Soybean Interaction with Engineered Nanomaterials: A Literature Review of Recent Data
Abstract
:1. Introduction
2. Engineered Nanomaterials and the Environment
3. Plant Exposure to ENMs and Toxicity Mechanisms
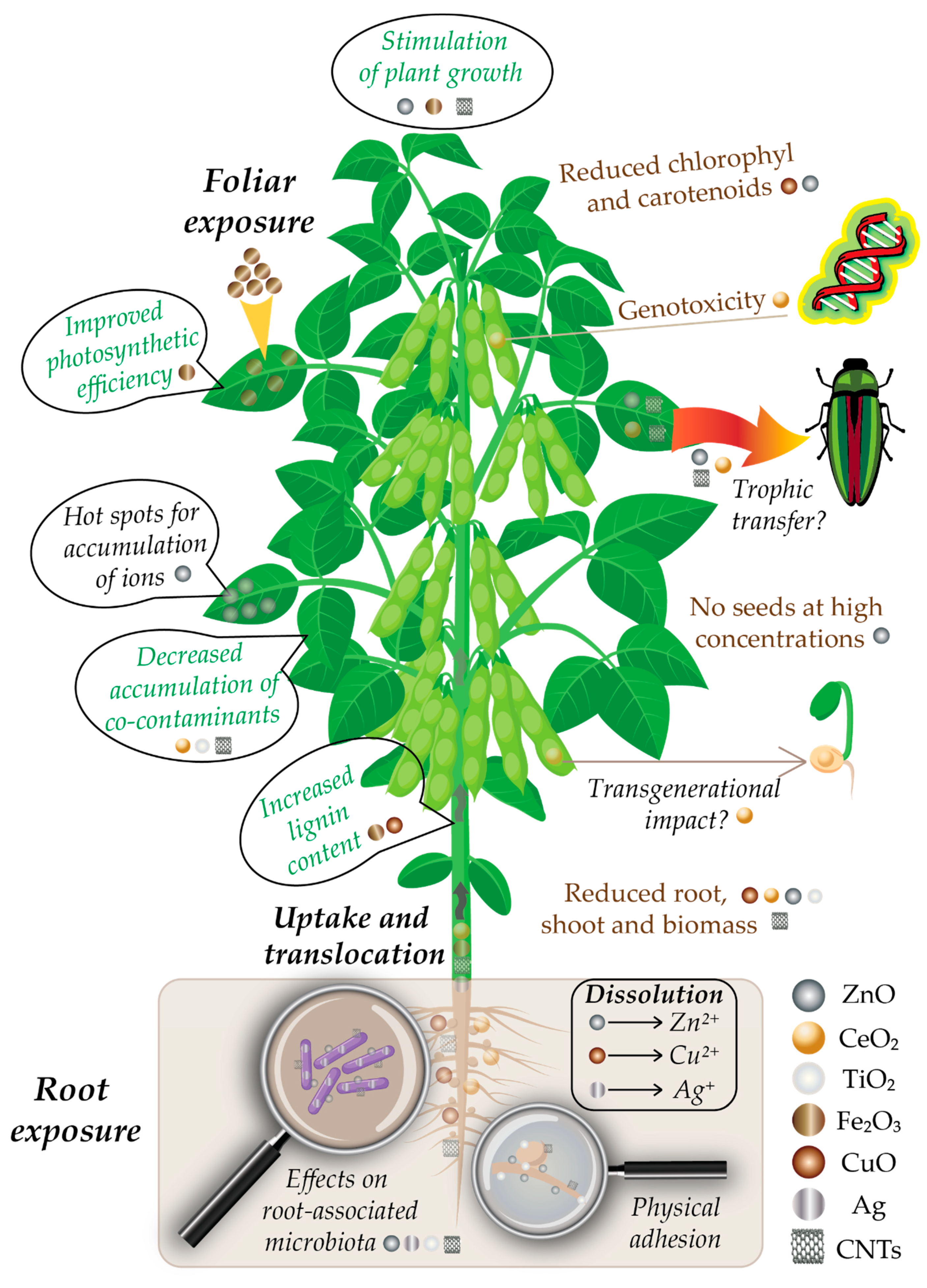
4. Soybean Interactions with ENMs
4.1. ZnO and CeO2 NPs
4.2. TiO2 NPs
4.3. Iron Oxides (Fe2O3 and Fe3O4) NPs
4.4. CuO NPs
4.5. Cr2O3 NPs
4.6. Ag NPs
4.7. Carbon Nanotubes and Fullerenes
5. Plant Microbiota and the Influence of ENMs
6. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Hochella, M.F., Jr.; Mogk, D.W.; Ranville, J.; Allen, I.C.; Luther, G.W.; Marr, L.C.; McGrail, B.P.; Murayama, M.; Qafoku, N.P.; Rosso, K.M.; et al. Natural, incidental, and engineered nanomaterials and their impacts on the Earth system. Science 2019, 363. [Google Scholar] [CrossRef] [PubMed]
- Salata, O.V. Applications of nanoparticles in biology and medicine. J. Nanobiotechnol. 2004, 2. [Google Scholar] [CrossRef] [PubMed]
- De Jong, W.H.; Borm, P.J.A. Drug delivery and nanoparticles: Applications and hazards. Int. J. Nanomed. 2008, 3, 133–149. [Google Scholar] [CrossRef] [Green Version]
- Kamat, P.V. Photophysical, photochemical and photocatalytic aspects of metal nanoparticles. J. Phys. Chem. B 2002, 106, 7729–7744. [Google Scholar] [CrossRef]
- Brigger, I.; Dubernet, C.; Couvreur, P. Nanoparticles in cancer therapy and diagnosis. Adv. Drug Del. Rev. 2002, 54, 631–651. [Google Scholar] [CrossRef]
- Liu, R.; Lal, R. Potentials of engineered nanoparticles as fertilizers for increasing agronomic productions. Sci. Total Environ. 2015, 514, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Misra, P.; Shukla, P.K.; Pramanik, K.; Gautam, S.; Kole, C. Nanotechnology for crop improvement. In Plant Nanotechnology: Principles and Practices; Springer: Berlin, Germany, 2016; pp. 219–256. [Google Scholar]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.M.; Hamilton, J.W.J.; Byrne, J.A.; O'Shea, K.; et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B Environ. 2012, 125, 331–349. [Google Scholar] [CrossRef] [Green Version]
- Keller, A.A.; Lazareva, A. Predicted Releases of Engineered Nanomaterials: From Global to Regional to Local. Environ. Sci. Technol. Lett. 2013, 1, 65–70. [Google Scholar] [CrossRef] [Green Version]
- Rico, C.M.; Majumdar, S.; Duarte-Gardea, M.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Interaction of nanoparticles with edible plants and their possible implications in the food chain. J. Agric. Food Chem. 2011, 59, 3485–3498. [Google Scholar] [CrossRef]
- Bar-On, Y.M.; Phillips, R.; Milo, R. The biomass distribution on Earth. Proc. Natl. Acad. Sci. USA 2018, 115, 6506–6511. [Google Scholar] [CrossRef] [Green Version]
- Nair, R.; Varghese, S.H.; Nair, B.G.; Maekawa, T.; Yoshida, Y.; Kumar, D.S. Nanoparticulate material delivery to plants. Plant Sci. 2010, 179, 154–163. [Google Scholar] [CrossRef]
- Lv, J.; Christie, P.; Zhang, S. Uptake, translocation, and transformation of metal-based nanoparticles in plants: Recent advances and methodological challenges. Environ. Sci. Nano 2019, 6, 41–59. [Google Scholar] [CrossRef]
- Ma, X.; Geiser-Lee, J.; Deng, Y.; Kolmakov, A. Interactions between engineered nanoparticles (ENPs) and plants: Phytotoxicity, uptake and accumulation. Sci. Total Environ. 2010, 408, 3053–3061. [Google Scholar] [CrossRef] [PubMed]
- Schmutz, J.; Cannon, S.B.; Schlueter, J.; Ma, J.; Mitros, T.; Nelson, W.; Hyten, D.L.; Song, Q.; Thelen, J.J.; Cheng, J.; et al. Genome sequence of the palaeopolyploid soybean. Nature 2010, 463, 178–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klaine, S.J.; Alvarez, P.J.J.; Batley, G.E.; Fernandes, T.F.; Handy, R.D.; Lyon, D.Y.; Mahendra, S.; McLaughlin, M.J.; Lead, J.R. Nanomaterials in the environment: Behavior, fate, bioavailability, and effects. Environ. Toxicol. Chem. 2008, 27, 1825–1851. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhou, G.; Liu, K.; Cui, Y. Design of Complex Nanomaterials for Energy Storage: Past Success and Future Opportunity. Acc. Chem. Res. 2017, 50, 2895–2905. [Google Scholar] [CrossRef] [PubMed]
- Pourzahedi, L.; Pandorf, M.; Ravikumar, D.; Zimmerman, J.B.; Seager, T.P.; Theis, T.L.; Westerhoff, P.; Gilbertson, L.M.; Lowry, G.V. Life cycle considerations of nano-enabled agrochemicals: Are today’s tools up to the task? Environ. Sci. Nano 2018, 5, 1057–1069. [Google Scholar] [CrossRef]
- Vance, M.E.; Kuiken, T.; Vejerano, E.P.; McGinnis, S.P.; Hochella, M.F., Jr.; Hull, D.R. Nanotechnology in the real world: Redeveloping the nanomaterial consumer products inventory. Beilstein J. Nanotechnol. 2015, 6, 1769–1780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keller, A.A.; McFerran, S.; Lazareva, A.; Suh, S. Global life cycle releases of engineered nanomaterials. J. Nanopart. Res. 2013, 15. [Google Scholar] [CrossRef]
- Bundschuh, M.; Filser, J.; Lüderwald, S.; McKee, M.S.; Metreveli, G.; Schaumann, G.E.; Schulz, R.; Wagner, S. Nanoparticles in the environment: Where do we come from, where do we go to? Environ. Sci. Eur. 2018, 30. [Google Scholar] [CrossRef] [PubMed]
- Gottschalk, F.; Nowack, B. The release of engineered nanomaterials to the environment. J. Environ. Monit. 2011, 13, 1145–1155. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.N. Do nanoparticles present ecotoxicological risks for the health of the aquatic environment? Environ. Int. 2006, 32, 967–976. [Google Scholar] [CrossRef] [PubMed]
- Borm, P.J.A.; Robbins, D.; Haubold, S.; Kuhlbusch, T.; Fissan, H.; Donaldson, K.; Schins, R.; Stone, V.; Kreyling, W.; Lademann, J.; et al. The potential risks of nanomaterials: A review carried out for ECETOC. Part. Fibre Toxicol. 2006, 3. [Google Scholar] [CrossRef] [PubMed]
- Hoet, P.H.M.; Brüske-Hohlfeld, I.; Salata, O.V. Nanoparticles—Known and unknown health risks. J. Nanobiotechnol. 2004, 2. [Google Scholar] [CrossRef] [PubMed]
- Mueller, N.C.; Nowack, B. Exposure modeling of engineered nanoparticles in the environment. Environ. Sci. Technol. 2008, 42, 4447–4453. [Google Scholar] [CrossRef] [PubMed]
- Nowack, B.; Bucheli, T.D. Occurrence, behavior and effects of nanoparticles in the environment. Environ. Pollut. 2007, 150, 5–22. [Google Scholar] [CrossRef]
