Fabrication of ZnO@Ag3PO4 Core-Shell Nanocomposite Arrays as Photoanodes and Their Photoelectric Properties
Abstract
:1. Introduction
2. Experiment
2.1. Materials
2.2. Cleaning the Conductive Glass (FTO) Substrate
2.3. Preparation of ZnO Seed Layer
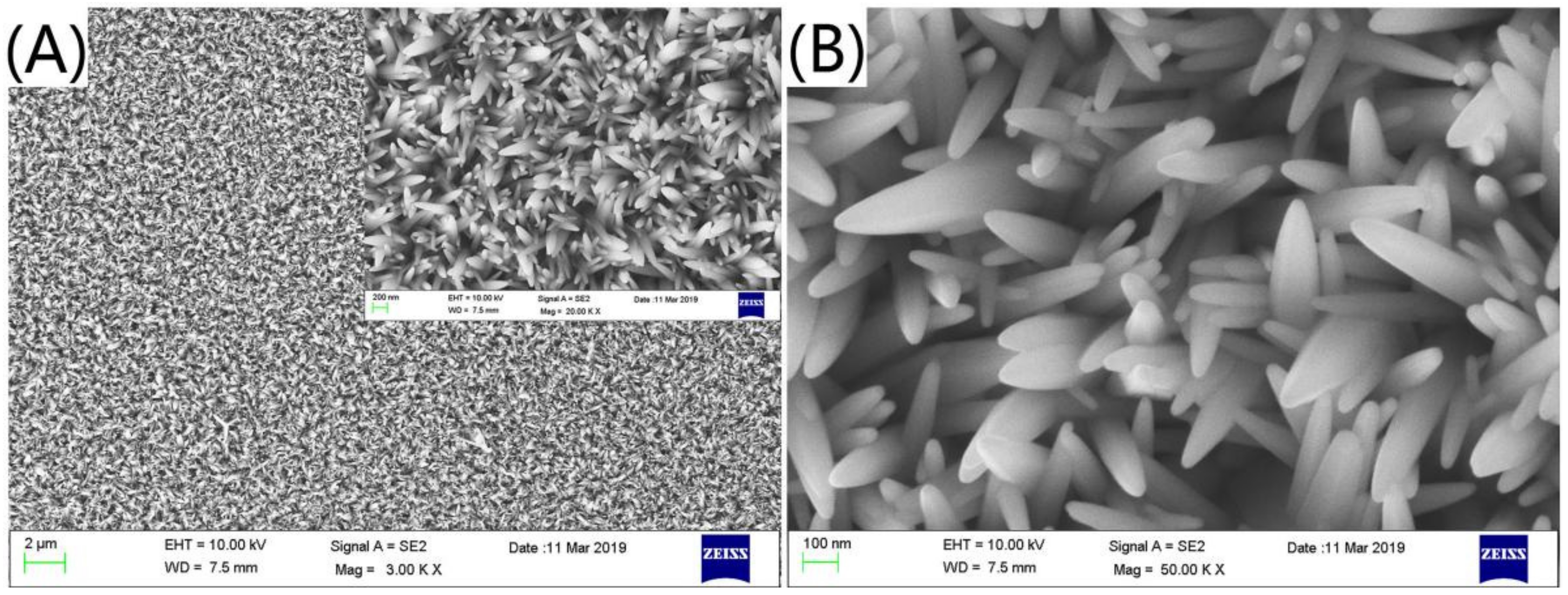
2.4. Preparation of ZnO Nanorods Array
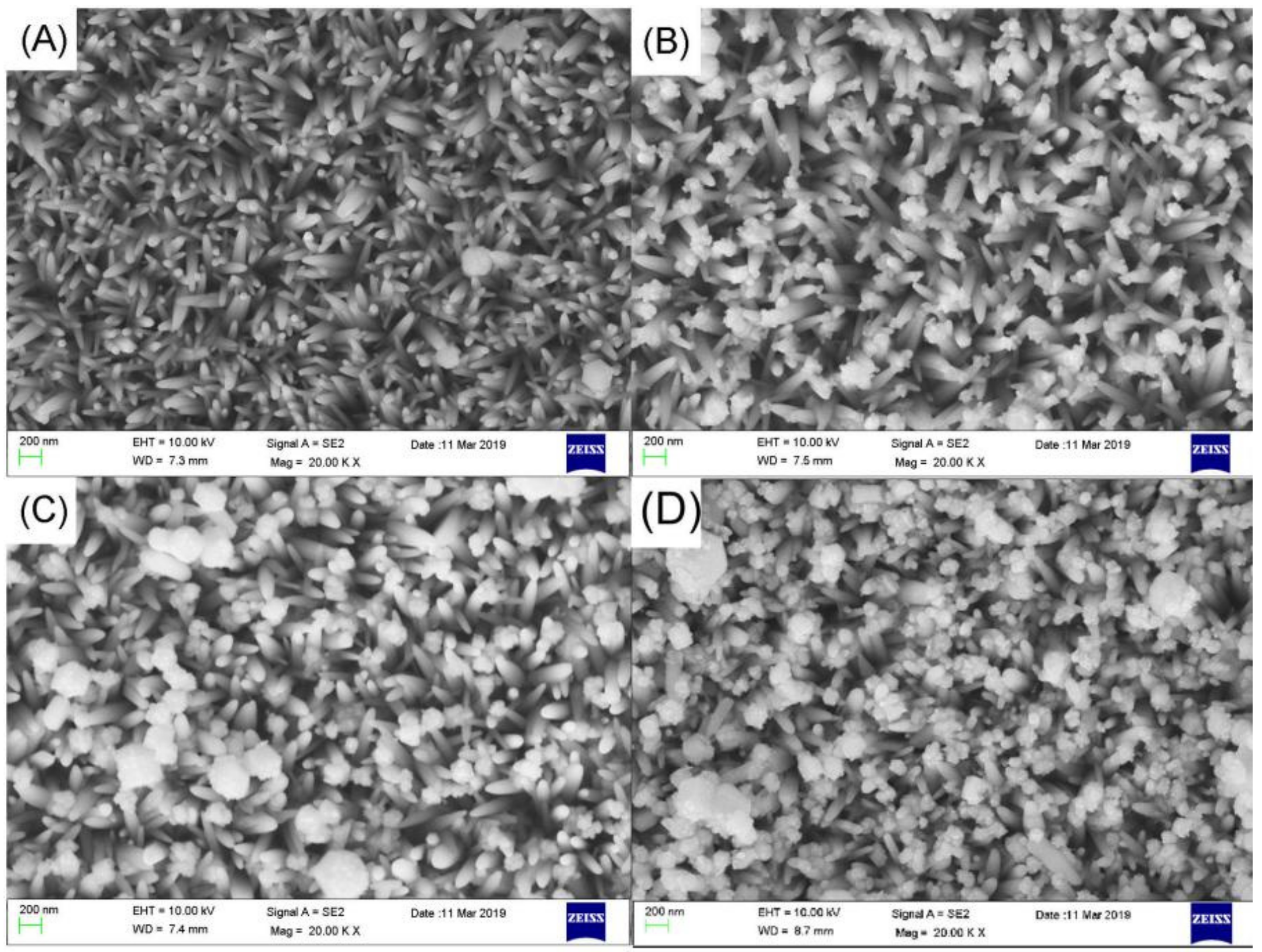
2.5. Preparation of Ag3PO4 by Stepwise Dipping Method
2.6. Annealing Experiment
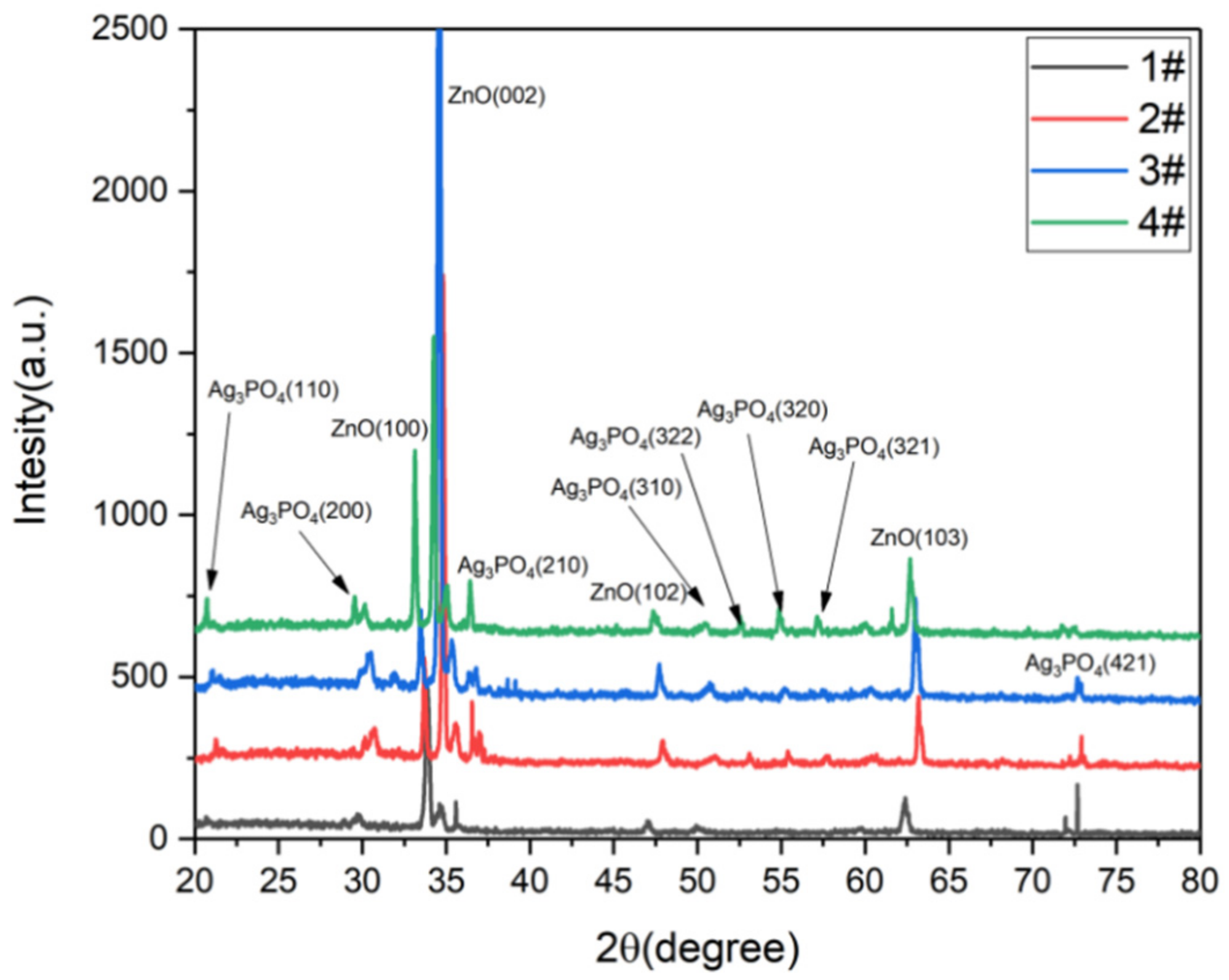
2.7. Characterization
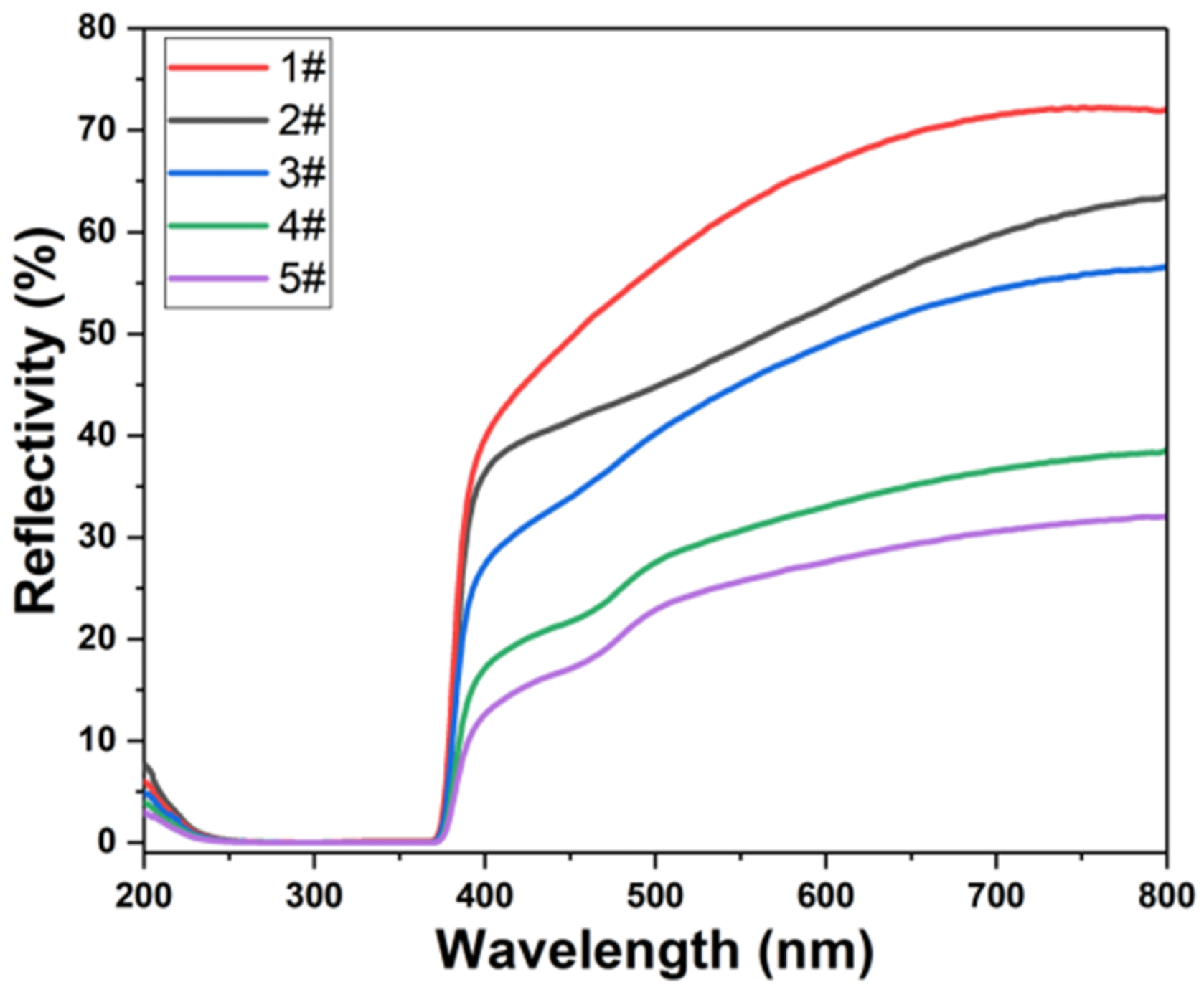
2.8. Optical Performance Test
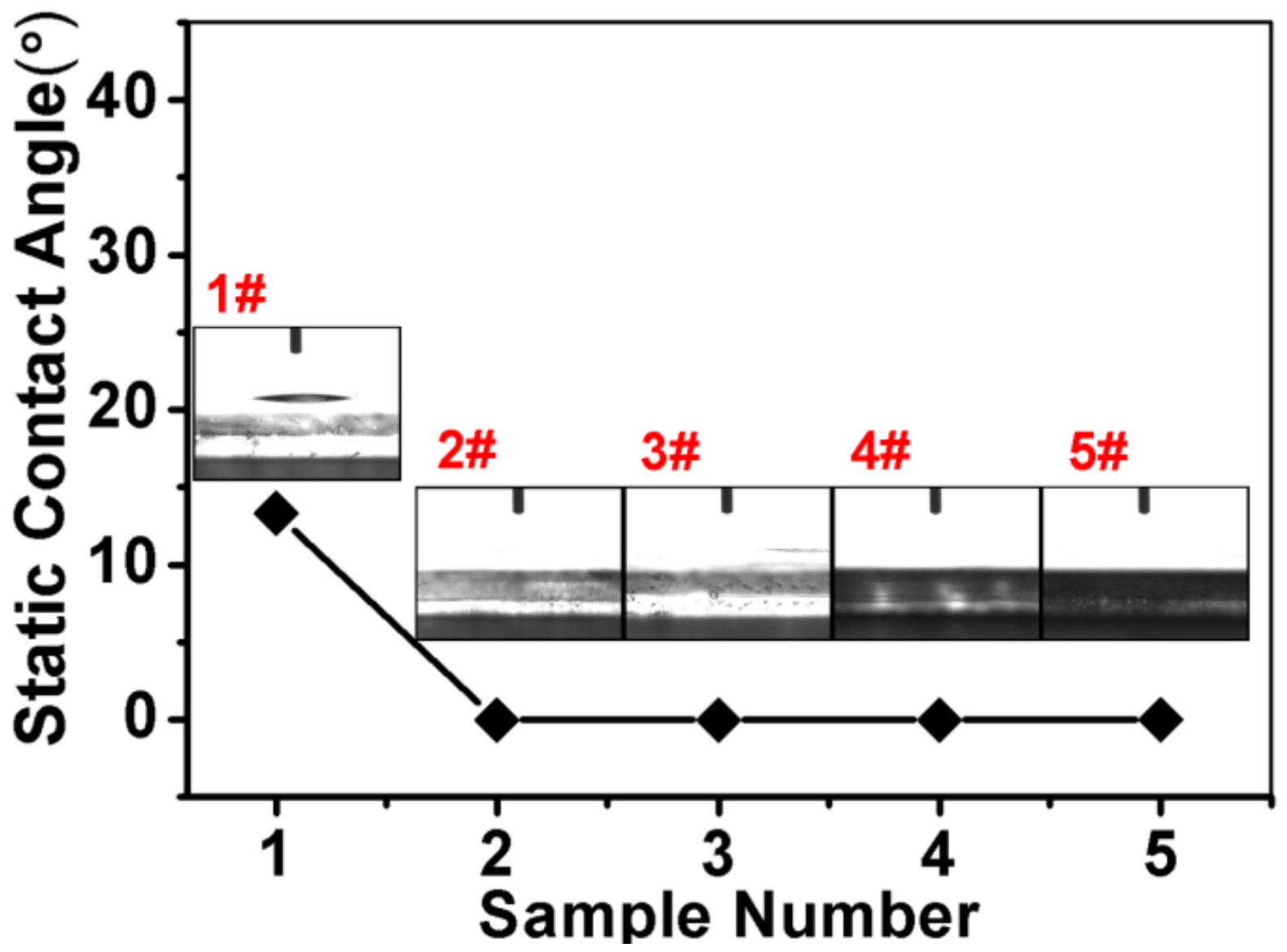
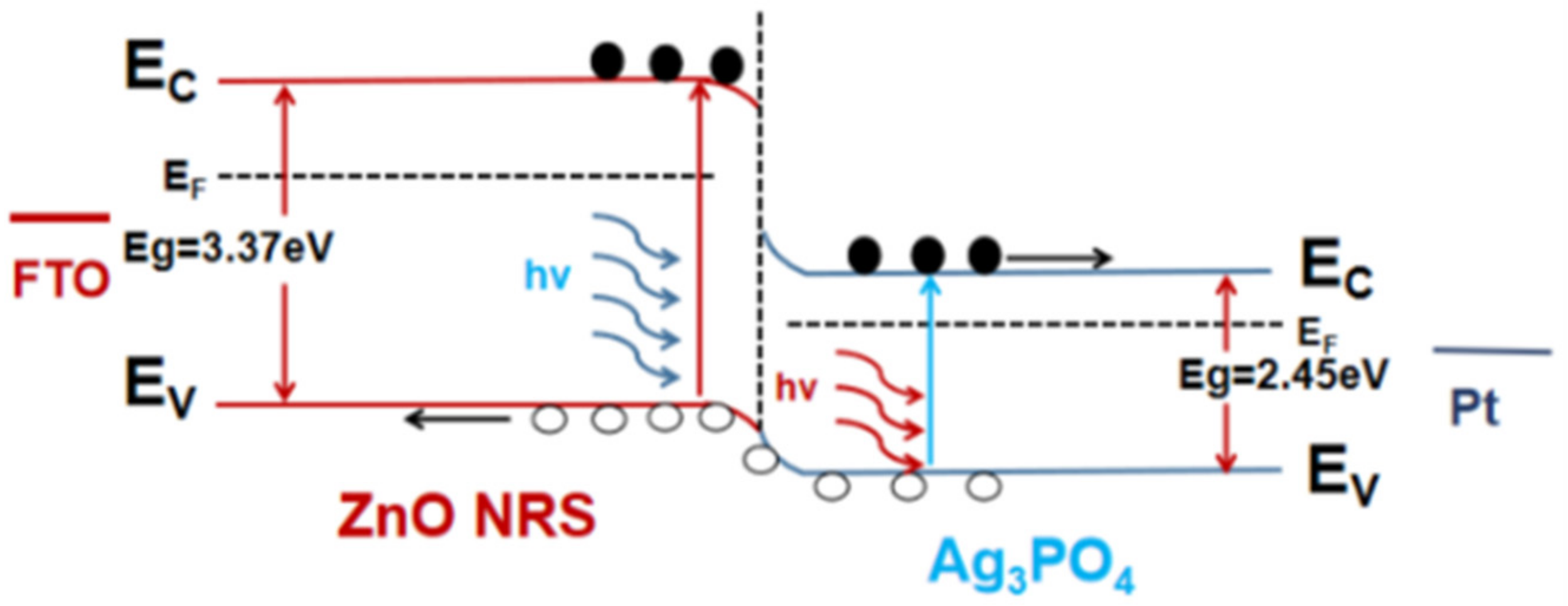
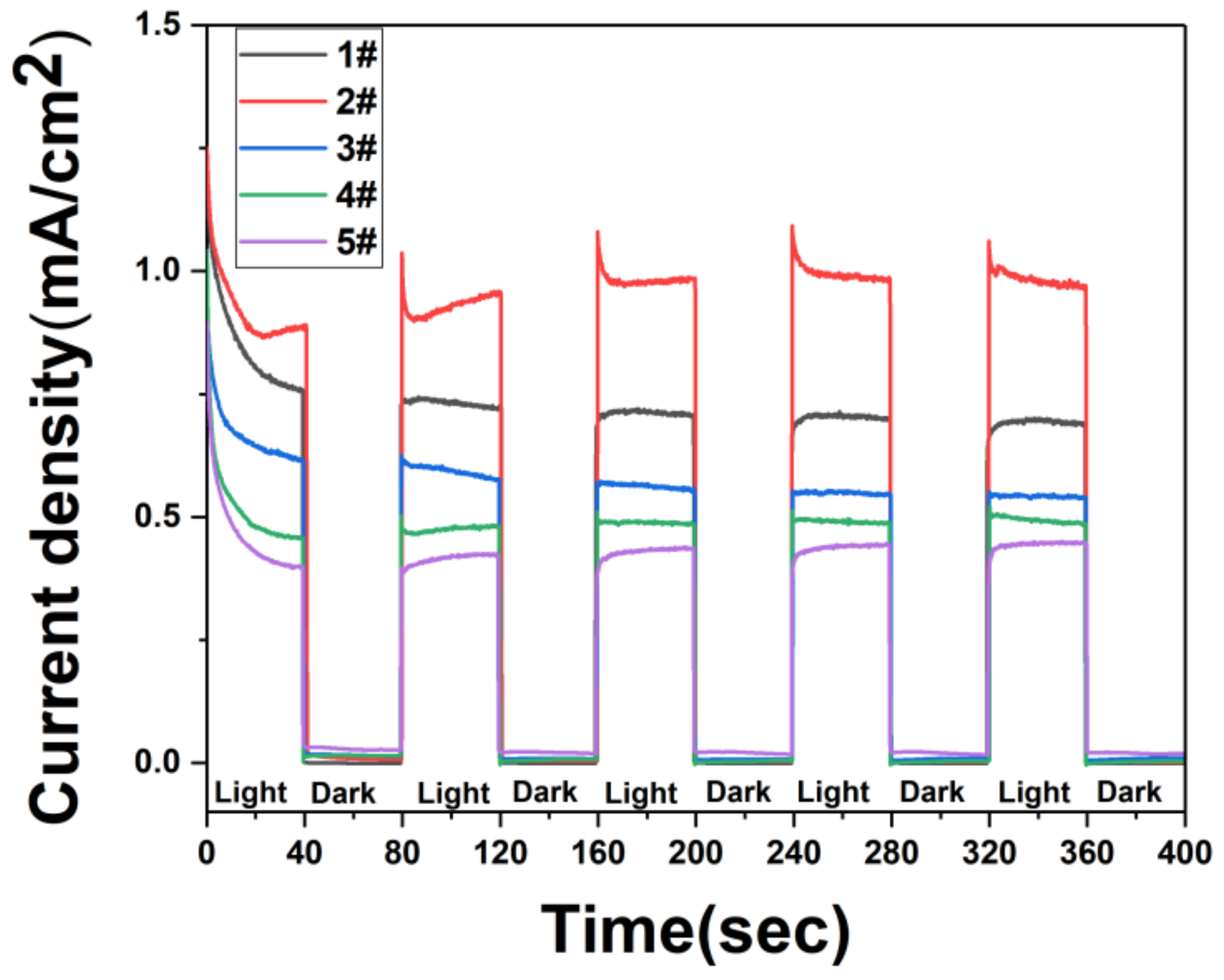
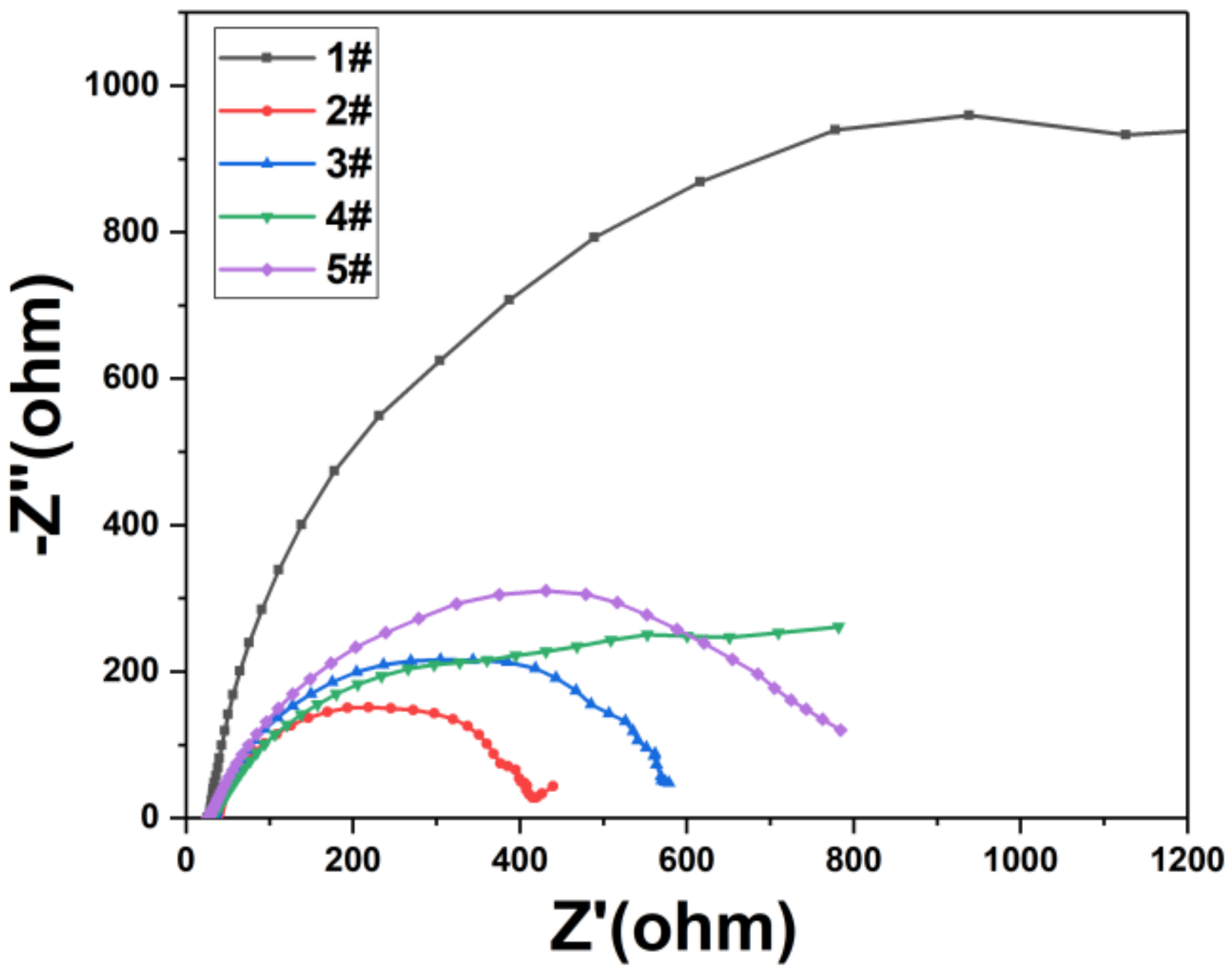
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Farhat, O.F.; Halim, M.M.; Ahmed, N.M.; Oglat, A.A.; Abuelsamen, A.A.; Bououdina, M.; Qaeed, M.A. A study of the effects of aligned vertically growth time on ZnO nanorods deposited for the first time on Teflon substrate. Appl. Surf. Sci. 2017, 426, 906–912. [Google Scholar] [CrossRef]
- Wang, Z.L. Zinc oxide nanostructures: Growth, properties and applications. J. Phys. Condens. Mater. 2004, 16, R829–R858. [Google Scholar] [CrossRef]
- Di, L.J.; Xian, T.; Sun, X.F.; Li, H.Q.; Zhou, Y.J.; Ma, J.; Yang, H. Facile preparation of CNT/Ag2S nanocomposites with improved visible and NIR light photocatalytic degradation activity and their catalytic mechanism. Micromachines 2019, 10, 503. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.X.; Yang, H.; Zhao, X.X.; Zhang, H.M.; Jiang, J.L. A hydrothermal route to the synthesis of CaTiO3 nanocuboids using P25 as the titanium source. J. Electron. Mater. 2018, 47, 3045–3050. [Google Scholar] [CrossRef]
- Wang, S.Y.; Yang, H.; Yi, Z.; Wang, X.X. Enhanced photocatalytic performance by hybridization of Bi2WO6 nanoparticles with honeycomb-like porous carbon skeleton. J. Environ. Manag. 2019, 248, 109341. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.X.; Yang, H.; Cui, Z.M.; Yi, Z.; Yu, H. Synergistically enhanced photocatalytic performance of Bi4Ti3O12 nanosheets by Au and Ag nanoparticles. J. Mater. Sci. Mater. Electron. 2019, 30, 13785–13796. [Google Scholar] [CrossRef]
