SERS-Active Cu Nanoparticles on Carbon Nitride Support Fabricated Using Pulsed Laser Ablation
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Formation and Characterization of Cu/g-CN Materials Produced by Pulsed Laser Ablation in Acetonitrile
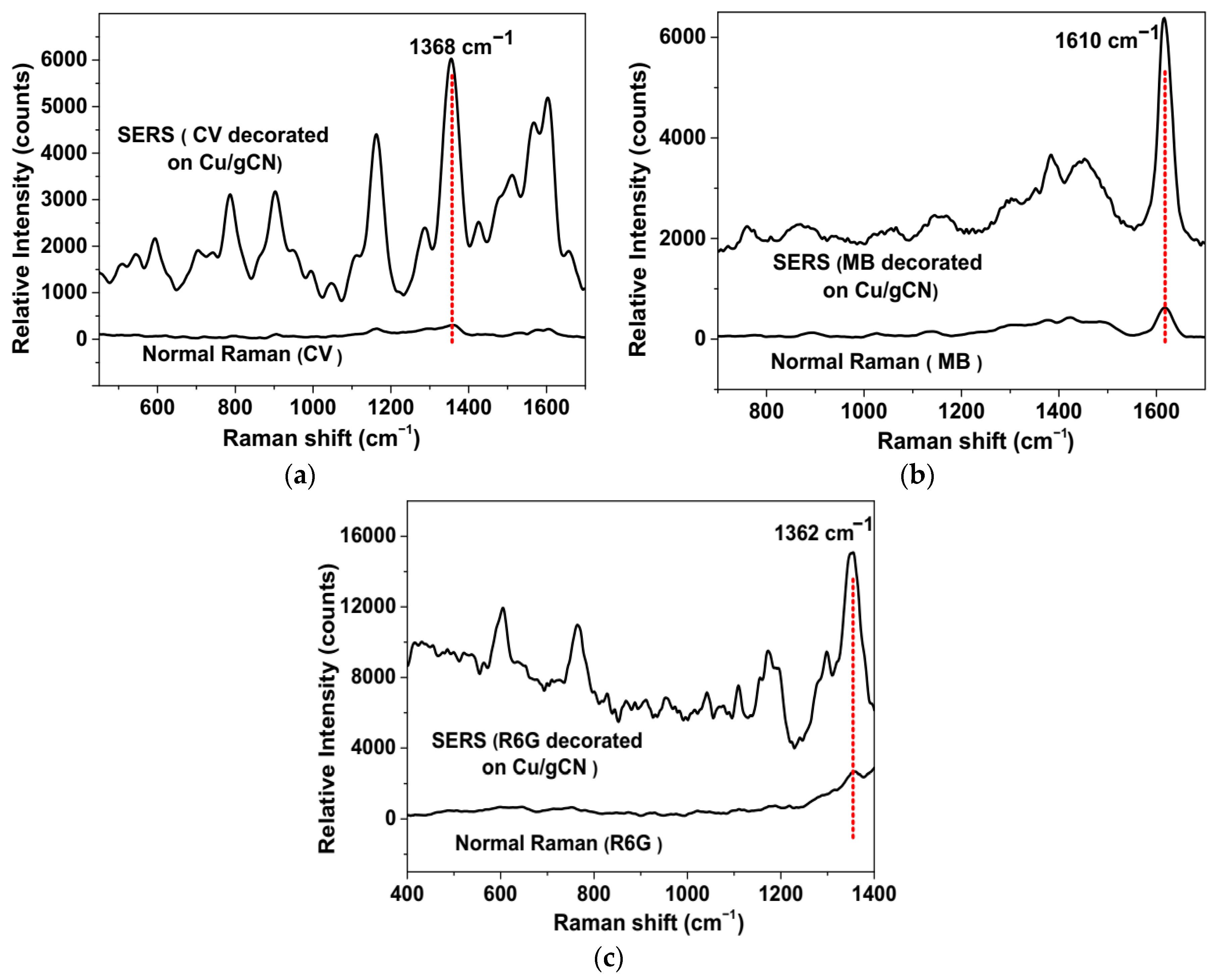
3.2. SERS/SERRS Activity of Analytes on Cu/gCN Hybrid Support Produced Using Pulsed Laser Deposition
3.3. Estimation of SERS/SERRS Enhancement Factors
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bell, S.E.J.; Sirimuthu, N.M.S. Surface-Enhanced Raman Spectroscopy (SERS) for Sub-Micromolar Detection of DNA/RNA Mononucleotides. J. Am. Chem. Soc. 2006, 128, 15580–15581. [Google Scholar] [CrossRef] [PubMed]
- Ambrosio, R.C.; Gewirth, A.A. Characterization of Water Structure on Silver Electrode Surfaces by SERS with Two-Dimensional Correlation Spectroscopy. Anal. Chem. 2010, 82, 1305–1310. [Google Scholar] [CrossRef] [PubMed]
- Roguska, A.; Kudelski, A.; Pisarek, M.; Lewandowska, M.; Kurzydłowski, K.; Janik-Czachor, M. In situ spectroelectrochemical surface-enhanced Raman scattering (SERS) investigations on composite Ag/TiO2-nanotubes/Ti substrates. Surf. Sci. 2009, 603, 2820–2824. [Google Scholar] [CrossRef]
- Qiu, C.; Zhang, L.; Wang, H.; Jiang, C. Surface-Enhanced Raman Scattering on Hierarchical Porous Cuprous Oxide Nanostructures in Nanoshell and Thin-Film Geometries. J. Phys. Chem. Lett. 2012, 3, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Fateixa, S.; Nogueira, H.I.S.; Trindade, T.; Caria, S.F.; Nogueira, H.S. Hybrid nanostructures for SERS: Materials development and chemical detection. Phys. Chem. Chem. Phys. 2015, 17, 21046–21071. [Google Scholar] [CrossRef] [PubMed]
- Han, X.X.; Ji, W.; Zhao, B.; Ozaki, Y. Semiconductor-enhanced Raman scattering: Active nanomaterials and applications. Nanoscale 2017, 9, 4847–4861. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, J.R.; Birke, R.L. A Unified Approach to Surface-Enhanced Raman Spectroscopy. J. Phys. Chem. C 2008, 112, 5605–5617. [Google Scholar] [CrossRef]
- Kneipp, K.; Wang, Y.; Kneipp, H.; Perelman, L.T.; Itzkan, I.; Dasari, R.R.; Feld, M.S. Single Molecule Detection Using Surface-Enhanced Raman Scattering (SERS). Phys. Rev. Lett. 1997, 78, 1667–1670. [Google Scholar] [CrossRef] [Green Version]
- Nie, S.; Emory, S.R. Probing single molecules and single nanoparticles by SERS. Science 1997, 275, 1102–1106. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Bjerneld, E.J.; Käll, M.; Börjesson, L. Spectroscopy of Single Hemoglobin Molecules by Surface Enhanced Raman Scattering. Phys. Rev. Lett. 1999, 83, 4357–4360. [Google Scholar] [CrossRef] [Green Version]