- Lewinski, N.; Colvin, V.; Drezek, R. Cytotoxicity of nanopartides. Small 2008, 4, 26–49. [Google Scholar] [CrossRef]
- Nel, A.; Xia, T.; Mädler, L.; Li, N. Toxic potential of materials at the nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef]
- Jiang, W.; Kim, B.Y.S.; Rutka, J.T.; Chan, W.C.W. Nanoparticle-mediated cellular response is size-dependent. Nat. Nanotechnol. 2008, 3, 145–150. [Google Scholar] [CrossRef]
- Cedervall, T.; Lynch, I.; Lindman, S.; Berggård, T.; Thulin, E.; Nilsson, H.; Dawson, K.A.; Linse, S. Understanding the nanoparticle-protein corona using methods to quntify exchange rates and affinities of proteins for nanoparticles. Proc. Natl. Acad. Sci. USA 2007, 104, 2050–2055. [Google Scholar] [CrossRef]
- Verma, A.; Stellacci, F. Effect of surface properties on nanoparticle-cell interactions. Small 2010, 6, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Ali, S.; Qayyum, M.F.; Ok, Y.S.; Adrees, M.; Ibrahim, M.; Zia-ur-Rehman, M.; Farid, M.; Abbas, F. Effect of metal and metal oxide nanoparticles on growth and physiology of globally important food crops: A critical review. J. Hazard. Mater. 2017, 322, 2–16. [Google Scholar] [CrossRef] [PubMed]
- Oberdörster, G.; Oberdörster, E.; Oberdörster, J. Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles. Environ. Health Perspect. 2005, 113, 823–839. [Google Scholar] [CrossRef] [PubMed]
- López-Moreno, M.L.; Cassé, C.; Correa-Torres, S.N. Engineered NanoMaterials interactions with living plants: Benefits, hazards and regulatory policies. Curr. Opin. Environ. Sci. Health 2018, 6, 36–41. [Google Scholar] [CrossRef]
- Zuverza-Mena, N.; Martínez-Fernández, D.; Du, W.; Hernandez-Viezcas, J.A.; Bonilla-Bird, N.; López-Moreno, M.L.; Komárek, M.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Exposure of engineered nanomaterials to plants: Insights into the physiological and biochemical responses-A review. Plant Physiol. Biochem. 2017, 110, 236–264. [Google Scholar] [CrossRef]
- Gottschalk, F.; Sonderer, T.; Scholz, R.W.; Nowack, B. Modeled environmental concentrations of engineered nanomaterials (TiO2, ZnO, Ag, CNT, fullerenes) for different regions. Environ. Sci. Technol. 2009, 43, 9216–9222. [Google Scholar] [CrossRef]
- Batley, G.E.; Kirby, J.K.; McLaughlin, M.J. Fate and risks of nanomaterials in aquatic and terrestrial environments. Acc. Chem. Res. 2013, 46, 854–862. [Google Scholar] [CrossRef]
- Brar, S.K.; Verma, M.; Tyagi, R.D.; Surampalli, R.Y. Engineered nanoparticles in wastewater and wastewater sludge—Evidence and impacts. Waste Manag. 2010, 30, 504–520. [Google Scholar] [CrossRef]
- Lowry, G.V.; Gregory, K.B.; Apte, S.C.; Lead, J.R. Transformations of nanomaterials in the environment. Environ. Sci. Technol. 2012, 46, 6893–6899. [Google Scholar] [CrossRef]
- Medina-Velo, I.A.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Assessing plant uptake and transport mechanisms of engineered nanomaterials from soil. MRS Bull. 2017, 42, 379–383. [Google Scholar] [CrossRef]
- Dimkpa, C.O. Soil properties influence the response of terrestrial plants to metallic nanoparticles exposure. Curr. Opin. Environ. Sci. Health 2018, 6, 1–8. [Google Scholar] [CrossRef]
- Bulgarelli, D.; Schlaeppi, K.; Spaepen, S.; Van Themaat, E.V.L.; Schulze-Lefert, P. Structure and functions of the bacterial microbiota of plants. Annu. Rev. Plant Biol. 2013, 64, 807–838. [Google Scholar] [CrossRef] [PubMed]
- Gardea-Torresdey, J.L.; Rico, C.M.; White, J.C. Trophic transfer, transformation, and impact of engineered nanomaterials in terrestrial environments. Environ. Sci. Technol. 2014, 48, 2526–2540. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ebbs, S.D.; Chen, Y.; Ma, X. Trans-generational impact of cerium oxide nanoparticles on tomato plants. Metallomics 2013, 5, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Bouwmeester, H.; Dekkers, S.; Noordam, M.Y.; Hagens, W.I.; Bulder, A.S.; de Heer, C.; ten Voorde, S.E.C.G.; Wijnhoven, S.W.P.; Marvin, H.J.P.; Sips, A.J.A.M. Review of health safety aspects of nanotechnologies in food production. Regul. Toxicol. Pharmacol. 2009, 53, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Boxall, A.B.A.; Tiede, K.; Chaudhry, Q. Engineered nanomaterials in soils and water: How do they behave and could they pose a risk to human health? Nanomedicine 2007, 2, 919–927. [Google Scholar] [CrossRef] [PubMed]
- Dobrovolskaia, M.A.; McNeil, S.E. Immunological properties of engineered nanomaterials. Nat. Nanotechnol. 2007, 2, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, K.S.; Husen, A. Plant Response to Engineered Metal Oxide Nanoparticles. Nanoscale Res. Lett. 2017, 12. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.K.; Das, A.K.; Patel, M.K.; Shah, A.; Kumar, V.; Gantait, S. Engineered nanomaterials for plant growth and development: A perspective analysis. Sci. Total Environ. 2018, 630, 1413–1435. [Google Scholar] [CrossRef]
- Cota-Ruiz, K.; Delgado-Rios, M.; Martínez-Martínez, A.; Núñez-Gastelum, J.A.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Current findings on terrestrial plants—Engineered nanomaterial interactions: Are plants capable of phytoremediating nanomaterials from soil? Curr. Opin. Environ. Sci. Health 2018, 6, 9–15. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef]
- Albanese, A.; Tang, P.S.; Chan, W.C.W. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu. Rev. Biomed. Eng. 2012, 14, 1–16. [Google Scholar] [CrossRef]
- Stampoulis, D.; Sinha, S.K.; White, J.C. Assay-dependent phytotoxicity of nanoparticles to plants. Environ. Sci. Technol. 2009, 43, 9473–9479. [Google Scholar] [CrossRef]
- Rajput, V.D.; Minkina, T.; Suskova, S.; Mandzhieva, S.; Tsitsuashvili, V.; Chapligin, V.; Fedorenko, A. Effects of Copper Nanoparticles (CuO NPs) on Crop Plants: A Mini Review. BioNanoScience 2018, 8, 36–42. [Google Scholar] [CrossRef]
- Van Aken, B. Gene expression changes in plants and microorganisms exposed to nanomaterials. Curr. Opin. Biotechnol. 2015, 33, 206–219. [Google Scholar] [CrossRef]
- Du, W.; Tan, W.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L.; Ji, R.; Yin, Y.; Guo, H. Interaction of metal oxide nanoparticles with higher terrestrial plants: Physiological and biochemical aspects. Plant Physiol. Biochem. 2017, 110, 210–225. [Google Scholar] [CrossRef]
