New Insight on Hydrogen Evolution Reaction Activity of MoP2 from Theoretical Perspective
Abstract
:1. Introduction
2. Computational Methods
3. Results and Discussion
3.1. Electronic Structures of Bulk
3.2. Hydrogen Adsorption
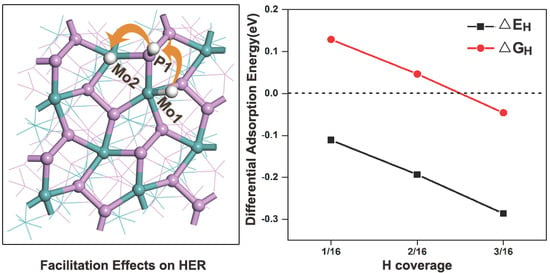
3.3. Doping on the (111) Surface
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cheng, X.; Shi, Z.; Glass, N.; Zhang, L.; Zhang, J.; Song, D.; Liu, Z.-S.; Wang, H.; Shen, J. A review of PEM hydrogen fuel cell contamination: Impacts, mechanisms, and mitigation. J. Power Sources 2007, 165, 739–756. [Google Scholar] [CrossRef]
- Cao, X.H.; Tan, C.L.; Sindoro, M.; Zhang, H. Hybrid micro-/nano-structures derived from metal-organic frameworks: Preparation and application in energy storage and convension. Chem. Soc. Rev. 2017, 46, 2660–2677. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Cui, W.; Liu, Q.; Xing, Z.C. Recent Progress in Cobalt-Based Heterogeneous Catalysts for Electrochemical Water Splitting. Adv. Mater. 2016, 28, 215–230. [Google Scholar] [CrossRef] [PubMed]
- Li, P.K.; Zhu, J.G.; Handoko, A.D.; Zhang, R.F.; Wang, H.T.; Legut, D.; Wen, X.D.; Fu, Z.; Shen, Z.; Zhang, Q. High-throughput theoretical optimization of hydrogen evolution reaction on MXens by transition mental modification. J. Mater. Chem. A 2018, 6, 4271–4278. [Google Scholar] [CrossRef]
- Kronberg, R.; Hakala, M.; Holmberg, N.; Laasonen, K. Hydrogen adsorption on MoS2 -surfaces: A DFT study on preferential sites and the effect of sulfur and hydrogen coverage. Phys. Chem. Chem. Phys. 2017, 19, 16231–16241. [Google Scholar] [CrossRef] [PubMed]
- Walter, M.G.; Warren, E.L.; McKone, J.R.; Boettcher, S.W.; Mi, Q.; Santori, E.A.; Lewis, N.S. Solar water splitting cells. Chem. Rev. 2010, 110, 6446–6473. [Google Scholar] [CrossRef]
- Chen, L.; Wang, M.; Han, K.; Zhang, P.; Gloaguen, F.; Sun, L. A super-efficient cobalt catalyst for electrochemical hydrogen production from neutral water with 80 mV overpotential. Energy Environ. Sci. 2014, 7, 329–334. [Google Scholar] [CrossRef]
- Abbas, M.A.; Bang, J.H. Rising Again: Opportunities and Challenges for Platinum-Free Electrocatalysts. Chem. Mater. 2015, 27, 7218–7235. [Google Scholar] [CrossRef]
- Islam, M.M.; Calatayud, M.; Pacchioni, G. Hydrogen Adsorption and Diffusion on the Anatase TiO2(101) Surface: A First-Principles Investigation. J. Phys. Chem. C 2011, 115, 6809–6814. [Google Scholar] [CrossRef]
- González-Navarrete, P.; Monica, C. On the reductive hydrogenation process of gas-phase metal dioxides: H2 activation or reduction of the metal center, what is more important? Theor. Chem. Acc. 2019, 138, 98. [Google Scholar] [CrossRef]
- Chen, W.-F.; Muckerman, J.T.; Fujita, E. ChemInform Abstract: Recent Developments in Transition Metal Carbides and Nitrides as Hydrogen Evolution Electrocatalysts. Chemin- 2013, 44, 8896–8909. [Google Scholar] [CrossRef]