- Schlücker, S. Surface-Enhanced Raman Spectroscopy: Concepts and Chemical Applications. Angew. Chem. Int. Ed. 2014, 53, 4756–4795. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Shi, W.; She, G.; Mu, L. Using Si and Ge Nanostructures as Substrates for Surface-Enhanced Raman Scattering Based on Photoinduced Charge Transfer Mechanism. J. Am. Chem. Soc. 2011, 133, 16518–16523. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Zhao, B.; Ozaki, Y. Semiconductor materials in analytical applications of surface-enhanced Raman scattering. J. Raman Spectrosc. 2016, 47, 51–58. [Google Scholar] [CrossRef]
- Musumeci, A.; Gosztola, D.; Schiller, T.; Dimitrijevic, N.M.; Mujica, V.; Martin, D.; Rajh, T. SERS of Semiconducting Nanoparticles (TiO2Hybrid Composites). J. Am. Chem. Soc. 2009, 131, 6040–6041. [Google Scholar] [CrossRef] [PubMed]
- Teguh, J.S.; Xing, B.; Yeow, E.K.L.; Liu, F.; Xing, P.D.B.; Yeow, P.D.E.K.L. Surface-Enhanced Raman Scattering (SERS) of Nitrothiophenol Isomers Chemisorbed on TiO2. Chem. Asian J. 2012, 7, 975–981. [Google Scholar] [CrossRef] [PubMed]
- Dendisova-Vyskovska, M.; Prokopec, V.; Člupek, M.; Matějka, P. Comparison of SERS effectiveness of copper substrates prepared by different methods: What are the values of enhancement factors? J. Raman Spectrosc. 2012, 43, 181–186. [Google Scholar] [CrossRef]
- McNay, G.; Eustace, D.; Smith, W.E.; Faulds, K.; Graham, D. Surface-enhanced Raman scattering (SERS) and resonance Raman scattering (SERRS): A review of applications. Appl. Spectrosc. 2011, 65, 825–837. [Google Scholar] [CrossRef] [PubMed]
- Bell, S.E.J.; McCourt, M.R. SERS enhancement by aggregated Au colloids: Effect of particle size. Phys. Chem. Chem. Phys. 2009, 11, 7455–7462. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.; Kim, S.K.; Lee, S.; Kim, J.H.; Woo, D. Ag nanodot array as a platform for surface-enhanced Raman scattering. J. Nanophotonics 2013, 7, 073798. [Google Scholar] [CrossRef] [Green Version]
- Jung, G.B.; Bae, Y.M.; Lee, Y.J.; Ryu, S.H.; Park, H.-K. Nanoplasmonic Au nanodot arrays as an SERS substrate for biomedical applications. Appl. Surf. Sci. 2013, 282, 161–164. [Google Scholar] [CrossRef]
- Ameer, F.S.; Pittman, C.U.; Zhang, D. Quantification of Resonance Raman Enhancement Factors for Rhodamine 6G (R6G) in Water and on Gold and Silver Nanoparticles: Implications for Single-Molecule R6G SERS. J. Phys. Chem. C 2013, 117, 27096–27104. [Google Scholar] [CrossRef]
- Roguska, A.; Kudelski, A.; Pisarek, M.; Opara, M.; Janik-Czachor, M. Surface-enhanced Raman scattering (SERS) activity of Ag, Au and Cu nanoclusters on TiO2 -nanotubes/Ti substrate. Appl. Surf. Sci. 2011, 257, 8182–8189. [Google Scholar] [CrossRef]