- Yanga, J.; Cao, W.; Rui, Y. Interactions between nanoparticles and plants: Phytotoxicity and defense mechanisms. J. Plant Interact. 2017, 12, 158–169. [Google Scholar] [CrossRef]
- Reddy, P.V.L.; Hernandez-Viezcas, J.A.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Lessons learned: Are engineered nanomaterials toxic to terrestrial plants? Sci. Total Environ. 2016, 568, 470–479. [Google Scholar] [CrossRef] [Green Version]
- Ma, C.; White, J.C.; Dhankher, O.P.; Xing, B. Metal-Based Nanotoxicity and Detoxification Pathways in Higher Plants. Environ. Sci. Technol. 2015, 49, 7109–7122. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, F.; Liu, Q.; Chen, M.; Liu, X.; Wang, Y.; Sun, Y.; Zhang, L. Nanomaterials and plants: Positive effects, toxicity and the remediation of metal and metalloid pollution in soil. Sci. Total Environ. 2019, 662, 414–421. [Google Scholar] [CrossRef]
- Cox, A.; Venkatachalam, P.; Sahi, S.; Sharma, N. Silver and titanium dioxide nanoparticle toxicity in plants: A review of current research. Plant Physiol. Biochem. 2016, 107, 147–163. [Google Scholar] [CrossRef]
- Miralles, P.; Church, T.L.; Harris, A.T. Toxicity, uptake, and translocation of engineered nanomaterials in vascular plants. Environ. Sci. Technol. 2012, 46, 9224–9239. [Google Scholar] [CrossRef]
- Nel, A.E.; Mädler, L.; Velegol, D.; Xia, T.; Hoek, E.M.V.; Somasundaran, P.; Klaessig, F.; Castranova, V.; Thompson, M. Understanding biophysicochemical interactions at the nano-bio interface. Nat. Mater. 2009, 8, 543–557. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Marslin, G.; Sheeba, C.J.; Franklin, G. Nanoparticles alter secondary metabolism in plants via ROS burst. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef]
- Mendoza, R.P.; Brown, J.M. Engineered nanomaterials and oxidative stress: Current understanding and future challenges. Curr. Opin. Toxicol. 2019, 13, 74–80. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Oxidant and antioxidant signalling in plants: A re-evaluation of the concept of oxidative stress in a physiological context. Plant Cell Environ. 2005, 28, 1056–1071. [Google Scholar] [CrossRef]
- Couturier, J.; Chibani, K.; Jacquot, J.P.; Rouhier, N. Cysteine-based redox regulation and signaling in plants. Front. Plant Sci. 2013, 4. [Google Scholar] [CrossRef]
- Jones, D.P. Radical-free biology of oxidative stress. Am. J. Physiol. Cell Physiol. 2008, 295, C849–C868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jorge de Souza, T.A.; Rosa Souza, L.R.; Franchi, L.P. Silver nanoparticles: An integrated view of green synthesis methods, transformation in the environment, and toxicity. Ecotoxicol. Environ. Saf. 2019, 171, 691–700. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, R.F., Jr.; Buckingham, S.; Holian, A. The effect of size on Ag nanosphere toxicity in macrophage cell models and lung epithelial cell lines is dependent on particle dissolution. Int. J. Mol. Sci. 2014, 15, 6815–6830. [Google Scholar] [CrossRef] [PubMed]
- Dimkpa, C.O.; Latta, D.E.; McLean, J.E.; Britt, D.W.; Boyanov, M.I.; Anderson, A.J. Fate of CuO and ZnO nano- and microparticles in the plant environment. Environ. Sci. Technol. 2013, 47, 4734–4742. [Google Scholar] [CrossRef] [PubMed]
- Dimkpa, C.O.; McLean, J.E.; Latta, D.E.; Manangón, E.; Britt, D.W.; Johnson, W.P.; Boyanov, M.I.; Anderson, A.J. CuO and ZnO nanoparticles: Phytotoxicity, metal speciation, and induction of oxidative stress in sand-grown wheat. J. Nanopart. Res. 2012, 14. [Google Scholar] [CrossRef]
- Ma, H.; Williams, P.L.; Diamond, S.A. Ecotoxicity of manufactured ZnO nanoparticles—A review. Environ. Pollut. 2013, 172, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Xing, B. Root uptake and phytotoxicity of ZnO nanoparticles. Environ. Sci. Technol. 2008, 42, 5580–5585. [Google Scholar] [CrossRef]
- García-Gómez, C.; Obrador, A.; González, D.; Babín, M.; Fernández, M.D. Comparative effect of ZnO NPs, ZnO bulk and ZnSO4 in the antioxidant defences of two plant species growing in two agricultural soils under greenhouse conditions. Sci. Total Environ. 2017, 589, 11–24. [Google Scholar] [CrossRef]
- Oberdörster, E.; Zhu, S.; Blickley, T.M.; McClellan-Green, P.; Haasch, M.L. Ecotoxicology of carbon-based engineered nanoparticles: Effects of fullerene (C60) on aquatic organisms. Carbon 2006, 44, 1112–1120. [Google Scholar] [CrossRef]
- Servin, A.D.; Castillo-Michel, H.; Hernandez-Viezcas, J.A.; Diaz, B.C.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Synchrotron micro-XRF and micro-XANES confirmation of the uptake and translocation of TiO2 nanoparticles in cucumber (Cucumis sativus) plants. Environ. Sci. Technol. 2012, 46, 7637–7643. [Google Scholar] [CrossRef]
- Larue, C.; Laurette, J.; Herlin-Boime, N.; Khodja, H.; Fayard, B.; Flank, A.M.; Brisset, F.; Carriere, M. Accumulation, translocation and impact of TiO2 nanoparticles in wheat (Triticum aestivum spp.): Influence of diameter and crystal phase. Sci. Total Environ. 2012, 431, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Docter, D.; Westmeier, D.; Markiewicz, M.; Stolte, S.; Knauer, S.K.; Stauber, R.H. The nanoparticle biomolecule corona: Lessons learned—Challenge accepted? Chem. Soc. Rev. 2015, 44, 6094–6121. [Google Scholar] [CrossRef] [PubMed]
- Asli, S.; Neumann, P.M. Colloidal suspensions of clay or titanium dioxide nanoparticles can inhibit leaf growth and transpiration via physical effects on root water transport. Plant Cell Environ. 2009, 32, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Han, J.; Xiao, J.Q.; Jin, Y. Uptake, translocation, and accumulation of manufactured iron oxide nanoparticles by pumpkin plants. J. Environ. Monit. 2008, 10, 713–717. [Google Scholar] [CrossRef] [PubMed]
- López-Moreno, M.L.; De La Rosa, G.; Hernández-Viezcas, J.A.; Castillo-Michel, H.; Botez, C.E.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Evidence of the differential biotransformation and genotoxicity of ZnO and CeO2 nanoparticles on soybean (Glycine max) plants. Environ. Sci. Technol. 2010, 44, 7315–7320. [Google Scholar] [CrossRef] [PubMed]