- Boscarino, S.; Filice, S.; Sciuto, A.; Libertino, S.; Scuderi, M.; Galati, C.; Scalese, S. Investigation of ZnO-decorated CNTs for UV Light Detection Applications. Nanomaterials 2019, 9, 1099. [Google Scholar] [CrossRef]
- Li, H.L.; Wang, G.Y.; Niu, J.B.; Wang, E.L.; Niu, G.; Xie, C.Q. Preparation of TiO2 nanotube arrays with efficient photocatalytic performance and super-hydrophilic properties utilizing anodized voltage method. Results Phys. 2019, 14, 102499. [Google Scholar] [CrossRef]
- Xiong, Z.; Cao, L. High magnetic-dielectric tunability in Ni nanocrystals embedded BaTiO3 films. J. Alloy. Compd. 2019, 785, 200–205. [Google Scholar] [CrossRef]
- Malka, D.; Adler Berke, B.; Tischler, Y.; Zalevsky, Z. Improving Raman spectra of pure silicon using super-resolved method. J. Opt. 2019, 21, 075801. [Google Scholar] [CrossRef]
- Malka, D.; Berkovic, G.; Hammer, Y.; Zalevsky, Z. Super-Resolved Raman Spectroscopy. Spectrosc. Lett. 2013, 46, 307–313. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, Y.; Su, M.; Xu, T.; Yang, H.; Bi, S.; Zhang, X.; Fang, Y.; Zhao, J. Design of Morphology-Controllable ZnO Nanorods/Nanopariticles Composite for Enhanced Performance of Dye-Sensitized Solar Cells. Nanomaterials 2019, 9, 931. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.M.; Li, P.; Huang, J.; Liu, H.D.; Zao, Y.; Hu, Z.L.; Zhang, L.; Chen, H.X.; Wang, M.S.; Peng, D.L.; et al. High performance columnar-like Fe2O3@ carbon composite anode via yolk@shell structural design. J. Energy Chem. 2020, 41, 126–134. [Google Scholar] [CrossRef]
- Wang, X.X.; Zhu, J.K.; Tong, H.; Yang, X.D.; Wu, X.X.; Pang, Z.Y.; Yang, H.; Qi, Y.P. A theoretical study of a plasmonic sensor comprising a gold nano-disk array on gold film with an SiO2 spacer. Chin. Phys. B 2019, 28, 044201. [Google Scholar] [CrossRef]
- Yi, Z.; Luo, J.; Ye, X.; Yi, Y.; Huang, J.; Yi, Y.; Duan, T.; Zhang, W.; Tang, Y. Effect of synthesis conditions on the growth of various ZnO nanostructures and corresponding morphology-dependent photocatalytic activities. Superlattices Microstruct. 2016, 100, 907–917. [Google Scholar] [CrossRef]
