blue from aqueous solution with magnetite loaded multi-wall carbon nanotube: Kinetic, isotherm and mechanism analysis. J. Hazard. Mater. 2011, 198, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Yang, Y.; Mai, J.; Sun, W.; Zhang, C.; Wei, D.; Chen, Q.; Ni, J. Adsorption behavior of methylene blue onto titanate nanotubes. Chem. Eng. J. 2010, 156, 313–320. [Google Scholar] [CrossRef]
- Wei, C.; Feng, D.; Xia, Y. Fast adsorption and removal of 2-methyl-4-chlorophenoxy acetic acid from aqueous solution with amine functionalized zirconium metal–organic framework. RSC Adv. 2016, 6, 96339–96346. [Google Scholar] [CrossRef]
- Wu, S.; Ge, Y.; Wang, Y.; Chen, X.; Li, F.; Xuan, H.; Li, X. Adsorption of Cr(VI) on nano Uio-66-NH2 MOFs in water. Environ. Technol. 2018, 39, 1937–1948. [Google Scholar] [CrossRef] [PubMed]
- Chizallet, C.; Lazare, S.; Bazer-Bachi, D.; Bonnier, F.; Lecocq, V.; Soyer, E.; Quoineaud, A.-A.; Bats, N. Catalysis of Transesterification by a Nonfunctionalized Metal−Organic Framework: Acido-Basicity at the External Surface of ZIF-8 Probed by FTIR and ab Initio Calculations. J. Am. Chem. Soc. 2010, 132, 12365–12377. [Google Scholar] [CrossRef]
- Fu, J.; Chen, Z.; Wang, M.; Liu, S.; Zhang, J.; Zhang, J.; Han, R.; Xu, Q. Adsorption of methylene blue by a high-efficiency adsorbent (polydopamine microspheres): Kinetics, isotherm, thermodynamics and mechanism analysis. Chem. Eng. J. 2015, 259, 53–61. [Google Scholar] [CrossRef]




| Sample | SBET (m2/g) | Smicro (m2/g) | Vmicro (cm3/g) | Pore Width (nm) |
|---|---|---|---|---|
| ZIF-8 | 1938.859 | 1841.897 | 0.646 | 0.524 |
| UiO-66-NH2 | 897.779 | 801.012 | 0.308 | 1.232 |
| 1-ZIF-8@UiO-66-NH2 | 651.895 | 596.664 | 0.233 | 0.548 |
| Adsorbent | Langmuir Isotherm | Freundlich Isotherm | ||||||
|---|---|---|---|---|---|---|---|---|
| qmax | KL | RL | R2 | KF | 1/n | n | R2 | |
| 1-ZIF@UiO | 173.01 | 1 | 0.0175 | 0.999 | 83.89 | 0.2297 | 4.35 | 0.937 |
| UiO-66-NH2 | 55.04 | 0.79 | 0.0324 | 0.999 | 34.50 | 0.1297 | 7.71 | 0.972 |
| Adsorbents | qmax (mg/g) | References |
|---|---|---|
| UiO-66 | 69.8 | [33] |
| UiO-66 | 90 | [34] |
| UiO-66-NH2 | 90.88 | [35] |
| Acid- promoted UiO-66 | 13.0 | [29] |
| UiO-66-P composite | 91.1 | [36] |
| Ce(III)-doped UiO-66 | 145.1 | [37] |
| UiO-66-NH2 | 55 | Our work |
| ZIF-8-loaded UiO-66-NH2 | 173 | Our work |
| Adsorbent | C0 | qe | Pseudo-First-Order Kinetic | Pseudo-Second-Order Kinetic | |||||
|---|---|---|---|---|---|---|---|---|---|