- Priester, J.H.; Ge, Y.; Mielke, R.E.; Horst, A.M.; Moritz, S.C.; Espinosa, K.; Gelb, J.; Walker, S.L.; Nisbet, R.M.; An, Y.J.; et al. Soybean susceptibility to manufactured nanomaterials with evidence for food quality and soil fertility interruption. Proc. Natl. Acad. Sci. USA 2012, 109, E2451–E2456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernandez-Viezcas, J.A.; Castillo-Michel, H.; Andrews, J.C.; Cotte, M.; Rico, C.; Peralta-Videa, J.R.; Ge, Y.; Priester, J.H.; Holden, P.A.; Gardea-Torresdey, J.L. In Situ synchrotron X-ray fluorescence mapping and speciation of CeO2 and ZnO nanoparticles in soil cultivated soybean (Glycine max). ACS Nano 2013, 7, 1415–1423. [Google Scholar] [CrossRef]
- Peralta-Videa, J.R.; Hernandez-Viezcas, J.A.; Zhao, L.; Diaz, B.C.; Ge, Y.; Priester, J.H.; Holden, P.A.; Gardea-Torresdey, J.L. Cerium dioxide and zinc oxide nanoparticles alter the nutritional value of soil cultivated soybean plants. Plant Physiol. Biochem. 2014, 80, 128–135. [Google Scholar] [CrossRef]
- Ge, Y.; Priester, J.H.; Van De Werfhorst, L.C.; Walker, S.L.; Nisbet, R.M.; An, Y.J.; Schimel, J.P.; Gardea-Torresdey, J.L.; Holden, P.A. Soybean plants modify metal oxide nanoparticle effects on soil bacterial communities. Environ. Sci. Technol. 2014, 48, 13489–13496. [Google Scholar] [CrossRef]
- Priester, J.H.; Moritz, S.C.; Espinosa, K.; Ge, Y.; Wang, Y.; Nisbet, R.M.; Schimel, J.P.; Susana Goggi, A.; Gardea-Torresdey, J.L.; Holden, P.A. Damage assessment for soybean cultivated in soil with either CeO2 or ZnO manufactured nanomaterials. Sci. Total Environ. 2017, 579, 1756–1768. [Google Scholar] [CrossRef]
- Yoon, S.J.; Kwak, J.I.; Lee, W.M.; Holden, P.A.; An, Y.J. Zinc oxide nanoparticles delay soybean development: A standard soil microcosm study. Ecotoxicol. Environ. Saf. 2014, 100, 131–137. [Google Scholar] [CrossRef]
- Hoe, P.T.; Mai, N.C.; Lien, L.Q.; Ban, N.K.; Van Minh, C.; Chau, N.H.; Buu, N.Q.; Hien, D.T.; Van, N.T.; Hien, L.T.T.; et al. Germination responses of soybean seeds under Fe, ZnO, Cu and Co nanoparticle treatments. Int. J. Agric. Biol. 2018, 20, 1562–1568. [Google Scholar] [CrossRef]
- Hashemi, S.; Asrar, Z.; Pourseyedi, S.; Nadernejad, N. Investigation of ZnO nanoparticles on proline, anthocyanin contents and photosynthetic pigments and lipid peroxidation in the soybean. IET Nanobiotechnol. 2019, 13, 66–70. [Google Scholar] [CrossRef]
- Andersen, C.P.; King, G.; Plocher, M.; Storm, M.; Pokhrel, L.R.; Johnson, M.G.; Rygiewicz, P.T. Germination and early plant development of ten plant species exposed to titanium dioxide and cerium oxide nanoparticles. Environ. Toxicol. Chem. 2016, 35, 2223–2229. [Google Scholar] [CrossRef]
- Dan, Y.; Ma, X.; Zhang, W.; Liu, K.; Stephan, C.; Shi, H. Single particle ICP-MS method development for the determination of plant uptake and accumulation of CeO 2 nanoparticles. Anal. Bioanal. Chem. 2016, 408, 5157–5167. [Google Scholar] [CrossRef]
- Cao, Z.; Stowers, C.; Rossi, L.; Zhang, W.; Lombardini, L.; Ma, X. Physiological effects of cerium oxide nanoparticles on the photosynthesis and water use efficiency of soybean (Glycine max (L.) Merr.). Environ. Sci. Nano 2017, 4, 1086–1094. [Google Scholar] [CrossRef]
- Cao, Z.; Rossi, L.; Stowers, C.; Zhang, W.; Lombardini, L.; Ma, X. The impact of cerium oxide nanoparticles on the physiology of soybean (Glycine max (L.) Merr.) under different soil moisture conditions. Environ. Sci. Pollut. Res. 2018, 25, 930–939. [Google Scholar] [CrossRef]
- Rossi, L.; Zhang, W.; Schwab, A.P.; Ma, X. Uptake, Accumulation, and in Planta Distribution of Coexisting Cerium Oxide Nanoparticles and Cadmium in Glycine max (L.) Merr. Environ. Sci. Technol. 2017, 51, 12815–12824. [Google Scholar] [CrossRef]
- Rossi, L.; Sharifan, H.; Zhang, W.; Schwab, A.P.; Ma, X. Mutual effects and: In planta accumulation of co-existing cerium oxide nanoparticles and cadmium in hydroponically grown soybean (Glycine max (L.) Merr.). Environ. Sci. Nano 2018, 5, 150–157. [Google Scholar] [CrossRef]
- Servin, A.D.; De la Torre-Roche, R.; Castillo-Michel, H.; Pagano, L.; Hawthorne, J.; Musante, C.; Pignatello, J.; Uchimiya, M.; White, J.C. Exposure of agricultural crops to nanoparticle CeO2 in biochar-amended soil. Plant Physiol. Biochem. 2017, 110, 147–157. [Google Scholar] [CrossRef] [Green Version]
- Stowers, C.; King, M.; Rossi, L.; Zhang, W.; Arya, A.; Ma, X. Initial Sterilization of Soil Affected Interactions of Cerium Oxide Nanoparticles and Soybean Seedlings (Glycine max (L.) Merr.) in a Greenhouse Study. ACS Sustain. Chem. Eng. 2018, 6, 10307–10314. [Google Scholar] [CrossRef]
- Burke, D.J.; Zhu, S.; Pablico-Lansigan, M.P.; Hewins, C.R.; Samia, A.C.S. Titanium oxide nanoparticle effects on composition of soil microbial communities and plant performance. Biol. Fertil. Soils 2014, 50. [Google Scholar] [CrossRef]
- Burke, D.J.; Pietrasiak, N.; Situ, S.F.; Abenojar, E.C.; Porche, M.; Kraj, P.; Lakliang, Y.; Samia, A.C.S. Iron oxide and titanium dioxide nanoparticle effects on plant performance and root associated microbes. Int. J. Mol. Sci. 2015, 16, 23630–23650. [Google Scholar] [CrossRef]
- Singh, J.; Lee, B.K. Influence of nano-TiO2 particles on the bioaccumulation of Cd in soybean plants (Glycine max): A possible mechanism for the removal of Cd from the contaminated soil. J. Environ. Manag. 2016, 170, 88–96. [Google Scholar] [CrossRef]
- Sheykhbaglou, R.; Sedghi, M.; Tajbakhsh Shishevan, M.; Seyed Sharifi, R. Effects of Nano-Iron Oxide Particles on Agronomic Traits of Soybean. Not. Sci. Biol. 2010, 2, 112–113. [Google Scholar] [CrossRef] [Green Version]
- Sheykhbaglou, R.; Sedghi, M.; Fathi-Achachlouie, B. The effect of ferrous nano-oxide particles on physiological traits and nutritional compounds of soybean (Glycine max L.) seed. An. Acad. Bras. Cienc. 2018, 90, 485–494. [Google Scholar] [CrossRef]
- Alidoust, D.; Isoda, A. Effect of γFe2O3 nanoparticles on photosynthetic characteristic of soybean (Glycine max (L.) Merr.): Foliar spray versus soil amendment. Acta Physiol. Plant. 2013, 35, 3365–3375. [Google Scholar] [CrossRef]
- Ghafariyan, M.H.; Malakouti, M.J.; Dadpour, M.R.; Stroeve, P.; Mahmoudi, M. Effects of magnetite nanoparticles on soybean chlorophyll. Environ. Sci. Technol. 2013, 47, 10645–10652. [Google Scholar] [CrossRef]