- Kong, D.; Wang, H.; Lu, Z.; Cui, Y. CoSe2 Nanoparticles Grown on Carbon Fiber Paper: An Efficient and Stable Electrocatalyst for Hydrogen Evolution Reaction. J. Am. Chem. Soc. 2014, 136, 4897–4900. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo, T.F.; Jørgensen, K.P.; Bonde, J.; Nielsen, J.H.; Horch, S.; Chorkendroff, I. Identification of active edge sites for electrochemical H2 evolution from MoS2 nanocatalysts. Science 2017, 317, 100–102. [Google Scholar] [CrossRef] [PubMed]
- Xiao, P.; Alam Sk, M.; Thia, L.; Ge, X.; Lim, R.J.; Wang, J.-Y.; Lim, K.H.; Wang, X. Molybdenum phosphide as an efficient electrocatalyst for the hydrogen evolution reaction. Energy Environ. Sci. 2014, 7, 2624–2629. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.F.; Sasaki, K.; Ma, C.; Frenkel, A.I.; Marinkovic, N.; Muckerman, J.T.; Zhu, Y.; Adzic, R.R. Hydrogen-Evolution Catalysts Based on Non-Noble Metal Nickel-Molybdenum Nitride Nanosheets. Angew. Chem. 2012, 124, 6235–6239. [Google Scholar] [CrossRef]
- Xiao, P.; Chen, W.; Wang, X. A Review of Phosphide-Based Materials for Electrocatalytic Hydrogen Evolution. Adv. Energy Mater. 2015, 5, 1500985. [Google Scholar] [CrossRef]
- Oyama, S.T.; Gott, T.; Zhou, H.Y.; Lee, Y.K. Transition metal phosphide hydroprocessing catalysts: A review. Calay. Today 2003, 143, 94–107. [Google Scholar] [CrossRef]
- McEnaney, J.M.; Crompton, J.C.; Callejas, J.F.; Popczun, E.J.; Read, C.G.; Lewis, N.S.; Schaak, R.E. Electrocatalytic hydrogen evolution using amorphous tungsten phosphide nanoparticles. Chem. Commun. 2014, 50, 11026–11028. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Pu, Z.; Tu, Z.; Amiinu, I.S.; Liu, S.; Wang, P.; Mu, S. Integrated design and construction of WP/W nanorod array electrodes toward efficient hydrogen evolution reaction. Chem. Eng. J. 2017, 327, 705–712. [Google Scholar] [CrossRef]
- Jiang, P.; Liu, Q.; Liang, Y.; Tian, J.; Asiri, A.M.; Sun, X. A Cost-Effective 3D Hydrogen Evolution Cathode with High Catalytic Activity: FeP Nanowire Array as the Active Phase. Angew. Chem. 2014, 126, 13069–13073. [Google Scholar] [CrossRef]
- Liang, Y.; Liu, Q.; Asiri, A.M.; Sun, X.; Luo, Y. Self-Supported FeP Nanorod Arrays: A Cost-Effective 3D Hydrogen Evolution Cathode with High Catalytic Activity. ACS Catal. 2014, 4, 4065–4069. [Google Scholar] [CrossRef]
- Zhou, Z.; Wei, L.; Wang, Y.; Karahan, H.E.; Chen, Z.; Lei, Y.; Chen, X.; Zhai, S.; Liao, X.; Chen, Y. Hydrogen evolution reaction activity of nickel phosphide is highly sensitive to electrolyte pH. J. Mater. Chem. A 2017, 5, 20390–20397. [Google Scholar] [CrossRef]
- Chen, X.; Wang, D.; Wang, Z.; Zhou, P.; Wu, Z.; Jiang, F. Molybdenum phosphide: A new highly efficient catalyst for the electrochemical hydrogen evolution reaction. Chem. Commun. 2014, 50, 11683–11685. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.N.; Li, S.H.; Tan, H.Q.; Khan, S.U.; Ma, Y.Y.; Zang, H.Y.; Wang, Y.H.; Li, Y.G. MoP/Mo2C@C: A New Combination of Electrocatalysts for Highly Efficient Hydrogen Evolution over the Entire pH Range. ACS Appl. Mater. Interfaces 2017, 9, 16270–16279. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.Y.; Wu, C.X.; Feng, X.J.; Tan, H.Q.; Yan, L.K.; Liu, Y.; Kang, Z.H.; Wang, E.B.; Li, Y.G. Highly efficient hydrogen evolution from seawater by a low-cost and stable CoMoPC electrocatalyst superior to Pt/C. Energy Environ. Sci. 2017, 10, 788–798. [Google Scholar] [CrossRef]