- Ding, Q.; Hang, L.; Ma, L. Controlled synthesis of Cu nanoparticle arrays with surface enhanced Raman scattering effect performance. RSC Adv. 2018, 8, 1753–1757. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.B.; Vu, T.K.T.; Nguyen, Q.D.; Nguyen, T.D.; Nguyen, T.A.; Trinh, T.H. Preparation of metal nanoparticles for surface enhanced Raman scattering by laser ablation method. Adv. Nat. Sci. Nanosci. Nanotechnol. 2012, 3, 025016. [Google Scholar] [CrossRef]
- Muniz-Miranda, M.; Gellini, C.; Giorgetti, E. Surface-Enhanced Raman Scattering from Copper Nanoparticles Obtained by Laser Ablation. J. Phys. Chem. C 2011, 115, 5021–5027. [Google Scholar] [CrossRef]
- Khan, A.; Rashid, A.; Younas, R.; Chong, R. A chemical reduction approach to the synthesis of copper nanoparticles. Int. Nano Lett. 2016, 6, 21–26. [Google Scholar] [CrossRef]
- Soomro, R.A.; Sirajuddin, S.T.H.S.; Memon, N.; Shah, M.R.; Kalwar, N.H.; Hallam, K.R.; Shah, A. Synthesis of Air Stable Copper Nanoparticles and Their Use in Catalysis. Adv. Mater. Lett. 2014, 5, 191–198. [Google Scholar] [CrossRef]
- Dang, T.M.D.; Le, T.T.T.; Fribourg-Blanc, E.; Dang, M.C. Synthesis and optical properties of copper nanoparticles prepared by a chemical reduction method. Adv. Nat. Sci. Nanosci. Nanotechnol. 2011, 2, 015009. [Google Scholar] [CrossRef]
- Matikainen, A.; Nuutinen, T.; Itkonen, T.; Heinilehto, S.; Puustinen, J.; Hiltunen, J.; Lappalainen, J.; Karioja, P.; Vahimaa, P. Atmospheric oxidation and carbon contamination of silver and its effect on surface-enhanced Raman spectroscopy (SERS). Sci. Rep. 2016, 6, 37192. [Google Scholar] [CrossRef] [Green Version]
- Zeng, F.; Xu, D.; Zhan, C.; Liang, C.; Zhao, W.; Zhang, J.; Feng, H.; Ma, X. Surfactant-Free Synthesis of Graphene Oxide Coated Silver Nanoparticles for SERS Biosensing and Intracellular Drug Delivery. ACS Appl. Nano Mater. 2018, 1, 2748–2753. [Google Scholar] [CrossRef]
- Neddersen, J.; Chumanov, G.; Cotton, T.M. Laser Ablation of Metals: A New Method for Preparing SERS Active Colloids. Appl. Spectrosc. 1993, 47, 1959–1964. [Google Scholar] [CrossRef]
- Gondal, M.A.; Qahtan, T.F.; Dastageer, M.A.; Maganda, Y.W.; Anjum, D.H. Synthesis of Cu/Cu2O nanoparticles by laser ablation in deionized water and their annealing transformation into CuO nanoparticles. J. Nanosci. Nanotechnol. 2013, 13, 5759–5766. [Google Scholar] [CrossRef] [PubMed]
- Aghdam, H.D.; Azadi, H.; Esmaeilzadeh, M.; Bellah, S.M.; Malekfar, R. Ablation time and laser fluence impacts on the composition, morphology and optical properties of copper oxide nanoparticles. Opt. Mater. 2019, 91, 433–438. [Google Scholar] [CrossRef]
- Abdulateef, S.A.; MatJafri, M.Z.; Omar, A.F.; Ahmed, N.M.; Azzez, S.A.; Ibrahim, I.M.; Al-Jumaili, B.E.B. Preparation of CuO nanoparticles by laser ablation in liquid. AIP Conf. Proc. 2016, 1733, 020035. [Google Scholar]
- Kudelski, A.; Grochala, W.; Janik-Czachor, M.; Bukowska, J.; Szummer, A.; Dolata, M. Surface-enhanced Raman scattering (SERS) at Copper(I) oxide. J. Raman Spectrosc. 1998, 29, 431–435. [Google Scholar] [CrossRef]