- Cunha Lopes, T.L.; de Cássia Siqueira-Soares, R.; Gonçalves de Almeida, G.H.; Romano de Melo, G.S.; Barreto, G.E.; de Oliveira, D.M.; dos Santos, W.D.; Ferrarese-Filho, O.; Marchiosi, R. Lignin-induced growth inhibition in soybean exposed to iron oxide nanoparticles. Chemosphere 2018, 211, 226–234. [Google Scholar] [CrossRef]
- Nair, P.M.G.; Chung, I.M. A Mechanistic Study on the Toxic Effect of Copper Oxide Nanoparticles in Soybean (Glycine max L.) Root Development and Lignification of Root Cells. Biol. Trace Elem. Res. 2014, 162, 342–352. [Google Scholar] [CrossRef]
- Li, J.; Song, Y.; Wu, K.; Tao, Q.; Liang, Y.; Li, T. Effects of Cr2O3 nanoparticles on the chlorophyll fluorescence and chloroplast ultrastructure of soybean (Glycine max). Environ. Sci. Pollut. Res. 2018, 25, 19446–19457. [Google Scholar] [CrossRef] [PubMed]
- De La Torre-Roche, R.; Hawthorne, J.; Musante, C.; Xing, B.; Newman, L.A.; Ma, X.; White, J.C. Impact of Ag nanoparticle exposure on p,p′ -DDE bioaccumulation by cucurbita pepo (Zucchini) and Glycine max (Soybean). Environ. Sci. Technol. 2013, 47, 718–725. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, G.; Sakata, K.; Hossain, Z.; Komatsu, S. Proteomic study on the effects of silver nanoparticles on soybean under flooding stress. J. Proteom. 2015, 122, 100–118. [Google Scholar] [CrossRef] [PubMed]
- Guilger, M.; Pasquoto-Stigliani, T.; Bilesky-Jose, N.; Grillo, R.; Abhilash, P.C.; Fraceto, L.F.; De Lima, R. Biogenic silver nanoparticles based on trichoderma harzianum: Synthesis, characterization, toxicity evaluation and biological activity. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Galazzi, R.M.; Lopes Júnior, C.A.; de Lima, T.B.; Gozzo, F.C.; Arruda, M.A.Z. Evaluation of some effects on plant metabolism through proteins and enzymes in transgenic and non-transgenic soybeans after cultivation with silver nanoparticles. J. Proteom. 2019, 191, 88–106. [Google Scholar] [CrossRef]
- Begum, P.; Ikhtiari, R.; Fugetsu, B.; Matsuoka, M.; Akasaka, T.; Watari, F. Phytotoxicity of multi-walled carbon nanotubes assessed by selected plant species in the seedling stage. Appl. Surf. Sci. 2012, 262, 120–124. [Google Scholar] [CrossRef]
- Lahiani, M.H.; Dervishi, E.; Chen, J.; Nima, Z.; Gaume, A.; Biris, A.S.; Khodakovskaya, M.V. Impact of carbon nanotube exposure to seeds of valuable crops. ACS Appl. Mater. Interfaces 2013, 5, 7965–7973. [Google Scholar] [CrossRef]
- Lahiani, M.H.; Nima, Z.A.; Villagarcia, H.; Biris, A.S.; Khodakovskaya, M.V. Assessment of Effects of the Long-Term Exposure of Agricultural Crops to Carbon Nanotubes. J. Agric. Food Chem. 2018, 66, 6654–6662. [Google Scholar] [CrossRef]
- Zaytseva, O.; Wang, Z.; Neumann, G. Phytotoxicity of carbon nanotubes in soybean as determined by interactions with micronutrients. J. Nanopart. Res. 2017, 19. [Google Scholar] [CrossRef]
- Zhai, G.; Gutowski, S.M.; Walters, K.S.; Yan, B.; Schnoor, J.L. Charge, Size, and Cellular Selectivity for Multiwall Carbon Nanotubes by Maize and Soybean. Environ. Sci. Technol. 2015, 49, 7380–7390. [Google Scholar] [CrossRef]
- De La Torre-Roche, R.; Hawthorne, J.; Deng, Y.; Xing, B.; Cai, W.; Newman, L.A.; Wang, Q.; Ma, X.; Hamdi, H.; White, J.C. Multiwalled carbon nanotubes and C60 fullerenes differentially impact the accumulation of weathered pesticides in four agricultural plants. Environ. Sci. Technol. 2013, 47, 12539–12547. [Google Scholar] [CrossRef] [PubMed]
- De La Torre-Roche, R.; Hawthorne, J.; Deng, Y.; Xing, B.; Cai, W.; Newman, L.A.; Wang, C.; Ma, X.; White, J.C. Fullerene-enhanced accumulation of p, p’-DDE in agricultural crop species. Environ. Sci. Technol. 2012, 46, 9315–9323. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Zhang, S.; Luo, L.; Han, W.; Zhang, J.; Yang, K.; Christie, P. Dissolution and microstructural transformation of ZnO nanoparticles under the influence of phosphate. Environ. Sci. Technol. 2012, 46, 7215–7221. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Nowack, B. Dynamic probabilistic material flow analysis of nano-SiO2, nano iron oxides, nano-CeO2, nano-Al2O3, and quantum dots in seven European regions. Environ. Pollut. 2018, 235, 589–601. [Google Scholar] [CrossRef] [PubMed]
- Shute, T.; Macfie, S.M. Cadmium and zinc accumulation in soybean: A threat to food safety? Sci. Total Environ. 2006, 371, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Azam, A.; Ahmed, A.S.; Oves, M.; Khan, M.S.; Habib, S.S.; Memic, A. Antimicrobial activity of metal oxide nanoparticles against Gram-positive and Gram-negative bacteria: A comparative study. Int. J. Nanomed. 2012, 7, 6003–6009. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Kovochich, M.; Liong, M.; Mädler, L.; Gilbert, B.; Shi, H.; Yeh, J.I.; Zink, J.I.; Nel, A.E. Comparison of the mechanism of toxicity of zinc oxide and cerium oxide nanoparticles based on dissolution and oxidative stress properties. ACS Nano 2008, 2, 2121–2134. [Google Scholar] [CrossRef] [PubMed]
- Reddy Pullagurala, V.L.; Adisa, I.O.; Rawat, S.; Kim, B.; Barrios, A.C.; Medina-Velo, I.A.; Hernandez-Viezcas, J.A.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Finding the conditions for the beneficial use of ZnO nanoparticles towards plants-A review. Environ. Pollut. 2018, 241, 1175–1181. [Google Scholar] [CrossRef] [PubMed]
- García-Gómez, C.; Obrador, A.; González, D.; Babín, M.; Fernández, M.D. Comparative study of the phytotoxicity of ZnO nanoparticles and Zn accumulation in nine crops grown in a calcareous soil and an acidic soil. Sci. Total Environ. 2018, 644, 770–780. [Google Scholar] [CrossRef] [PubMed]
- Tangaa, S.R.; Selck, H.; Winther-Nielsen, M.; Khan, F.R. Trophic transfer of metal-based nanoparticles in aquatic environments: A review and recommendations for future research focus. Environ. Sci. Nano 2016, 3, 966–981. [Google Scholar] [CrossRef]
- Skjolding, L.M.; Winther-Nielsen, M.; Baun, A. Trophic transfer of differently functionalized zinc oxide nanoparticles from crustaceans (Daphnia magna) to zebrafish (Danio rerio). Aquat. Toxicol. 2014, 157, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Karimi, M.; Sadeghi, R.; Kokini, J. Human exposure to nanoparticles through trophic transfer and the biosafety concerns that nanoparticle-contaminated foods pose to consumers. Trends Food Sci. Technol. 2018, 75, 129–145. [Google Scholar] [CrossRef]