- Li, X.; Chen, X.; Yi, Z.; Zhou, Z.; Tang, Y.; Yi, Y. Fabriction of ZnO Nanorods with Strong UV Absorption and Different Hydrophobicity on Foamed Nickel under Different Hydrothermal Conditions. Micromachines 2019, 10, 164. [Google Scholar] [CrossRef] [PubMed]
- Yi, Z.; Xu, X.; Kang, X.; Zhao, Y.; Zhang, S.; Yao, W.; Yi, Y.; Luo, J.; Wang, C.; Yi, Y.; et al. Fabrication of well-aligned ZnO@Ag nanorod arrays with effective charge transfer for surface-enhanced Raman scattering. Surf. Coat. Technol. 2017, 324, 257–263. [Google Scholar] [CrossRef]
- Lee, K.M.; Lai, C.W.; Ngai, K.S.; Juan, J.C. Recent developments of zinc oxide based photocatalyst in water treatment technology: A review. Water Res. 2016, 88, 428–448. [Google Scholar] [CrossRef]
- Ong, C.B.; Ng, L.Y.; Mohammad, A.W. A review of ZnO nanoparticles as solar photocatalysts: Synthesis, mechanisms and applications. Renew. Sustain. Energy Rev. 2018, 81, 536–551. [Google Scholar] [CrossRef]
- Pirhashemi, M.; Habibi-Yangjeh, A.; Rahim Pouran, S. Review on the criteria anticipated for the fabrication of highly efficient ZnO-based visible-light-driven photocatalysts. J. Ind. Eng. Chem. 2018, 62, 1–25. [Google Scholar] [CrossRef]
- Vernardou, D.; Kenanakis, G. Electrochemistry Studies of Hydrothermally Grown ZnO on 3D-Printed Graphene. Nanomaterials 2019, 9, 1056. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.J.; Balamurugan, C.; Lee, D.W. Enhanced CO2 gas-sensing performance of ZnO nanopowder by La loaded during simple hydrothermal method. Sens. Actuators B Chem. 2016, 229, 288–296. [Google Scholar] [CrossRef]
- Hassanpour, A.; Bogdan, N.; Capobianco, J.A.; Bianucci, P. Hydrothermal selective growth of low aspect ratio isolated ZnO nanorods. Mater. Des. 2017, 119, 464–469. [Google Scholar] [CrossRef]
- Tam, K.H.; Cheung, C.K.; Leung, Y.H.; Djurišić, A.B.; Ling, C.C.; Beling, C.D.; Fung, S.; Kwok, W.M.; Chan, W.K.; Phillips, D.L.; et al. Defects in ZnO Nanorods Prepared by a Hydrothermal Method. J. Phys. Chem. B 2006, 110, 20865–20871. [Google Scholar] [CrossRef] [PubMed]
- Jiaqiang, X.; Yuping, C.; Yadong, L.; Jianian, S. Gas sensing properties of ZnO nanorods prepared by hydrothermal method. J. Mater. Sci. 2005, 40, 2919–2921. [Google Scholar] [CrossRef]