- Amendola, V.; Rizzi, G.A.; Polizzi, S.; Meneghetti, M. Synthesis of Gold Nanoparticles by Laser Ablation in Toluene: Quenching and Recovery of the Surface Plasmon Absorption. J. Phys. Chem. B 2005, 109, 23125–23128. [Google Scholar] [CrossRef] [PubMed]
- Nikov, R.G.; Nedyalkov, N.N.; Atanasov, P.A.; Karashanova, D.B. Laser-assisted fabrication and size distribution modification of colloidal gold nanostructures by nanosecond laser ablation in different liquids. Appl. Phys. A 2017, 123, 490. [Google Scholar] [CrossRef]
- Cho, H.-G. Matrix Infrared Spectra and DFT Computations of CH2CNH and CH2NCH Produced from CH3CN by Laser-Ablation Plume Radiation. Bull. Korean Chem. Soc. 2013, 34, 1361–1365. [Google Scholar] [CrossRef]
- Andrews, L.; Citra, A. Infrared Spectra and Density Functional Theory Calculations on Transition Metal Nitrosyls. Vibrational Frequencies of Unsaturated Transition Metal Nitrosyls. Chem. Rev. 2002, 102, 885–912. [Google Scholar] [CrossRef]
- Cho, H.-G.; Andrews, L. Matrix Infrared Spectra and Density Functional Calculations of the H2CCN and H2CNC Radicals Produced from CH3CN. J. Phys. Chem. A 2011, 115, 8638–8642. [Google Scholar] [CrossRef]
- Amendola, V.; Meneghetti, M. What controls the composition and the structure of nanomaterials generated by laser ablation in liquid solution? Phys. Chem. Chem. Phys. 2013, 15, 3027–3046. [Google Scholar] [CrossRef] [PubMed]
- Hamad, S.; Podagatlapalli, G.K.; Rao, S.V.; Syed, H.; Soma, V.R. Explosives Detection with Copper Nanostructures Fabricated using Ultrafast Laser Ablation in Acetonitrile. Light Energy Environ. 2014. [Google Scholar] [CrossRef]
- Kessler, F.K.; Zheng, Y.; Schwarz, D.; Merschjann, C.; Schnick, W.; Wang, X.; Bojdys, M.J. Functional carbon nitride materials—Design strategies for electrochemical devices. Nat. Rev. Mater. 2017, 2, 17030. [Google Scholar] [CrossRef]
- Miller, T.; Jorge, A.B.; Suter, T.M.; Sella, A.; Corà, F.; McMillan, P.F. Carbon nitrides: Synthesis and characterization of a new class of functional materials. Phys. Chem. Chem. Phys. 2017, 19, 15613–15638. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Xie, J.; Chen, X.; Li, X. A review on g-C 3 N 4 -based photocatalysts. Appl. Surf. Sci. 2017, 391, 72–123. [Google Scholar] [CrossRef]
- Mansor, N.; Miller, T.S.; Dedigama, I.; Jorge, A.B.; Jia, J.; Brázdová, V.; Mattevi, C.; Gibbs, C.; Hodgson, D.; Shearing, P.R.; et al. Graphitic Carbon Nitride as a Catalyst Support in Fuel Cells and Electrolyzers. Electrochim. Acta 2016, 222, 44–57. [Google Scholar] [CrossRef] [Green Version]