| qe | K1 | R2 | qe | K2 | R2 | Rate | |||
| 1-ZIF@UiO | 10 | 90 | 33.64 | 0.0066 | 0.727 | 92.25 | 0.00050 | 0.999 | 0.046 |
| 20 | 132 | 50.68 | 0.0099 | 0.897 | 135.32 | 0.00039 | 0.999 | 0.053 | |
| 30 | 160 | 62.90 | 0.0076 | 0.866 | 164.47 | 0.00030 | 0.999 | 0.049 | |
| UiO-66-NH2 | 5 | 36 | 21.48 | 0.0053 | 0.984 | 36.44 | 0.00068 | 0.995 | 0.025 |
| 10 | 43 | 21.03 | 0.0079 | 0.937 | 44.74 | 0.00083 | 0.999 | 0.037 | |
| 20 | 50 | 39.23 | 0.0129 | 0.941 | 53.22 | 0.00062 | 0.999 | 0.033 | |
| Adsorbent | C (mg/L) | Intra-Particle Diffusion Kinetic Model | |||||
|---|---|---|---|---|---|---|---|
| K1 | C1 | R2 | K2 | C2 | R2 | ||
| 1-ZIF@UiO | 10 | 8.27 | 1.16 | 0.983 | 0.47 | 77.48 | 0.913 |
| 20 | 12.91 | 2.54 | 0.959 | 0.79 | 112.42 | 0.961 | |
| 30 | 14.17 | 11.27 | 0.972 | 1.28 | 130.13 | 0.950 | |
| UiO-66-NH2 | 5 | 1.53 | 7.97 | 0.985 | 0.34 | 26.40 | 0.982 |
| 10 | 2.52 | 8.29 | 0.981 | 0.26 | 36.58 | 0.995 | |
| 20 | 2.81 | 10.48 | 0.982 | 0.26 | 44.31 | 0.964 | |
| Adsorbent | CONCENTRATION (mg/L) | ΔH° (kJ/mol) | ΔS° (J/mol K) | ΔG° (kJ/mol) | |||
|---|---|---|---|---|---|---|---|
| 298 K | 308 K | 318 K | R2 | ||||
| 1−ZIF@UiO | 10 | −18.46 | −27.89 | −10.15 | −9.87 | −9.59 | 0.977 |
| 20 | −9.83 | −7.18 | −7.69 | −7.62 | −7.55 | 0.970 | |
| 30 | −9.11 | −10.29 | −6.05 | −5.95 | −5.84 | 0.996 | |
| UiO−66−NH2 | 5 | −21.31 | −44.93 | −7.92 | −7.47 | −7.02 | 0.850 |
| 10 | −20.11 | −50.84 | −4.96 | −4.46 | −3.95 | 0.936 | |
| 20 | −21.29 | −60.86 | −3.15 | −2.54 | −1.93 | 0.963 | |
| Adsorbate | Molecular Structure | Size (nm) |
|---|---|---|
| Methylene blue (MB+) | 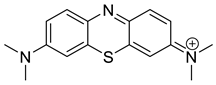 | 1.38 × 0.64 |
| Methyl orange (MO−) | 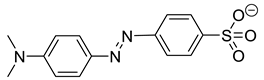 | 1.54 × 0.48 |
| Rhodamine B (RhB+) | 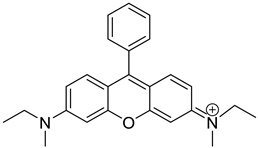 | 1.41 × 0.98 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Shi, X.; Li, J.; Kumar, P.; Liu, B. Selective Dye Adsorption by Zeolitic Imidazolate Framework-8 Loaded UiO-66-NH2. Nanomaterials 2019, 9, 1283. https://doi.org/10.3390/nano9091283
Zhang H, Shi X, Li J, Kumar P, Liu B. Selective Dye Adsorption by Zeolitic Imidazolate Framework-8 Loaded UiO-66-NH2. Nanomaterials. 2019; 9(9):1283. https://doi.org/10.3390/nano9091283
Chicago/Turabian StyleZhang, Hao, Xiaobo Shi, Jialiang Li, Parveen Kumar, and Bo Liu. 2019. "Selective Dye Adsorption by Zeolitic Imidazolate Framework-8 Loaded UiO-66-NH2" Nanomaterials 9, no. 9: 1283. https://doi.org/10.3390/nano9091283