- Li, D.; Baydoun, H.; Verani, C.N.; Brock, S.L. Efficient Water Oxidation Using CoMnP Nanoparticles. J. Am. Chem. Soc. 2016, 138, 4006–4009. [Google Scholar] [CrossRef] [PubMed]
- Xing, Z.; Liu, Q.; Asiri, A.M.; Sun, X. Closely Interconnected Network of Molybdenum Phosphide Nanoparticles: A Highly Efficient Electrocatalyst for Generating Hydrogen from Water. Adv. Mater. 2014, 26, 5702–5707. [Google Scholar] [CrossRef]
- Wu, T.L.; Pi, M.Y.; Zhang, D.K.; Chen, S.J. Three-dimensional porous structural MoP2 nanoparticles as a novel and superior catalyst for electrochemical hydrogen evolution. J. Power Sources 2016, 328, 551–557. [Google Scholar] [CrossRef]
- Sheng, M.; Yano, J.; You, B.; Jiang, N.; Gul, S.; Sun, Y. High-Performance Overall Water Splitting Electrocatalysts Derived from Cobalt-Based Metal–Organic Frameworks. Chem. Mater. 2015, 27, 7636–7642. [Google Scholar]
- Du, H.; Gu, S.; Liu, R.; Li, C.M. Tungsten diphosphide nanorods as an efficient catalyst for electrochemical hydrogen evolution. J. Power Sources 2015, 278, 540–545. [Google Scholar] [CrossRef]
- Xu, Y.; Wu, R.; Zhang, J.; Shi, Y.; Zhang, B. Anion-exchange synthesis of nanoporous FeP nanosheets as electrocatalysts for hydrogen evolution reaction. Chem. Commun. 2013, 49, 6656–6658. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.L.; Chen, S.J.; Zhang, D.K.; Hou, J.K. Facile preparation of semimetallic MoP2 as a novel visible light driven photocatalyst with high photocatalytic activity. J. Mater. Chem. A 2015, 3, 0360–10367. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, B. Recent advances in transition metal phosphide nanomaterials: Synthesis and applications in hydrogen evolution reaction. Chem. Soc. Rev. 2016, 45, 1529–1541. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Sun, Y.; Xu, N.; Manna, K.; Yao, M.; Süss, V.; Leermakers, I.; Young, O.; Forster, T.; Schmidt, M.; et al. Extremely high magnetoresistance and conductivity in the type-II Weyl semimetals WP2 and MoP2. Nat. Commun. 2017, 8, 1642. [Google Scholar] [CrossRef] [PubMed]
- Pu, Z.; Saana, A.I.; Wang, M.; Yang, Y.; Mu, S. Semimetallic MoP2: An active and stable hydrogen evolution electrocatalyst over the whole pH range. Nanoscale 2016, 8, 8500–8504. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhu, W.; Tang, C.; Wang, J.; Asiri, A.M.; Sun, X. A self-standing nanoporous MoP 2 nanosheet array: An advanced pH-universal catalytic electrode for the hydrogen evolution reaction. J. Mater. Chem. A 2016, 4, 7169–7173. [Google Scholar]
- Gao, Y.; Zhang, M.; Ding, J.; Hong, S.; Masa, J.; Liu, S.; Sun, Z. Simple synthesis of two-dimensional MoP2 nanosheets for efficient electrocatalytic hydrogen evolution. Electrochem. Commun. 2018, 97, 27–31. [Google Scholar] [CrossRef]
- Owens-Baird, B.; Kolen’ko, Y.V.; Kovnir, K. Structure-Activity Relationships for Pt-Free Metal Phosphide Hydrogen Evolution Electrocatalysts. Chem. Eur. J. 2018, 24, 7298–7311. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 1993, 47, 558–561. [Google Scholar] [CrossRef]