- Ma, X.; Wang, Q.; Rossi, L.; Zhang, W. Cerium Oxide Nanoparticles and Bulk Cerium Oxide Leading to Different Physiological and Biochemical Responses in Brassica rapa. Environ. Sci. Technol. 2016, 50, 6793–6802. [Google Scholar] [CrossRef] [PubMed]
- Hawthorne, J.; De La Torre Roche, R.; Xing, B.; Newman, L.A.; Ma, X.; Majumdar, S.; Gardea-Torresdey, J.; White, J.C. Particle-size dependent accumulation and trophic transfer of cerium oxide through a terrestrial food chain. Environ. Sci. Technol. 2014, 48, 13102–13109. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Yao, Y.; Yang, J.; He, X.; Ding, Y.; Zhang, P.; Zhang, J.; Wang, G.; Xie, C.; Luo, W.; et al. Trophic Transfer and Transformation of CeO2 Nanoparticles along a Terrestrial Food Chain: Influence of Exposure Routes. Environ. Sci. Technol. 2018, 52, 7921–7927. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, S.; Trujillo-Reyes, J.; Hernandez-Viezcas, J.A.; White, J.C.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Cerium Biomagnification in a Terrestrial Food Chain: Influence of Particle Size and Growth Stage. Environ. Sci. Technol. 2016, 50, 6782–6792. [Google Scholar] [CrossRef] [PubMed]
- Peralta-Videa, J.R.; Zhao, L.; Lopez-Moreno, M.L.; de la Rosa, G.; Hong, J.; Gardea-Torresdey, J.L. Nanomaterials and the environment: A review for the biennium 2008-2010. J. Hazard. Mater. 2011, 186, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Aslani, F.; Bagheri, S.; Muhd Julkapli, N.; Juraimi, A.S.; Hashemi, F.S.G.; Baghdadi, A. Effects of engineered nanomaterials on plants growth: An overview. Sci. World J. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Chichiriccò, G.; Poma, A. Penetration and toxicity of nanomaterials in higher plants. Nanomaterials 2015, 5, 851–873. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Interaction of titanium dioxide nanoparticles with soil components and plants: Current knowledge and future research needs-a critical review. Environ. Sci. Nano 2018, 5, 257–278. [Google Scholar] [CrossRef]
- Servin, A.D.; Morales, M.I.; Castillo-Michel, H.; Hernandez-Viezcas, J.A.; Munoz, B.; Zhao, L.; Nunez, J.E.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Synchrotron verification of TiO2 accumulation in cucumber fruit: A possible pathway of TiO2 nanoparticle transfer from soil into the food chain. Environ. Sci. Technol. 2013, 47, 11592–11598. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ma, X.; Zhang, W.; Pei, H.; Chen, Y. The impact of cerium oxide nanoparticles on tomato (Solanum lycopersicum L.) and its implications for food safety. Metallomics 2012, 4, 1105–1112. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhu, Y.; Yang, K.; Zhu, L.; Lin, D. Nanoparticle TiO 2 size and rutile content impact bioconcentration and biomagnification from algae to daphnia. Environ. Pollut. 2019. [Google Scholar] [CrossRef]
- Hou, J.; Wang, L.; Wang, C.; Zhang, S.; Liu, H.; Li, S.; Wang, X. Toxicity and mechanisms of action of titanium dioxide nanoparticles in living organisms. J. Environ. Sci. 2019, 75, 40–53. [Google Scholar] [CrossRef] [PubMed]
- Ruttkay-Nedecky, B.; Krystofova, O.; Nejdl, L.; Adam, V. Nanoparticles based on essential metals and their phytotoxicity. J. Nanobiotechnol. 2017, 15. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yue, L.; Wang, Z.; Xing, B. Processes and mechanisms of photosynthesis augmented by engineered nanomaterials. Environ. Chem. 2019. [Google Scholar] [CrossRef]
- Lin, C.C.; Chen, L.M.; Liu, Z.H. Rapid effect of copper on lignin biosynthesis in soybean roots. Plant Sci. 2005, 168, 855–861. [Google Scholar] [CrossRef]
- Atha, D.H.; Wang, H.; Petersen, E.J.; Cleveland, D.; Holbrook, R.D.; Jaruga, P.; Dizdaroglu, M.; Xing, B.; Nelson, B.C. Copper oxide nanoparticle mediated DNA damage in terrestrial plant models. Environ. Sci. Technol. 2012, 46, 1819–1827. [Google Scholar] [CrossRef]
- Moreau, J.W.; Weber, P.K.; Martin, M.C.; Gilbert, B.; Hutcheon, I.D.; Banfield, J.F. Extracellular proteins limit the dispersal of biogenic nanoparticles. Science 2007, 316, 1600–1603. [Google Scholar] [CrossRef]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramírez, J.T.; Yacaman, M.J. The bactericidal effect of silver nanoparticles. Nanotechnology 2005, 16, 2346–2353. [Google Scholar] [CrossRef] [Green Version]
- Rai, M.; Yadav, A.; Gade, A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.K.; Yngard, R.A.; Lin, Y. Silver nanoparticles: Green synthesis and their antimicrobial activities. Adv. Colloid Interface Sci. 2009, 145, 83–96. [Google Scholar] [CrossRef]
- Bjorkland, R.; Tobias, D.A.; Petersen, E.J. Increasing evidence indicates low bioaccumulation of carbon nanotubes. Environ. Sci. Nano 2017, 4, 747–766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verma, S.K.; Das, A.K.; Gantait, S.; Kumar, V.; Gurel, E. Applications of carbon nanomaterials in the plant system: A perspective view on the pros and cons. Sci. Total Environ. 2019, 667, 485–499. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Petersen, E.J.; Cornelis, G.; Wang, X.; Guo, X.; Tao, S.; Xing, B. Retention of 14C-labeled multiwall carbon nanotubes by humic acid and polymers: Roles of macromolecule properties. Carbon 2016, 99, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Sturz, A.V.; Christie, B.R.; Nowak, J. Bacterial endophytes: Potential role in developing sustainable systems of crop production. Crit. Rev. Plant Sci. 2000, 19, 1–30. [Google Scholar] [CrossRef]
- Hayat, R.; Ali, S.; Amara, U.; Khalid, R.; Ahmed, I. Soil beneficial bacteria and their role in plant growth promotion: A review. Ann. Microbiol. 2010, 60, 579–598. [Google Scholar] [CrossRef]
- Compant, S.; Duffy, B.; Nowak, J.; Clément, C.; Barka, E.A. Use of plant growth-promoting bacteria for biocontrol of plant diseases: Principles, mechanisms of action, and future prospects. Appl. Environ. Microbiol. 2005, 71, 4951–4959. [Google Scholar] [CrossRef]
- Rodríguez, H.; Fraga, R. Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol. Adv. 1999, 17, 319–339. [Google Scholar] [CrossRef]
- Richardson, A.E.; Barea, J.M.; McNeill, A.M.; Prigent-Combaret, C. Acquisition of phosphorus and nitrogen in the rhizosphere and plant growth promotion by microorganisms. Plant Soil 2009, 321, 305–339. [Google Scholar] [CrossRef]