- Algara-Siller, G.; Severin, N.; Chong, S.Y.; Björkman, T.; Palgrave, R.G.; Laybourn, A.; Antonietti, M.; Khimyak, Y.Z.; Krasheninnikov, A.V.; Rabe, J.P.; et al. Triazine-Based Graphitic Carbon Nitride: A Two-Dimensional Semiconductor. Angew. Chem. 2014, 126, 7580–7585. [Google Scholar] [CrossRef]
- Wirnhier, E.; Doblinger, M.; Gunzelmann, D.; Senker, J.; Lotsch, B.V.; Schnick, W. Poly(triazine imide) with intercalation of lithium and chloride ions [(C3N3)2(NHxLi1-x)3•LiCl]: A crystalline 2D carbon nitride network. Chem. Eur. J. 2011, 17, 3213–3221. [Google Scholar] [CrossRef]
- Ladva, S.A.; Travis, W.; Quesada-Cabrera, R.; Rosillo-Lopez, M.; Afandi, A.; Li, Y.; Jackman, R.B.; Bear, J.C.; Parkin, I.P.; Blackman, C.; et al. Nanoscale, conformal films of graphitic carbon nitride deposited at room temperature: A method for construction of heterojunction devices. Nanoscale 2017, 9, 16586–16590. [Google Scholar] [CrossRef]
- Suter, T.M.; Brazdova, V.; McColl, K.; Miller, T.S.; Nagashima, H.; Salvadori, E.; Sella, A.; Howard, C.A.; Kay, C.W.M.; Cora, F.; et al. Synthesis, Structure and Electronic Properties of Graphitic Carbon Nitride Films. J. Phys. Chem. C 2018, 122, 25183–25194. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.J.; Fan, S.; Lieber, C.M. Growth and composition of covalent carbon nitride solids. Appl. Phys. Lett. 1995, 66, 3582–3584. [Google Scholar] [CrossRef] [Green Version]
- Rodil, S.E.; Muhl, S.; Maca, S.; Ferrari, A. Optical gap in carbon nitride films. Thin Solid Films 2003, 433, 119–125. [Google Scholar] [CrossRef]
- Inagaki, M.; Toyoda, M.; Soneda, Y.; Morishita, T. Nitrogen-doped carbon materials. Carbon 2018, 132, 104–140. [Google Scholar] [CrossRef]
- González, P.; Soto, R.; Parada, E.; Redondas, X.; Chiussi, S.; Serra, J.; Pou, J.; Leon, B.; Pérez-Amor, M. Carbon nitride films prepared by excimer laser ablation. Appl. Surf. Sci. 1997, 109, 380–383. [Google Scholar] [CrossRef]
- Bulíř, J.; Jelínek, M.; Vorlicek, V.; Zemek, J.; Perina, V. Study of nitrogen pressure effect on the laser-deposited amorphous carbon films. Thin Solid Films 1997, 292, 318–323. [Google Scholar] [CrossRef]
- Zhao, X.; Ong, C.W.; Tsang, Y.C.; Wong, Y.W.; Chan, P.W.; Choy, C.L. Reactive pulsed laser deposition of CNx films. Appl. Phys. Lett. 1995, 66, 2652–2654. [Google Scholar] [CrossRef]
- Ong, C.W.; Zhao, X.-A.; Tsang, Y.; Choy, C.; Chan, P. Effects of substrate temperature on the structure and properties of reactive pulsed laser deposited CNx films. Thin Solid Films 1996, 280, 1–4. [Google Scholar] [CrossRef]
- Alexandrescu, R.; Huisken, F.; Pugna, G.; Crunteanu, A.; Petcu, S.; Cojocaru, S.; Cireasa, R.; Morjan, I.; Cojocaru, C.S. Preparation of carbon nitride fine powder by laser induced gas-phase reactions. Appl. Phys. A 1997, 65, 207–213. [Google Scholar] [CrossRef]