- Nørskov, J.K.; Bligaard, T.; Logadottir, A. Trends in the Exchange Current for Hydrogen Evolution. J. Electrochem. Soc. 2015, 152, J23–J26. [Google Scholar] [CrossRef]
- Tang, W.; Sanville, E.; Henkelman, G. A grid-based Bader analysis algorithm without lattice bias. J. Phys. Condens. Matter 2009, 21, 084204. [Google Scholar] [CrossRef] [PubMed]
- Mo, L.B.; Wang, Y.; Bai, Y.; Xiang, Q.Y.; Li, Q.; Yao, W.Q.; Wang, J.O.; Ibrahim, K.; Wang, H.H.; Wan, C.H.; et al. Hydrogen Impurity Defects in Rutile TiO2. Sci. Rep. 2015, 5, 17634. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Jiang, D.E. Mechanism of Hydrogen Evolution Reaction on 1T-MoS2 from First Principles. ACS Catal. 2016, 6, 4953–4961. [Google Scholar] [CrossRef]
- An, Y.R.; Fan, X.L.; Liu, H.J.; Luo, Z.F. Improved catalytic performance of monolayer nano-triangles WS2 and MoS2 on HER by 3d metals doping. Comp. Mater. Sci. 2019, 159, 333–340. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Perdew, burke, and ernzerhof reply. Phys. Rev. Lett. 1998, 80, 891. [Google Scholar] [CrossRef]
- Hafner, J.; Kresse, G. The Vienna AB-Initio Simulation Program VASP: An Efficient and Versatile Tool for Studying the Structural, Dynamic, and Electronic Properties of Materials. In Properties of Complex Inorganic Solids; Springer: Boston, MA, USA, 1997; pp. 69–82. [Google Scholar]
- Huber, K.P.; Herzberg, G. Molecular spectra and molecular structure: IV constants of diatomic molecules; Van Rostrand-Reinhold: New York, NY, USA, 1997. [Google Scholar]
- Rogal, J.; Reuter, K. Ab Initio Atomistic Thermodynamics for Surfaces: A Primer; Max-planck-gesellschaft zur foerderung der wissenschaften ev berlin (germany fr) fritz-haber-inst: Berlin, Germany, 2006. [Google Scholar]
- Rundqvist, S.; Lundström, T. X-ray Studies of Molybdenum and Tungsten Phosphides. Acta Chem. Scand. 1963, 17, 37–46. [Google Scholar] [CrossRef]
- Winkler, B.; Knorr, K.; Hytha, M.; Milman, V.; Soto, V.; Avalos, M.; Avalos-Borja, M. Crystal chemistry of molybdenum phosphides from density functional theory calculations. J. Phys. Chem. Solids 2003, 64, 405–411. [Google Scholar] [CrossRef]
- Mou, J.; Gao, Y.; Wang, J.; Ma, J.; Ren, H. Hydrogen evolution reaction activity related to the facet-dependent electrocatalytic performance of NiCoP from first principles. RSC Adv. 2019, 9, 11755–11761. [Google Scholar] [CrossRef] [Green Version]
- Scaranto, J.; Idriss, H. DFT studies of bulk and surfaces of the electrocatalyst cobalt phosphide CoP2. Chem. Phys. Lett. X 2019, 2, 100008. [Google Scholar] [CrossRef]
- Liang, Z.; Zhong, X.L.; Li, T.Q.; Chen, M.; Feng, G. DFT Study on the Hydrogen Evolution Reaction for Different Facets of Co2P. Chem. Electro. Chem. 2019, 6, 260–267. [Google Scholar]
- George, P.P.; Genish, I.; Maklouf, S.B.; Koltypin, Y.; Gedanken, A. A New Approach to the Synthesis of Transition Metal Phosphide Nanocrystallites (MoP, MoP2, Cu3P and CuP2) by Using Reaction under Autogenic Pressure at Elevated Temperatures (RAPET) Technique. Int. J. Nanosci. 2017, 16, 1650030. [Google Scholar] [CrossRef]