- Vance, C.P. Symbiotic nitrogen fixation and phosphorus acquisition. Plant nutrition in a world of declining renewable resources. Plant Physiol. 2001, 127, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Vessey, J.K. Plant growth promoting rhizobacteria as biofertilizers. Plant Soil 2003, 255, 571–586. [Google Scholar] [CrossRef]
- Johansson, J.F.; Paul, L.R.; Finlay, R.D. Microbial interactions in the mycorrhizosphere and their significance for sustainable agriculture. FEMS Microbiol. Ecol. 2004, 48, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Tilman, D.; Cassman, K.G.; Matson, P.A.; Naylor, R.; Polasky, S. Agricultural sustainability and intensive production practices. Nature 2002, 418, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Bonfante, P.; Anca, I.A. Plants, mycorrhizal fungi, and bacteria: A network of interactions. Annu. Rev. Microbiol. 2009, 63, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Schimel, J.P.; Holdena, P.A. Identification of soil bacteria susceptible to TiO2 and ZnO nanoparticles. Appl. Environ. Microbiol. 2012, 78, 6749–6758. [Google Scholar] [CrossRef] [PubMed]
- Stoimenov, P.K.; Klinger, R.L.; Marchin, G.L.; Klabunde, K.J. Metal oxide nanoparticles as bactericidal agents. Langmuir 2002, 18, 6679–6686. [Google Scholar] [CrossRef]
- Frenk, S.; Ben-Moshe, T.; Dror, I.; Berkowitz, B.; Minz, D. Effect of metal oxide nanoparticles on microbial community structure and function in two different soil types. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Rousk, J.; Ackermann, K.; Curling, S.F.; Jones, D.L. Comparative toxicity of nanoparticulate CuO and ZnO to soil bacterial communities. PLoS ONE 2012, 7. [Google Scholar] [CrossRef]
- Khodakovskaya, M.V.; Kim, B.S.; Kim, J.N.; Alimohammadi, M.; Dervishi, E.; Mustafa, T.; Cernigla, C.E. Carbon nanotubes as plant growth regulators: Effects on tomato growth, reproductive system, and soil microbial community. Small 2013, 9, 115–123. [Google Scholar] [CrossRef]
- Kerfahi, D.; Tripathi, B.M.; Singh, D.; Kim, H.; Lee, S.; Lee, J.; Adams, J.M. Effects of functionalized and raw multi-walled carbon nanotubes on soil bacterial community composition. PLoS ONE 2015, 10, e0123042. [Google Scholar] [CrossRef] [PubMed]
- Liné, C.; Larue, C.; Flahaut, E. Carbon nanotubes: Impacts and behaviour in the terrestrial ecosystem—A review. Carbon 2017, 123, 767–785. [Google Scholar] [CrossRef]
- Deng, Y.; Petersen, E.J.; Challis, K.E.; Rabb, S.A.; Holbrook, R.D.; Ranville, J.F.; Nelson, B.C.; Xing, B. Multiple Method Analysis of TiO2 Nanoparticle Uptake in Rice (Oryza sativa L.) Plants. Environ. Sci. Technol. 2017, 51, 10615–10623. [Google Scholar] [CrossRef] [PubMed]
| NM | Form; Size | Conc. | Growth Conditions | Effects | Ref. |
|---|---|---|---|---|---|
| ZnO | NPs hexagonal; 8 nm | 0–4 g/L | Germination tests | ZnO NPs dissolved as Zn2+ had no effect on germination. | [86] |
| NPs powder in soil; ~10 nm | 0.5–5 g/kg soil | Garden pots with 2.4 kg of soil | Zn bioaccumulated in all tissues and especially in the leaves. ZnO slightly stimulated plant growth. | [87] | |
| NPs powder in soil; ~10 nm | 0.5 g/kg soil | Same as in [87] | ZnO NPS were dissolved and accumulated in the seeds in a form resembling Zn citrate. | [88] | |
| NPs powder in soil; ~10 nm | 0–0.5 g/kg soil | Same as in [87] | ZnO impacted the accumulation of essential elements (K, Mg). Zn accumulation was significantly increased in all plant organs. | [89] | |
| NPs powder in soil; ~10 nm | 0–0.5 g/kg soil | Same as in [87] | ZnO significantly altered soil microbiota both in unplanted and planted soils; the presence of plants reduced effects on soil bacteria. | [90] | |
| NPs powder in soil; ~10 nm | 0–0.5 g/kg soil | Same as in [87] | ZnO NPs decreased chlorophyll concentrations and had some genotoxic effects at the highest concentration (0.5 g/kg soil). | [91] | |
| NPs; <50 nm | 0–0.5 g/kg soil | Soil (65 d) | ZnO NPs reduced roots and shoots (area and volume). Plants treated with high conc. (0.5 g/kg) had no seeds. | [92] | |
| NPs; 40–60 nm | 0.025–0.5 g/L | Germination tests | ZnO promoted the growth of primary roots and supported the development of first trifoliate leaves earlier than the control. | [93] | |
| NPs; 41 nm | 0–400 ppm | Hoagland medium (21 d) | ZnO NPs reduced chlorophyll and carotenoids, and increased anthocyanin, malondialdehyde, H2O2 and phenylalanine ammonia-lyase activity. | [94] | |
| CeO2 | NPs cubic; 7 nm | 0–4 g/L | Germination tests | CeO2 NPs remained intact in roots and had genotoxic effects. | [86] |
| NPs powder in soil; ~8 nm | 1–10 g/kg soil | Garden pots with 2.4 kg of soil | CeO2 NPs decreased plant growth and yield, stopped nitrogen fixation at high conc.; no effect on seed production. | [87] | |
| NPs powder in soil; ~8 nm | 1 g/kg soil | Same as in [87] | CeO2 NPs were in the root nodule including root epidermis and pods. NPs were also shown to potentially transfer to next plant generation via the reproductive organs. | [88] | |
| NPs powder in soil; ~8 nm | 0–1 g/kg soil | Same as in [87] | CeO2 interfered with the uptake of elements involved in nitrogen metabolism and photosynthesis (Ca, Mg, P, K, and S). | [89] | |
| NPs powder in soil; ~8 nm | 0–1 g/kg soil | Same as in [87] | CeO2 had no effect in unplanted soils; the presence of plants promoted the altering of bacterial communities in planted soils. | [90] | |
| NPs powder in soil; ~8 nm | 0–1 g/kg soil | Same as in [87] | CeO2 NPs caused signs of oxidative damage in leaves with consequences to the entire plant. | [91] | |
| NPs; ~25 nm | 0–1 g/kg soil | Germination tests | CeO2 NPs did not significantly affect germination and root. | [95] | |
| NPs; 30–50 nm | 7 mg/L | Hydroponic growth | Dissolved Ce was found for the first time in plant seedling shoots exposed to NPs hydroponically. | [96] | |
| NPs; 10–30 nm | 0–0.5 g/kg soil | Greenhouse (3 wk) | At 0.1 g/kg, CeO2 NPs stimulated plant growth and photosynthesis (+54%). Photosynthesis rate decreased at 0.5 g/kg (~36%). | [97] | |
| NPs; 10–30 nm | 0.1 g/kg soil | Greenhouse (3 wk) | CeO2 NPs was dependent on soil moisture, with positive effects (increased fresh biomass) above 70% moisture content. | [98] | |