- Sharma, A.K.; Ayyub, P.; Multani, M.S.; Adhi, K.P.; Ogale, S.B.; Sunderaraman, M.; Upadhyay, D.D.; Banerjee, S. Synthesis of crystalline carbon nitride thin films by laser processing at a liquid–solid interface. Appl. Phys. Lett. 1996, 69, 3489–3491. [Google Scholar] [CrossRef]
- Yang, L.; May, P.W.; Yin, L.; Brown, R.; Scott, T.B. Direct Growth of Highly Organized Crystalline Carbon Nitride from Liquid-Phase Pulsed Laser Ablation. Chem. Mater. 2006, 18, 5058–5064. [Google Scholar] [CrossRef]
- Li, H.; Xu, Y.; Sitinamaluwa, H.; Wasalathilake, K.; Galpaya, D.; Yan, C. Cu nanoparticles supported on graphitic carbon nitride as an efficient electrocatalyst for oxygen reduction reaction. Chin. J. Catal. 2017, 38, 1006–1010. [Google Scholar] [CrossRef] [Green Version]
- Le, S.; Fang, B.; Jiang, T.; Zhao, Q.; Liu, X.; Li, Y.; Gong, M. Cu-doped mesoporous graphitic carbon nitride for enhanced visible-light driven photocatalysis. RSC Adv. 2016, 6, 38811–38819. [Google Scholar] [CrossRef]
- Muniandy, L.; Adam, F.; Mohamed, A.R.; Iqbal, A.; Rahman, N.R.A. Cu2+ coordinated graphitic carbon nitride (Cu-g-C3N4) nanosheets from melamine for the liquid phase hydroxylation of benzene and VOCs. Appl. Surf. Sci. 2017, 398, 43–55. [Google Scholar] [CrossRef]
- Gao, J.; Wang, J.; Qian, X.; Dong, Y.; Xu, H.; Song, R.; Yan, C.; Zhu, H.; Zhong, Q.; Qian, G.; et al. One-pot synthesis of copper-doped graphitic carbon nitride nanosheet by heating Cu–melamine supramolecular network and its enhanced visible-light-driven photocatalysis. J. Solid State Chem. 2015, 228, 60–64. [Google Scholar] [CrossRef]
- Tahir, B.; Tahir, M.; Amin, N.A.S. Photo-induced CO2 reduction by CH4/H2O to fuels over Cu-modified g-C3N4 nanorods under simulated solar energy. Appl. Surf. Sci. 2017, 419, 875–885. [Google Scholar] [CrossRef]
- Shi, G.; Yang, L.; Liu, Z.; Chen, X.; Zhou, J.; Yu, Y. Photocatalytic reduction of CO2 to CO over copper decorated g-C3N4 nanosheets with enhanced yield and selectivity. Appl. Surf. Sci. 2018, 427, 1165–1173. [Google Scholar] [CrossRef]
- Wolosiuk, A.; Tognalli, N.G.; Martínez, E.D.; Granada, M.; Fuertes, M.C.; Troiani, H.; Bilmes, S.A.; Fainstein, A.; Soler-Illia, G.J.A.A. Silver Nanoparticle-Mesoporous Oxide Nanocomposite Thin Films: A Platform for Spatially Homogeneous SERS-Active Substrates with Enhanced Stability. ACS Appl. Mater. Interfaces 2014, 6, 5263–5272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, K.-H.; Liu, Y.-C.; Hsu, T.-C.; Juang, M.-Y. Strategy to improve stability of surface-enhanced raman scattering-active Ag substrates. J. Mater. Chem. 2010, 20, 7530–7535. [Google Scholar] [CrossRef]