- Wang, H.; Tsai, C.; Kong, D.; Chan, K.; Abild-Pedersen, F.; Nørskov, J.K.; Cui, Y. Transition-metal doped edge sites in vertically aligned MoS2 catalysts for enhanced hydrogen evolution. Nano Res. 2015, 8, 566–575. [Google Scholar] [CrossRef]
- Hong, X.; Chan, K.; Tsai, C.; Norskov, J.K. How doped MoS2 breaks transition-metal scaling relations for CO2 electrochemical reduction. ACS Catal. 2016, 6, 4428–4437. [Google Scholar] [CrossRef]
- Dai, X.; Du, K.; Li, Z.; Liu, M.; Ma, Y.; Sun, H.; Zhang, X.; Yang, Y. Co-Doped MoS2 Nanosheets with the Dominant CoMoS Phase Coated on Carbon as an Excellent Electrocatalyst for Hydrogen Evolution. ACS Appl. Mater. Interfaces 2015, 7, 27242–27253. [Google Scholar] [CrossRef] [PubMed]
- Tedstone, A.A.; Lewis, D.J.; O’Brien, P. Synthesis, Properties, and Applications of Transition Metal-Doped Layered Transition Metal Dichalcogenides. Chem. Mater. 2016, 28, 1965–1974. [Google Scholar] [CrossRef]
- Merki, D.; Vrubel, H.; Fierro, S.; Hu, X.; Rovelli, L. Fe, Co, and Ni ions promote the catalytic activity of amorphous molybdenum sulfide films for hydrogen evolution. Chem. Sci. 2012, 3, 2515–2525. [Google Scholar] [CrossRef] [Green Version]
- Kibsgaard, J.; Jaramillo, T.F. Molybdenum Phosphosulfide: An Active, Acid-Stable, Earth-Abundant Catalyst for the Hydrogen Evolution Reaction. Angew. Chem. 2014, 126, 14661–14665. [Google Scholar] [CrossRef]
- Anjum, M.A.R.; Lee, J.S. Sulfur and Nitrogen Dual-Doped Molybdenum Phosphide Nanocrystallites as an Active and Stable Hydrogen Evolution Reaction Electrocatalyst in Acidic and Alkaline Media. ACS Catal. 2017, 7, 3030–3038. [Google Scholar] [CrossRef]







| a (Å) | b (Å) | c (Å) | |
|---|---|---|---|
| Present | 3.147 | 11.242 | 5.009 |
| Calculation a | 3.142 | 11.132 | 4.949 |
| Experiment b | 3.145 a | 11.184 | 4.984 |
| (111) | (110) | (101) | (011) | (100) | (001) | |
|---|---|---|---|---|---|---|
| Surface energy(meVÅ−2) | 104.78 | 134.34 | 134.85 | 146.73 | 147.35 | 173.52 |
| SPD (atom nm−2) | 18 | 17 | 15 | 13 | 11 | 11 |
| Clean facet | 1/16 H atom | 2/16 H atoms | 3/16 H atoms | |
|---|---|---|---|---|
| (Å) | 2.313 | 2.330 | 2.380 | 2.394 |
| (Å) | 2.382 | 2.366 | 2.435 | 2.411 |
| (e) | −0.360 | −0.354 | 0.146 | 0.200 |
| (e) | 0.659 | 0.707 | 0.722 | 0.736 |
| (e) | 0.696 | 0.670 | 0.681 | 0.698 |
| Adsorption Energy | Mo1 site | P1 site | Mo2 site |
|---|---|---|---|
| −0.106 | 0 | 0 | |
| 0 | −0.038 | 0 | |
| 0 | 0 | 0.083 | |
| 1 | −0.194 | 0 | |
| 1 | 1 | −0.286 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Li, H.; Wang, J.; Ma, J.; Ren, H. New Insight on Hydrogen Evolution Reaction Activity of MoP2 from Theoretical Perspective. Nanomaterials 2019, 9, 1270. https://doi.org/10.3390/nano9091270
Gao Y, Li H, Wang J, Ma J, Ren H. New Insight on Hydrogen Evolution Reaction Activity of MoP2 from Theoretical Perspective. Nanomaterials. 2019; 9(9):1270. https://doi.org/10.3390/nano9091270
Chicago/Turabian StyleGao, Yuyue, Hongyan Li, Jingyu Wang, Jianyi Ma, and Haisheng Ren. 2019. "New Insight on Hydrogen Evolution Reaction Activity of MoP2 from Theoretical Perspective" Nanomaterials 9, no. 9: 1270. https://doi.org/10.3390/nano9091270