- Yang, W.; Liu, Z.; Peng, D.L.; Zhang, F.; Huang, H.; Xie, Y.; Wu, Z. Room-temperature deposition of transparent conducting Al-doped ZnO films by RF magnetron sputtering method. Appl. Surf. Sci. 2009, 255, 5669–5673. [Google Scholar] [CrossRef]
- Li, M.W.; Liang, C.P.; Zhang, Y.B.; Yi, Z.; Chen, X.F.; Zhou, Z.G.; Yang, H.; Tang, Y.J.; Yi, Y.G. Terahertz wideband perfect absorber based on open loop with cross nested structure. Results Phys. 2019, 15, 102603. [Google Scholar] [CrossRef]
- Chin, H.S.; Chao, L.S.; Wu, C.C. Crystal, optical, and electrical characteristics of transparent conducting gallium-doped zinc oxide films deposited on flexible polyethylene naphthalate substrates using radio frequency magnetron sputtering. Mater. Res. Bull. 2016, 79, 90–96. [Google Scholar] [CrossRef]
- Wen, X.; He, Y.; Chen, C.; Liu, X.; Wang, L.; Yang, B.; Leng, M.; Song, H.; Zeng, K.; Li, D.; et al. Magnetron sputtered ZnO buffer layer for Sb2Se3 thin film solar cells. Sol. Energy Mater. Sol. Cells 2017, 172, 74–81. [Google Scholar] [CrossRef]
- Chen, J.; Tang, C.J.; Mao, P.; Peng, C.; Gao, D.P.; Yu, Y.; Wang, Q.G.; Zhang, L.B. Surface-plasmon-polaritons-assisted enhanced magnetic response at optical frequencies in metamaterials. IEEE Photonics J. 2016, 8, 4800107. [Google Scholar] [CrossRef]
- Barreca, D.; Bekermann, D.; Comini, E.; Devi, A.; Fischer, R.A.; Gasparotto, A.; Maccato, C.; Sberveglieri, G.; Tondello, E. 1D ZnO nano-assemblies by Plasma-CVD as chemical sensors for flammable and toxic gases. Sens. Actuators B Chem. 2010, 149, 1–7. [Google Scholar] [CrossRef]
- Ye, Z.; Wang, T.; Wu, S.; Ji, X.; Zhang, Q. Na-doped ZnO nanorods fabricated by chemical vapor deposition and their optoelectrical properties. J. Alloy. Compd. 2017, 690, 189–194. [Google Scholar] [CrossRef]
- Zhan, Z.; Xu, L.; An, J.; Du, H.; Weng, Z.; Lu, W. Direct Catalyst-Free Chemical Vapor Deposition of ZnO Nanowire Array UV Photodetectors with Enhanced Photoresponse Speed. Adv. Eng. Mater. 2017, 19, 1700101. [Google Scholar] [CrossRef]
- Liang, C.P.; Yi, Z.; Chen, X.F.; Tang, Y.J.; Yi, Y.; Zhou, Z.G.; Wu, X.G.; Huang, Z.; Yi, Y.G.; Zhang, G.F. Dual-band infrared perfect absorber based on a Ag-dielectric-Ag multilayer films with nanoring grooves arrays. Plasmonics 2019. [Google Scholar] [CrossRef]