- Mai, F.-D.; Yang, K.-H.; Liu, Y.-C.; Hsu, T.-C. Improved stabilities on surface-enhanced Raman scattering-active Ag/Al2O3 films on substrates. Analyst 2012, 137, 5906–5912. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Zhu, L.; Zou, J.; Ou-Yang, L.; Zheng, A.; Tang, H. Micro/nano-structured graphitic carbon nitride—Ag nanoparticle hybrids as surface-enhanced Raman scattering substrates with much improved long-term stability. Carbon 2015, 87, 193–205. [Google Scholar] [CrossRef]
- Jiang, J.; Zou, J.; Wee, A.T.S.; Zhang, W. Use of Single-Layer g-C3N4/Ag Hybrids for Surface-Enhanced Raman Scattering (SERS). Sci. Rep. 2016, 6, 34599. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.; Gerson, A.R.; Smart, R.S. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Liu, P.; Wang, H.; Li, X.; Rui, M.; Zeng, H. Localized surface plasmon resonance of Cu nanoparticles by laser ablation in liquid media. RSC Adv. 2015, 5, 79738–79745. [Google Scholar] [CrossRef]
- Zambon, A.; Mouesca, J.M.; Gheorghiu, C.; Bayle, P.A.; Pécaut, J.; Claeys-Bruno, M.; Gambarelli, S.; Dubois, L. s-Heptazine oligomers: Promising structural models for graphitic carbon nitride. Chem. Sci. 2016, 7, 945–950. [Google Scholar] [CrossRef] [PubMed]
- Fina, F.; Callear, S.K.; Carins, G.M.; Irvine, J.T.S. Structural Investigation of Graphitic Carbon Nitride via XRD and Neutron Diffraction. Chem. Mater. 2015, 27, 2612–2618. [Google Scholar] [CrossRef] [Green Version]
- Tyborski, T.; Merschjann, C.; Orthmann, S.; Yang, F.; Lux-Steiner, M.-C.; Schedel-Niedrig, T. Crystal structure of polymeric carbon nitride and the determination of its process-temperature-induced modifications. J. Phys. Condens. Matter 2013, 25, 395402. [Google Scholar] [CrossRef] [Green Version]
- Bonner, O.; Curry, J.D. Infrared spectra of liquid H2O and D2O. Infrared Phys. 1970, 10, 91–94. [Google Scholar] [CrossRef]
- Rodil, S.E.; Ferrari, A.; Robertson, J.; Muhl, S. Infrared spectra of carbon nitride films. Thin Solid Films 2002, 420, 122–131. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Rodil, S.E.; Robertson, J. Interpretation of infrared and Raman spectra of amorphous carbon nitrides. Phys. Rev. B 2003, 67, 155306. [Google Scholar] [CrossRef] [Green Version]
- Angeloni, L.; Smulevich, G.; Marzocchi, M.P. Resonance Raman spectrum of crystal violet. J. Raman Spectrosc. 1979, 8, 305–310. [Google Scholar] [CrossRef]
- Anastasopoulos, J.; Beobide, A.S.; Manikas, A.; Voyiatzis, G. Quantitative surface-enhanced resonance Raman scattering analysis of methylene blue using silver colloid. J. Raman Spectrosc. 2017, 48, 1762–1770. [Google Scholar] [CrossRef]
- Mo, Y.; Lei, J.; Li, X.; Wächter, P. Surface enhanced Raman scattering of rhodamine 6G and dye 1555 adsorbed on roughened copper surfaces. Solid State Commun. 1988, 66, 127–131. [Google Scholar] [CrossRef]
- Ujihara, M.; Dang, N.M.; Imae, T. Surface-Enhanced Resonance Raman Scattering of Rhodamine 6G in Dispersions and on Films of Confeito-Like Au Nanoparticles. Sensors 2017, 17, 2563. [Google Scholar] [CrossRef] [PubMed]