| NPs; ~42 nm | 0–0.5 g/kg soil | Greenhouse (30 d) | CeO2 NPs enhanced the plant light energy use efficiency by photosystem II. The presence of Cd significantly increased Ce accumulation in plant tissues. | [99] | |
| NPs; ~42 nm | 0.1 g/L (+Cd) | Hydroponic growth | CeO2 NPs and Cd interacted significantly, affecting their accumulation: CeO2 reduced the translocation of Cd from roots to shoots by 70%; Cd lowered the conc. of Ce in roots by 45% but increased it in shoots by 60%. | [100] | |
| NPs; 20–200 nm | 0–2 g/kg soil | Greenhouse | Root biomass was reduced by 60% and by 81%, while shoot biomass increased by 65% and 92% at 0.5 and 2 g/kg CeO2. | [101] | |
| NPs; ~10 nm | 0–0.5 g/kg soil | Greenhouse (27 d) | Initial soil sterilization affected interaction of CeO2 NPs with plants and CeO2 accumulation. The net photosynthesis rate was higher at 0.1 g/kg but lower at 0.5 g/kg, as compared to the unsterilized soil. | [102] | |
| TiO2 | NPs; ~25 nm | 0–1 g/kg soil | Germination tests | TiO2 NPs showed a marginal effect on germination. | [95] |
| NPs; <60 nm. | <0.2 g/kg soil | Greenhouse (6 wk) | TiO2 was found in roots; no effects on plant growth, nutrient content, or the composition of root-associated microbiota. | [103] | |
| NPs; 22–25 nm | 0–0.2 g/kg soil | Greenhouse (6 wk) | TiO2 significantly reduced plant growth. | [104] | |
| NPs; <100 nm | 0.1–0.3 g/kg soil (+Cd) | Plant growth chamber | TiO2 NPs restricted Cd-induce toxicity by increasing the photosynthetic rate and growth parameters of the plants. | [105] | |
| Fe2O3 | Sprayed nano-Fe2O3 | 0.25–1 g/L | Clay soil (pH 7.6) | Fe2O3 NPs enhanced pod and grain biomass by 48%. | [106] |
| Fe2O3 NPs at 0.75 g/L enhanced protein (33.8%) and lipid (25.4%) content vs control; increased fatty acids, minerals and chlorophyll. | [107] | ||||
| NPs; 6 nm. Foliar vs soil treatment. | 0.05–2 g/L | Wagner pots | Fe2O3 NPs produced positive effects on root elongation, shoot weight, leaf area, and soil plant analysis development values. | [108] | |
| Superparamagnetic NPs; 9 nm. | 0.2–2 g/L | Hydroponic growth | NPs significantly enhanced the chlorophyll content in subapical leaves, with no trace of toxicity. | [109] | |
| NPs; <50 nm | 0.2–1.5 g/L | Hoagland nutrient solution | Fe2O3 NPs increased the lignin content of roots and stems, followed by the stiffening of the cell wall and growth inhibition. | [110] | |
| Fe3O4 | NPs; 18 nm | 0–0.2 g/kg soil | Greenhouse (6 wk). | Fe3O4 NPs increased plant growth and leaf C but reduced P content. Negatively charged Fe3O4 NPs increased leaf P content, and decreased root colonization of rhizobia, vs positive NPs. | [104] |
| CuO | NPs; 50 nm | 0–0.5 g/L | Murashige and Skoog medium | CuO NPs increased lignification of root cells via improving root peroxidases activity; and reduced the shoot growth, weight, and total chlorophyll content. | [111] |
| Cr2O3 | NPs; 50 nm | 0.01–0.5 g/L | Suspensions with NPs | Cr2O3 NPs inhibited plant growth by damaging photosynthesis, destroying the chloroplast thylakoid structure and inhibiting electron acceptors. | [112] |
| Ag | NPs or bulk; 68–91 nm. | 0.5–2 g/L | 125 mL jars of vermiculite | Ag NP-exposed plants had 1.9−2.2 x higher Ag content and transport to shoot tissues. Ag altered DDE (a co-contaminant) accumulation and translocation. | [113] |
| NPs; 2–80 nm | 0.2–20 ppm | Soybean exposed to flooding stress | Ag NPs positively influenced the growth performance of soybeans under flooding stress. | [114] | |
| NPs; ~60 nm | 0.15–0.31*1012 NPs/mL | Standard germination tests | Ag NPs did not present any negative effects on the germination and growth. | [115] | |
| NPs; ~60 nm | 50 mg/kg vs AgNO3 | Greenhouse (21 d). | Ag NPs decreased the mass production of non-transgenic plants by 25% by generating oxidative stress. | [116] | |
| C | MWCNTs, od 13 nm, id ∼4 nm, l >1 μm | 0–2 g/L | Hydroponics (15 d) | MWCNTs induced very little or no effect on root and shoot growth, cell death, and electrolyte leakage at the seedling stage. | [117] |
| MWCNTs, od 15–40 nm; l - several μm | 0–200 μg/mL | Agar medium | MWCNTs accelerated seed germination, increased roots, and showed no negative effects on plant development. | [118] | |
| 50 μg/mL | Hydroponics (20 wk) | MWCNTs decreased the roots weight; no influence on shoot reduction or the development of other organs was observed; CNTs enhanced photosynthesis. | [119] | ||
| MWCNTs; od 20–70 nm, id 5–10 nm, l >2 μm | 1 g/L | Suspensions with CNTs (36 h) | MWCNTs treatment reduced the total root length by 29%, induced oxidative stress. | [120] | |
| MWCNTs, d 20−30 nm, l - 0.05−2.0 μm | 10–50.0 mg/L | Hydroponics (18 d) | MWCNTs inhibited growth and transpiration; increased dry weight biomass (@20 mg/L); the effect was influenced by the MWCNTs charge. | [121] | |
| MWCNTs, hd 3.5–3.9 μm (0.5–1 g/L) and 17.7 μm (5 g/L) | 0–5 g/kg soil | Soil (28 d) | MWCNTs induced phytotoxicity; reduced biomass (19.2–26.9%), reduced net growth (29.8–31.9%). Co-exposure with contaminants decreased chlordane and DDx accumulation. | [122] | |
| C60 (fullerenes), 1450-1900 nm | 0–5 g/kg soil | Soil (28 d) | C60 induced phytotoxicity; reduced biomass and net growth by 25.0–40.4% and 27.7–42.6%. Co-exposure with contaminants increased chlordane uptake. | [122] | |
| 40 mg/80 mL | Vermiculite (3 wk) | Fullerene treatment decreased accumulation of p,p′-DDE contaminant in shoots (48%); root and total plant p,p’-DDE increased. | [123] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coman, V.; Oprea, I.; Leopold, L.F.; Vodnar, D.C.; Coman, C. Soybean Interaction with Engineered Nanomaterials: A Literature Review of Recent Data. Nanomaterials 2019, 9, 1248. https://doi.org/10.3390/nano9091248
Coman V, Oprea I, Leopold LF, Vodnar DC, Coman C. Soybean Interaction with Engineered Nanomaterials: A Literature Review of Recent Data. Nanomaterials. 2019; 9(9):1248. https://doi.org/10.3390/nano9091248
Chicago/Turabian StyleComan, Vasile, Ioana Oprea, Loredana Florina Leopold, Dan Cristian Vodnar, and Cristina Coman. 2019. "Soybean Interaction with Engineered Nanomaterials: A Literature Review of Recent Data" Nanomaterials 9, no. 9: 1248. https://doi.org/10.3390/nano9091248