- Ren, X.; Zi, W.; Ma, Q.; Xiao, F.; Gao, F.; Hu, S.; Zhou, Y.; Liu, S. Topology and texture controlled ZnO thin film electrodeposition for superior solar cell efficiency. Sol. Energy Mater. Sol. Cells 2015, 134, 54–59. [Google Scholar] [CrossRef]
- Sarangi, S.N. Controllable growth of ZnO nanorods via electrodeposition technique: Towards UV photo-detection. J. Phys. D Appl. Phys. 2016, 49, 355103. [Google Scholar] [CrossRef]
- Li, H.L.; Niu, J.B.; Wang, G.Y. Dual-band, polarization-insensitive metamaterial perfect absorber based on monolayer graphene in the mid-infrared range. Results Phys. 2019, 13, 102313. [Google Scholar] [CrossRef]
- Yi, Z.; Liang, C.P.; Chen, X.F.; Zhou, Z.G.; Tang, Y.J.; Ye, X.; Yi, Y.G.; Wang, J.Q.; Wu, P.H. Dual-band plasmonic perfect absorber based on graphene metamaterials for refractive index sensing application. Micromachines 2019, 10, 443. [Google Scholar] [CrossRef] [PubMed]
- Cen, C.L.; Zhang, Y.B.; Liang, C.P.; Chen, X.F.; Yi, Z.; Duan, T.; Tang, Y.J.; Ye, X.; Yi, Y.G.; Xiao, S.Y. Numerical investigation of a tunable dual-band metamaterial perfect absorber consisting of two-intersecting graphene nanorings arrays. Phys. Lett. A 2019, 383, 3030–3035. [Google Scholar] [CrossRef]
- Cen, C.L.; Yi, Z.; Zhang, G.F.; Zhang, Y.B.; Liang, C.P.; Chen, X.F.; Tang, Y.J.; Ye, X.; Yi, Y.G.; Wang, J.Q.; et al. Theoretical design of a triple-band perfect metamaterial absorber in the THz frequency range. Results Phys. 2019, 14, 102463. [Google Scholar] [CrossRef]
- Zhang, Y.B.; Cen, C.L.; Liang, C.P.; Yi, Z.; Chen, X.F.; Li, M.W.; Zhou, Z.G.; Tang, Y.J.; Yi, Y.G.; Zhang, G.F. Dual-band switchable terahertz metamaterial absorber based on metal nanostructure. Results Phys. 2019, 14, 102422. [Google Scholar] [CrossRef]
- Liu, C.; Su, W.Q.; Wang, F.M.; Li, X.L.; Yang, L.; Sun, T.; Mu, H.W.; Chu, P.K. Theoretical assessment of a highly sensitive photonic crystal fibre based on surface plasmon resonance sensor operating in the near-infrared wavelength. J. Mod. Opt. 2019, 66, 1–6. [Google Scholar] [CrossRef]