- Le Ru, E.C.; Blackie, E.; Meyer, M.; Etchegoin, P.G. Surface Enhanced Raman Scattering Enhancement Factors: A Comprehensive Study. J. Phys. Chem. C 2007, 111, 13794–13803. [Google Scholar] [CrossRef]
- Jiang, L.; You, T.; Yin, P.; Shang, Y.; Zhang, D.; Guo, L.; Yang, S. Surface-enhanced Raman scattering spectra of adsorbates on Cu2O nanospheres: Charge-transfer and electromagnetic enhancement. Nanoscale 2013, 5, 2784–2789. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Chua, J.; Tan, E.K.M.; Kah, J.C.Y. Optimizing the SERS enhancement of a facile gold nanostar immobilized paper-based SERS substrate. RSC Adv. 2017, 7, 16264–16272. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Konrad, M.P.; Trotter, J.L.; McCoy, C.P.; Bell, S.E.J. Rapid One-Pot Preparation of Large Freestanding Nanoparticle-Polymer Films. Small 2016, 13, 1602163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, S.; Zhang, C.; Yuwen, L.; Chao, J.; Zuo, X.; Liu, X.; Song, C.; Fan, C.; Wang, L. Creating SERS Hot Spots on MoS2 Nanosheets with in Situ Grown Gold Nanoparticles. ACS Appl. Mater. Interfaces 2014, 6, 18735–18741. [Google Scholar] [CrossRef]
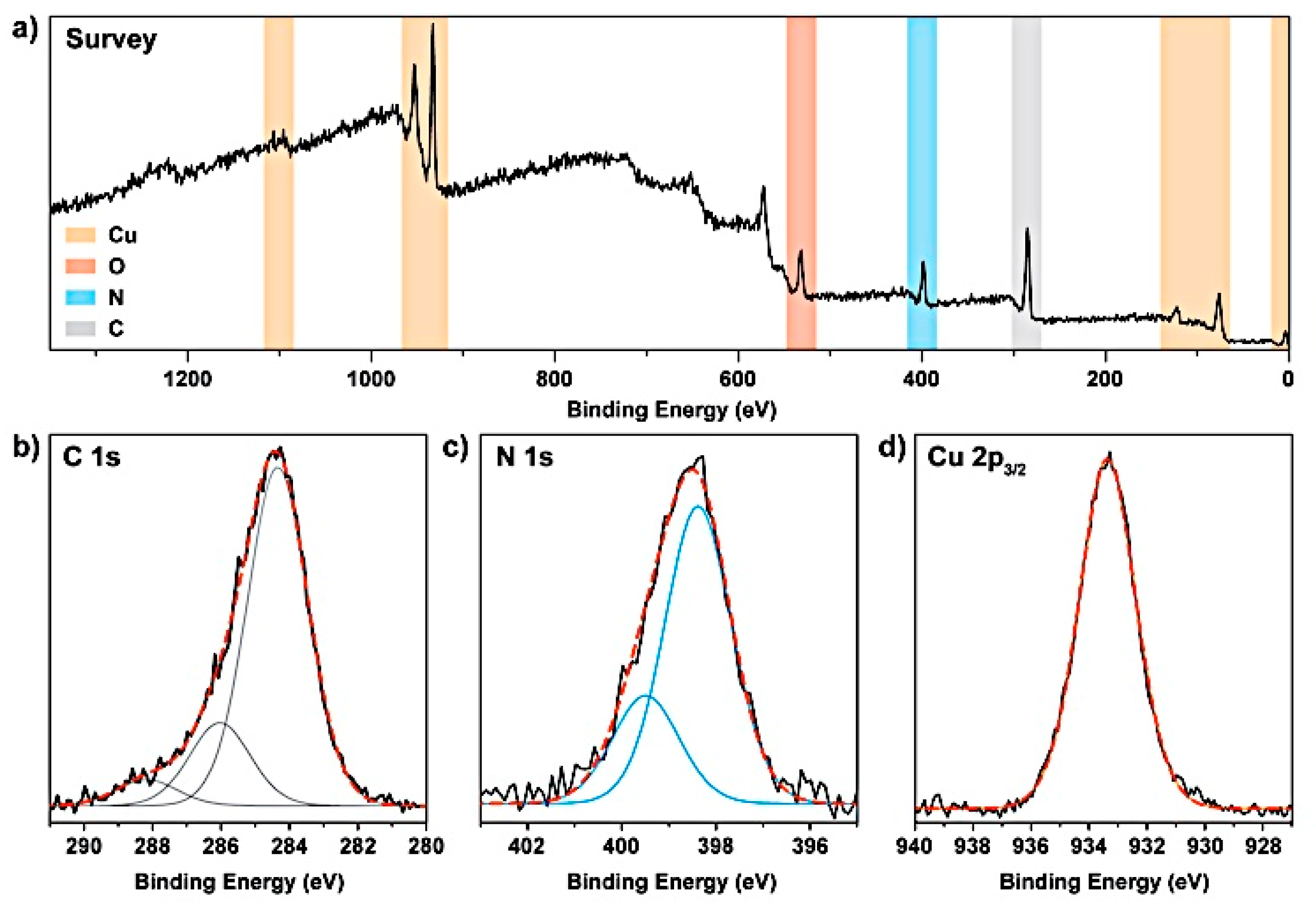
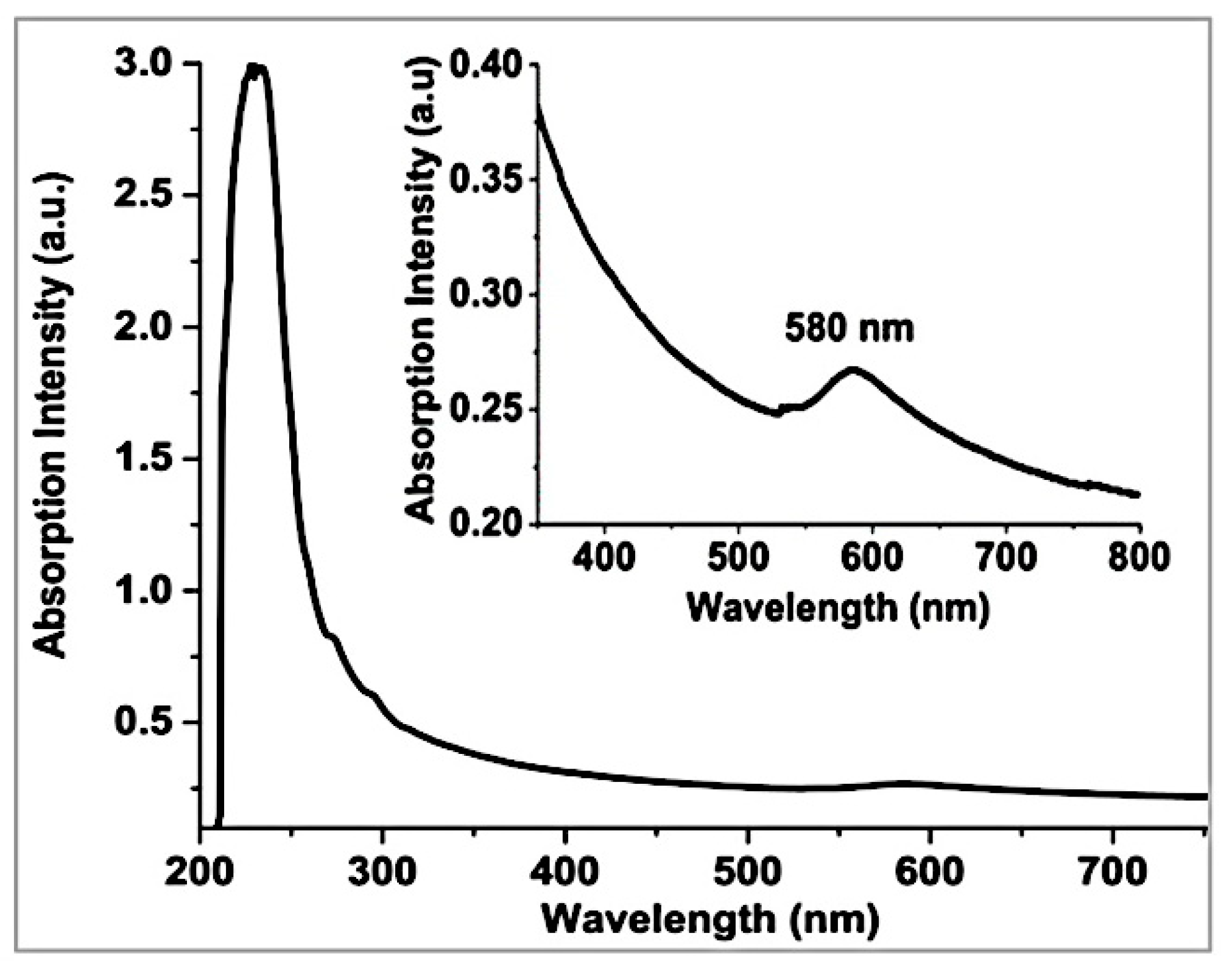
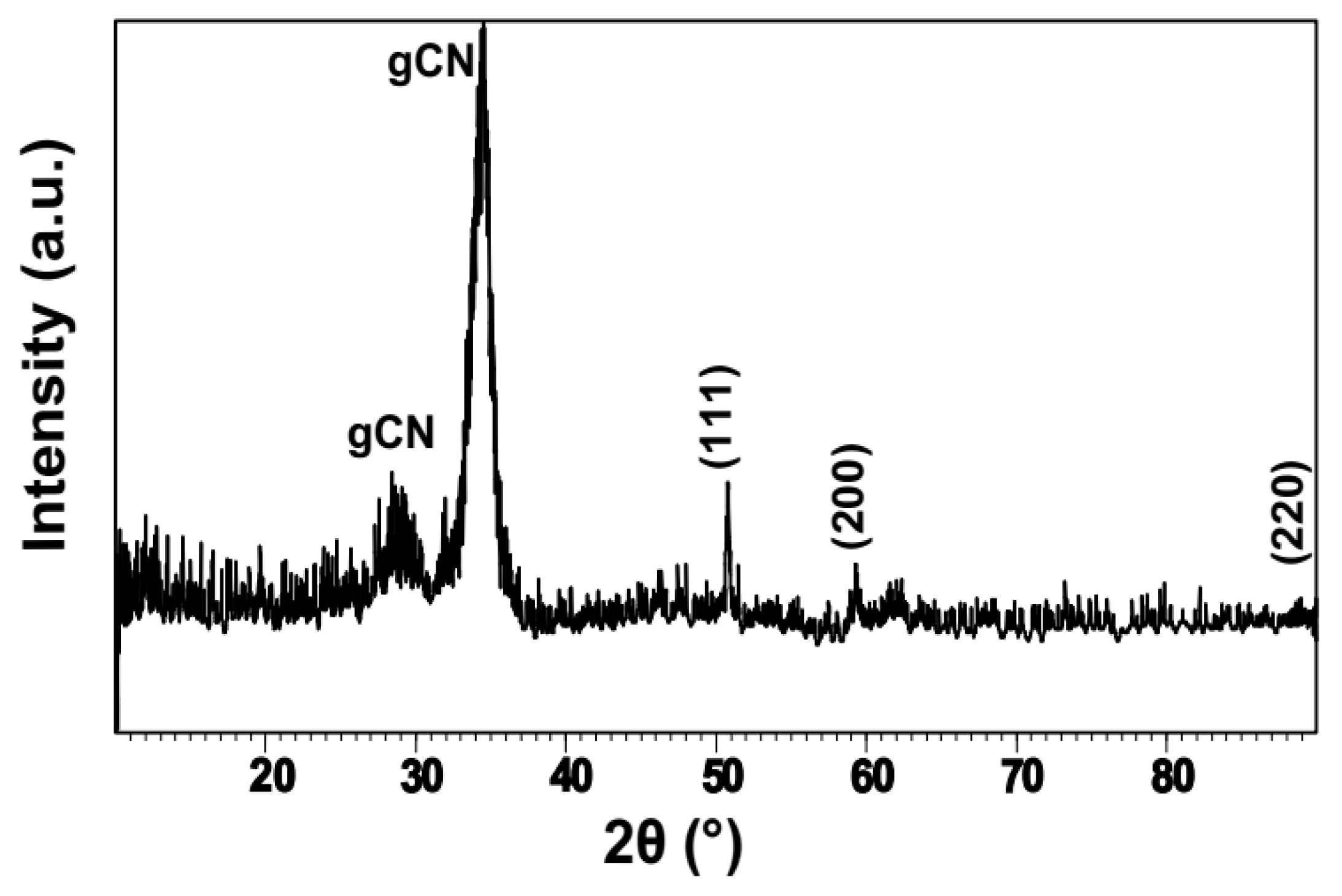
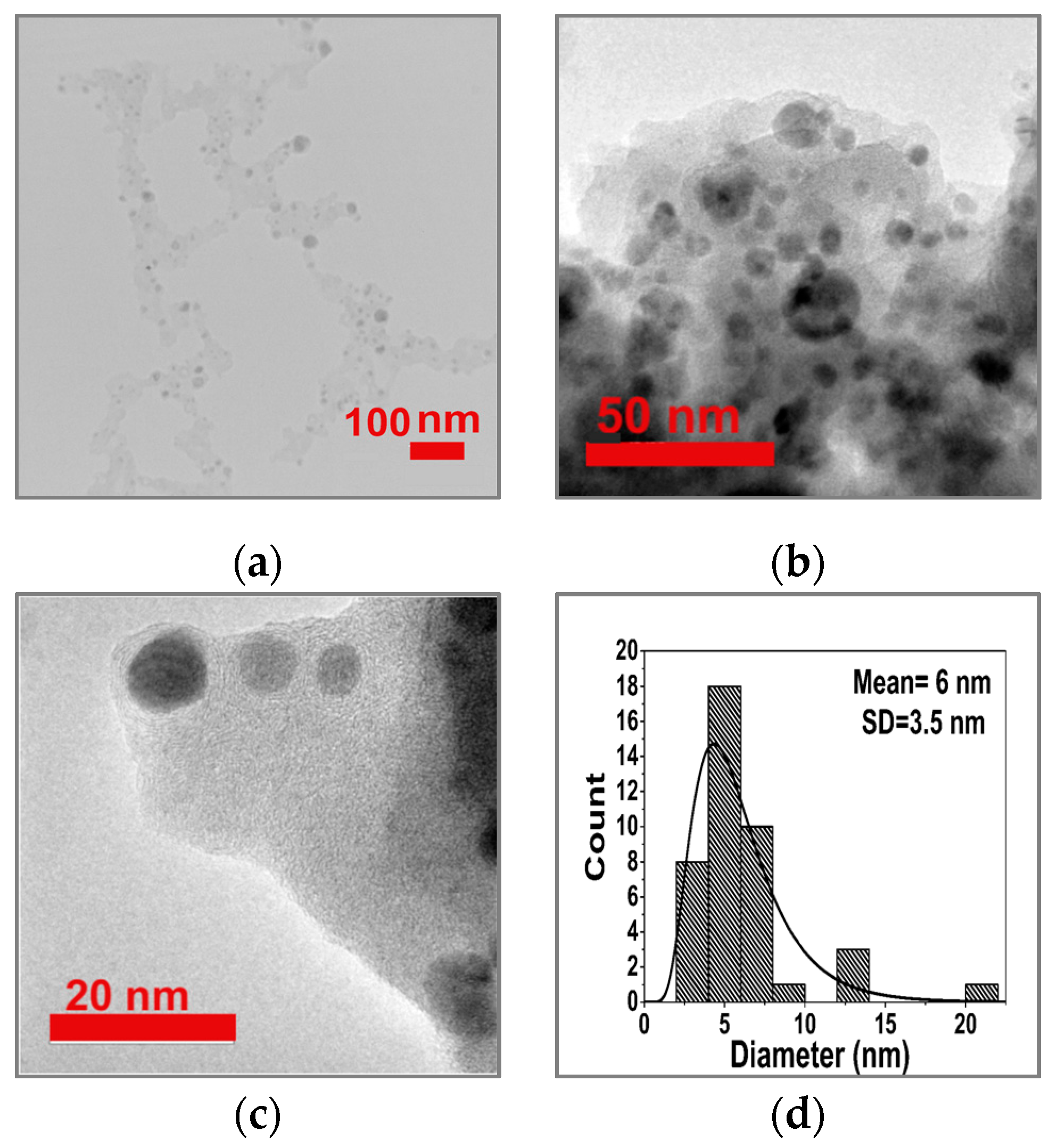


| Angle (2θ) | {hkl} | FWHM (2θ) | d-spacing (Å) | Height (counts) | Identification |
|---|---|---|---|---|---|
| 28.9 | * | 3.3 | 3.59 | 27.2 | gCN |
| 34.3 | * | 1.6 | 3.03 | 153.9 | gCN |
| 50.7 | {111} | 0.3 | 2.09 | 36.9 | Cu |
| 59.2 | {200} | 0.4 | 1.08 | 14.6 | Cu |
| 89.1 | {220} | 3.2 | 1.27 | 5.4 | Cu |
| Analyte | Density (g/mL) | Surface Area (nm2) | nRaman/nSERS | ISERS/IRaman | Cu/g-CN EFs |
|---|---|---|---|---|---|
| Crystal violet | 1 | 1.2 | 5.4 × 1015π/1.5 × 109π | 20 | 7.2 × 107 |
| Methylene blue | 0.98 | 1.3 | 6.6 × 1015π/1.4 × 109π | 6.6 | 2.3 × 107 |
| Rhodamine 6G | 1.26 | 0.6 | 6 × 1015π/3 × 109π | 6.4 | 1.3 × 107 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dizajghorbani-Aghdam, H.; Miller, T.S.; Malekfar, R.; McMillan, P.F. SERS-Active Cu Nanoparticles on Carbon Nitride Support Fabricated Using Pulsed Laser Ablation. Nanomaterials 2019, 9, 1223. https://doi.org/10.3390/nano9091223
Dizajghorbani-Aghdam H, Miller TS, Malekfar R, McMillan PF. SERS-Active Cu Nanoparticles on Carbon Nitride Support Fabricated Using Pulsed Laser Ablation. Nanomaterials. 2019; 9(9):1223. https://doi.org/10.3390/nano9091223
Chicago/Turabian StyleDizajghorbani-Aghdam, Hossein, Thomas S. Miller, Rasoul Malekfar, and Paul F. McMillan. 2019. "SERS-Active Cu Nanoparticles on Carbon Nitride Support Fabricated Using Pulsed Laser Ablation" Nanomaterials 9, no. 9: 1223. https://doi.org/10.3390/nano9091223