- Liu, C.; Yang, L.; Lu, X.L.; Liu, Q.; Wang, F.M.; Lv, J.W.; Sun, T.; Mu, H.W.; Chu, P.K. Mid-infrared surface plasmon resonance sensor based on photonic crystal fibers. Opt. Express 2017, 25, 14227–14237. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yang, L.; Liu, Q.; Wang, F.M.; Sun, Z.J.; Sun, T.; Mu, H.W.; Chu, P.K. Analysis of a Surface Plasmon Resonance Probe Based on Photonic Crystal Fibers for Low Refractive Index Detection. Plasmonics 2018, 13, 779–784. [Google Scholar] [CrossRef]
- Liu, C.; Fu, G.L.; Wang, F.M.; Yi, Z.; Xu, C.H.; Yang, L.; Liu, Q.; Liu, W.; Li, X.L.; Mu, H.W.; et al. Ex-centric core photonic crystal fiber sensor with gold nanowires based on surface plasmon resonance. Optik 2019, 196, 163173. [Google Scholar] [CrossRef]
- Liu, C.; Wang, L.Y.; Yang, L.; Wang, F.M.; Xu, C.H.; Lv, J.W.; Fu, G.L.; Li, X.L.; Liu, Q.; Mu, H.W.; et al. The single-polarization filter composed of gold-coated photonic crystal fiber. Phys. Lett. A 2019, 383, 3200–3206. [Google Scholar] [CrossRef]
- Wang, X.X.; Zhu, J.K.; Wen, X.L.; Wu, X.X.; Wu, Y.; Su, Y.W.; Tong, H.; Qi, Y.P.; Yang, H. Wide range refractive index sensor based on a coupled structure of Au nanocubes and Au film. Opt. Mater. Express 2019, 9, 3079–3088. [Google Scholar] [CrossRef]
- Tong, H.; Xu, Y.Q.; Su, Y.W.; Wang, X.X. Theoretical study for fabricating elliptical subwavelength nanohole arrays by higher-order waveguide-mode interference. Results Phys. 2019, 14, 102460. [Google Scholar] [CrossRef]
- Wang, X.X.; Pang, Z.Y.; Yang, H.; Qi, Y.P. Theoretical study of subwavelength circular grating fabrication based on continuously exposed surface plasmon interference lithography. Results Phys. 2019, 14, 102446. [Google Scholar] [CrossRef]
- Wang, X.X.; Bai, X.L.; Pang, Z.Y.; Zhu, J.K.; Wu, Y.; Yang, H.; Qi, Y.P.; Wen, X.L. Surface-enhanced Raman scattering by composite structure of gold nanocube-PMMA-gold film. Opt. Mater. Express 2019, 9, 1872–1881. [Google Scholar] [CrossRef]
- Ty, J.T.D.; Yanagi, H. Electrochemical deposition of zinc oxide nanorods for hybrid solar cells. Jpn. J. Appl. Phys. 2015, 54, 04DK05. [Google Scholar] [CrossRef]
- Dong, C.; Wu, K.L.; Li, M.R.; Liu, L.; Wei, X.W. Synthesis of Ag3PO4–ZnO nanorod composites with high visible-light photocatalytic activity. Catal. Commun. 2014, 46, 32–35. [Google Scholar] [CrossRef]
- Yi, Z.; Ye, J.; Kikugawa, N.; Kako, T.; Ouyang, S.; Stuart-Williams, H.; Yang, H.; Cao, J.; Luo, W.; Li, Z.; et al. An orthophosphate semiconductor with photooxidation properties under visible-light irradiation. Nat. Mater. 2010, 9, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.J.; Zheng, C.X.; Yang, H.; Niu, X.W.; Wang, S.F. Construction of a CQDs/Ag3PO4/BiPO4 heterostructure photocatalyst with enhanced photocatalytic degradation of rhodamine B under simulated solar irradiation. Micromachines 2019, 10, 557. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Hu, P.; Chen, S. Photocatalytic activity of Ag3PO4 nanoparticle/TiO2 nanobelt heterostructures. Appl. Surf. Sci. 2012, 258, 9805–9809. [Google Scholar] [CrossRef]
- Gunjakar, J.L.; Jo, Y.K.; Kim, I.Y.; Lee, J.M.; Patil, S.B.; Pyun, J.C.; Hwang, S.J. A chemical bath deposition route to facet-controlled Ag3PO4 thin films with improved visible light photocatalytic activity. J. Solid State Chem. 2016, 240, 115–121. [Google Scholar] [CrossRef]
- Liu, Z.Q.; Tang, P.; Liu, X.S.; Yi, Z.; Liu, G.Q.; Wang, Y.; Liu, M.L. Truncated titanium/semiconductor cones for wide-band solar absorbers. Nanotechnology 2019, 30, 305203. [Google Scholar] [CrossRef]
- Chen, J.; Tang, P.; Liu, G.Q.; Yi, Z.; Liu, X.S.; Pan, P.P.; Liu, Z.Q. Si nano-cavity enabled SERS signal amplification. Nanotechnology 2019, 30. [Google Scholar] [CrossRef]
- Liu, G.; Liu, Y.; Tang, L.; Liu, X.; Fu, G.; Liu, Z. Semiconductor-enhanced Raman scattering sensors via quasi-three-dimensional Au/Si/Au structures. Nanophotonics 2019, 8, 1095–1107. [Google Scholar] [CrossRef]
- Guo, J.; Zhou, H.; Ouyang, S.; Kako, T.; Ye, J. An Ag3PO4/nitridized Sr2Nb2O7 composite photocatalyst with adjustable band structures for efficient elimination of gaseous organic pollutants under visible light irradiation. Nanoscale 2014, 6, 7303–7311. [Google Scholar] [CrossRef]
- He, Z.X.; Li, M.M.; Li, Y.H.; Li, C.C.; Yi, Z.; Zhu, J.; Dai, L.; Meng, W.; Zhou, H.Z.; Wang, L. ZrO2 nanoparticle embedded carbon nanofibers by electrospinning technique as advanced negative electrode materials for vanadium redox flow battery. Electrochim. Acta 2019, 309, 166–176. [Google Scholar] [CrossRef]
- Zhang, Q.B.; Liao, J.; Liao, M.; Dai, J.Y.; Ge, H.L.; Duan, T.; Yao, W.T. One-dimensional Fe7S8@C nanorods as anode materials for high-rate and long-life lithium-ion batteries. Appl. Surf. Sci. 2019, 473, 799–806. [Google Scholar] [CrossRef]
- Chen, X.F.; Cen, C.L.; Zhou, L.; Cao, R.F.; Yi, Z.; Tang, Y.J. Magnetic properties and reverse magnetization process of anisotropic nanocomposite permanent magnet. J. Magn. Magn. Mater. 2019, 483, 152–157. [Google Scholar] [CrossRef]
- Liu, G.; Chen, J.; Pan, P.; Liu, Z. Hybrid Metal-Semiconductor Meta-Surface Based Photo-Electronic Perfect Absorber. IEEE J. Sel. Top. Quantum Electron. 2019, 25, 1–7. [Google Scholar] [CrossRef]
- Shi, D.; Xiong, Z.; Li, J.; Luo, B.; Fang, L.; Xia, Y.; Gao, Z. Electron transition and electron-hole recombination processes in epitaxial BaTiO3 films with embedded Co nanocrystals. Mater. Res. Express 2019. [Google Scholar] [CrossRef]
- Yi, Z.; Zeng, Y.; Wu, H.; Chen, X.F.; Fan, Y.X.; Yang, H.; Tang, Y.J.; Yi, Y.G.; Wang, J.Q.; Wu, P.H. Synthesis, surface properties, crystal structure and dye-sensitized solar cell performance of TiO2 nanotube arrays anodized under different parameters. Results Phys. 2019, 15, 102609. [Google Scholar] [CrossRef]
- Yan, Y.X.; Yang, H.; Yi, Z.; Xian, T.; Wang, X.X. Direct Z-scheme CaTiO3@BiOBr composite photocatalysts with enhanced photodegradation of dyes. Environ. Sci. Pollut. Res. 2019. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yi, Z.; Li, X.; Wu, H.; Chen, X.; Yang, H.; Tang, Y.; Yi, Y.; Wang, J.; Wu, P. Fabrication of ZnO@Ag3PO4 Core-Shell Nanocomposite Arrays as Photoanodes and Their Photoelectric Properties. Nanomaterials 2019, 9, 1254. https://doi.org/10.3390/nano9091254
Yi Z, Li X, Wu H, Chen X, Yang H, Tang Y, Yi Y, Wang J, Wu P. Fabrication of ZnO@Ag3PO4 Core-Shell Nanocomposite Arrays as Photoanodes and Their Photoelectric Properties. Nanomaterials. 2019; 9(9):1254. https://doi.org/10.3390/nano9091254
Chicago/Turabian StyleYi, Zao, Xin Li, Hui Wu, Xifang Chen, Hua Yang, Yongjian Tang, Yougen Yi, Junqiao Wang, and Pinghui Wu. 2019. "Fabrication of ZnO@Ag3PO4 Core-Shell Nanocomposite Arrays as Photoanodes and Their Photoelectric Properties" Nanomaterials 9, no. 9: 1254. https://doi.org/10.3390/nano9091254




